Occurrence and Distribution of Antibiotics in the Water, Sediment, and Biota of Freshwater and Marine Environments: A Review
Abstract
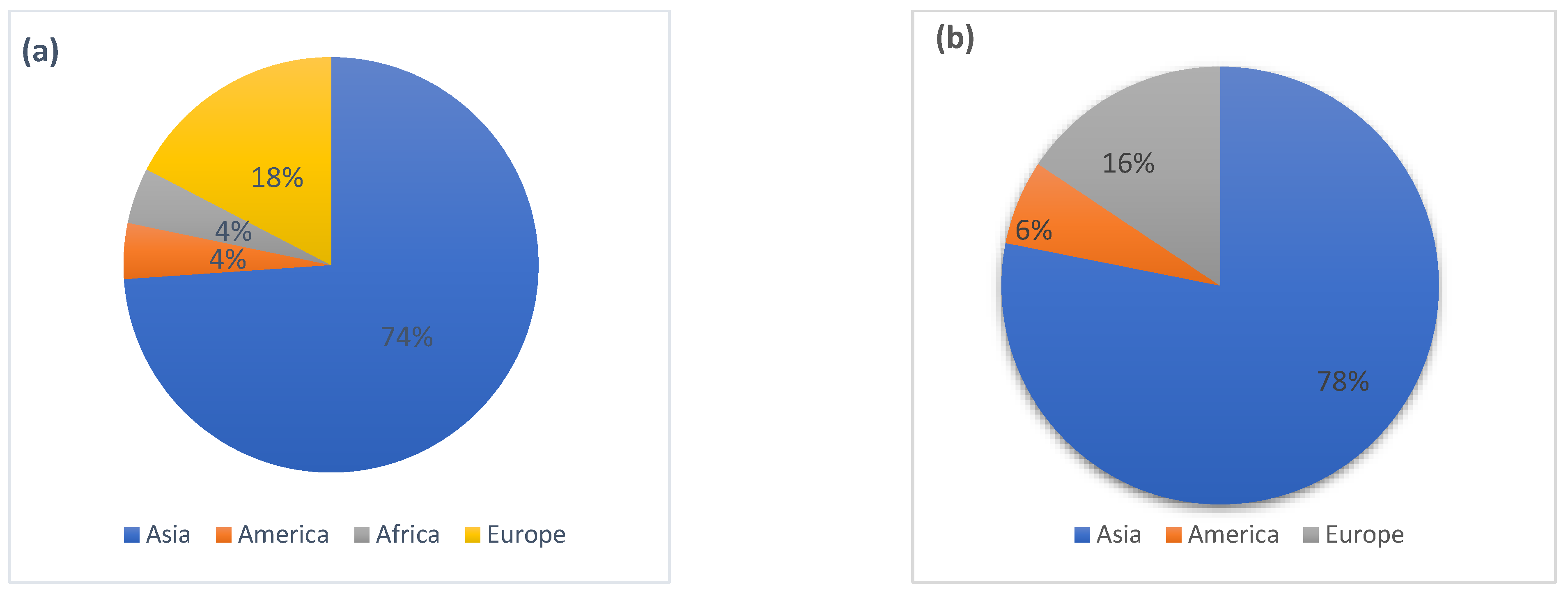
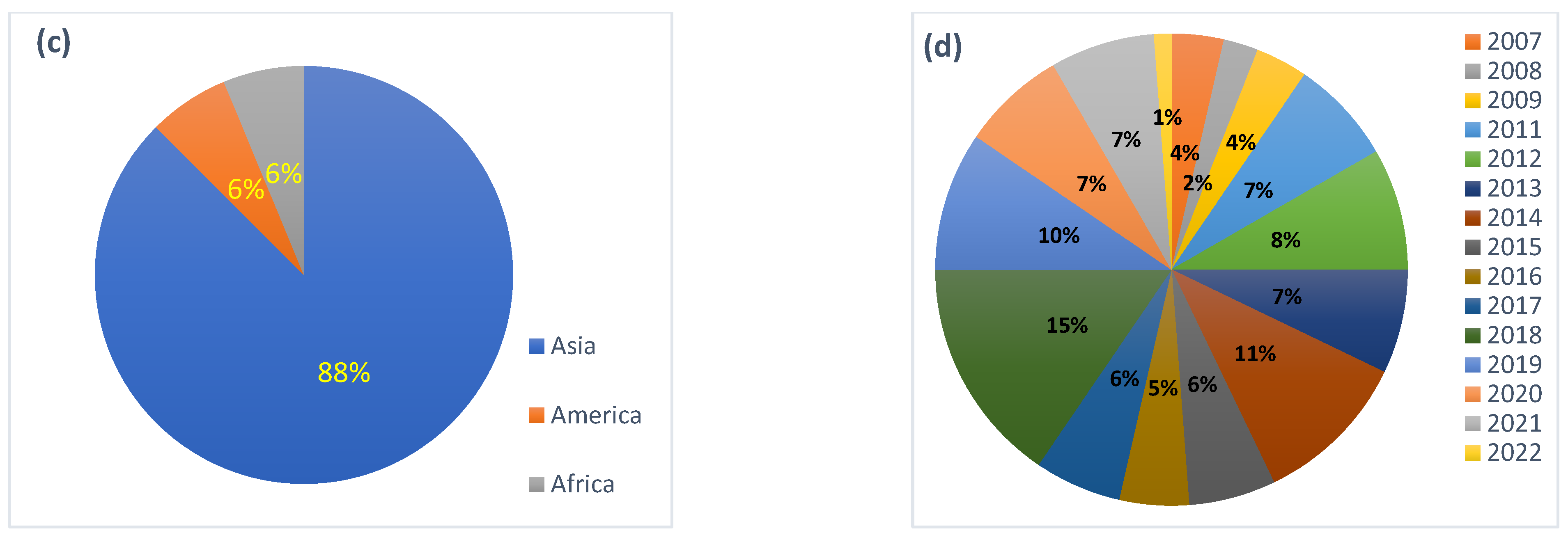
1. Introduction
2. Methodology
3. Results and Discussion
3.1. Classification of Antibiotics
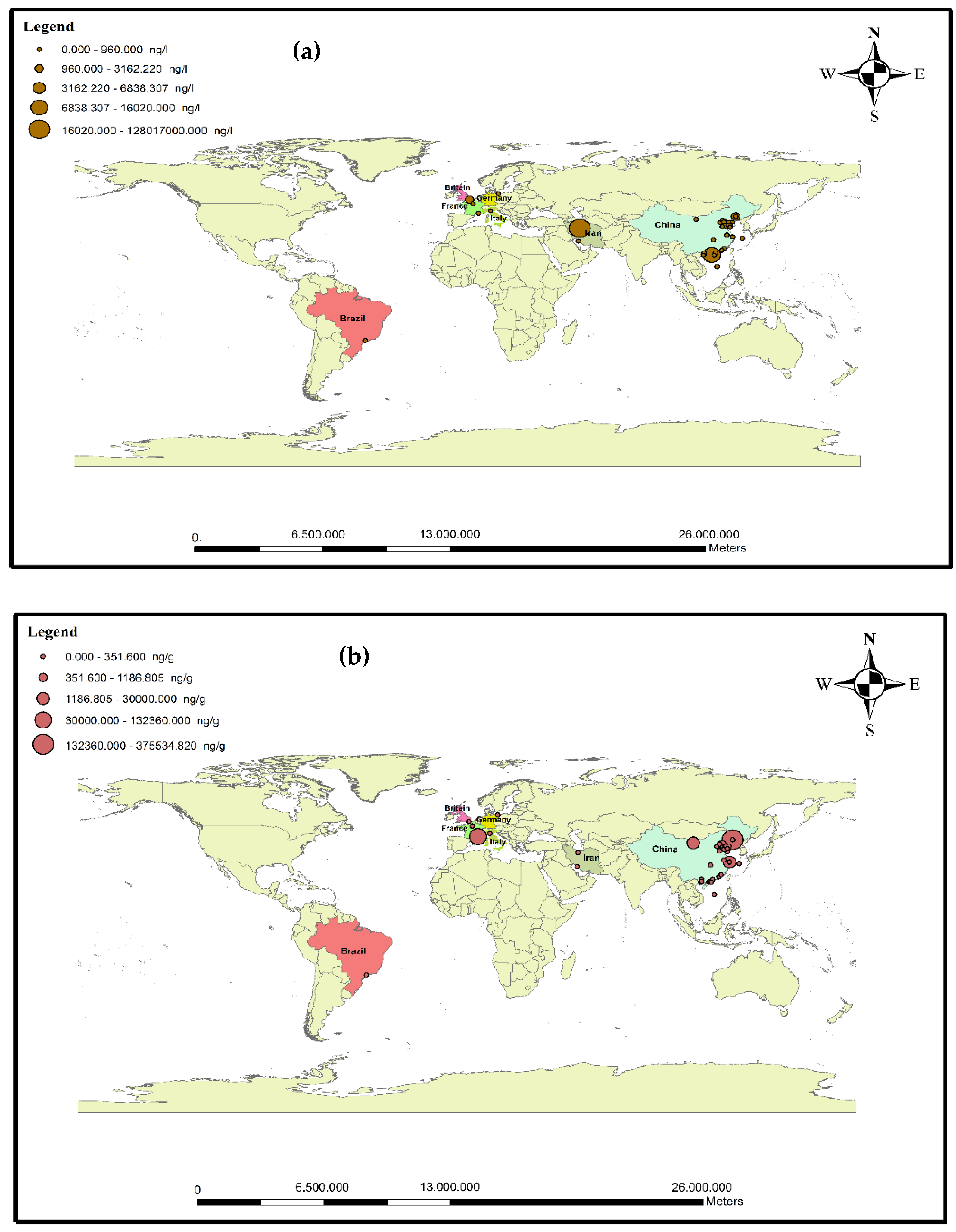
3.2. Occurrence of Antibiotic Pollution in Seawater
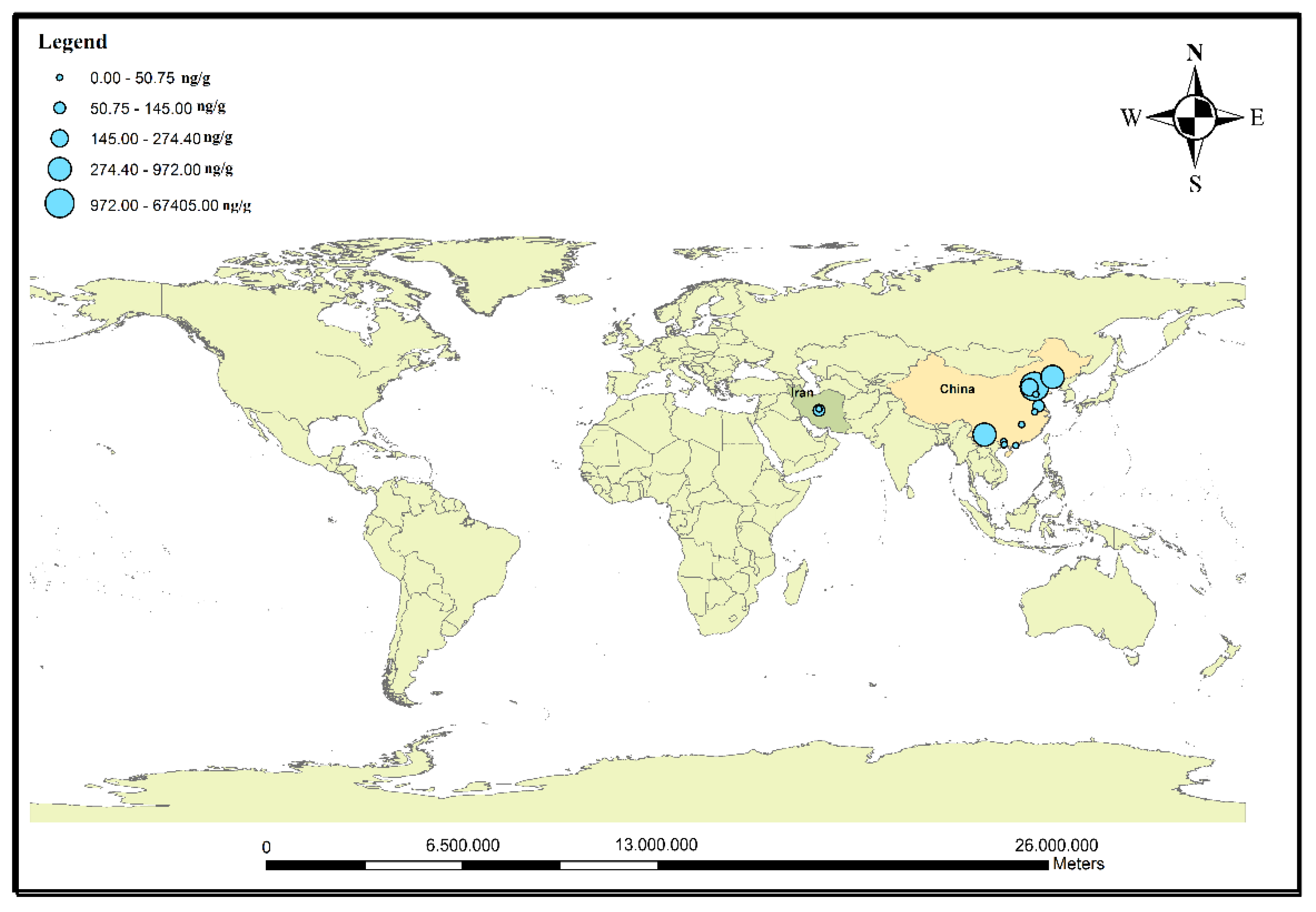
3.3. Occurrence of Antibiotics in Sediments
3.4. Occurrence of Antibiotics in Rivers
3.5. Occurrence of Antibiotics in Lakes
3.6. Occurrence of Antibiotics in Biota
4. Conclusions and Remarks
- Detect the concentrations of antibiotics in more aquatic organisms.
- The function of antibiotics is different in different organisms; therefore, it is necessary to study the concentrations of antibiotics in different organisms (fish, algae, etc.) simultaneously.
- Since antibiotics can accumulate in sediments of aquatic environments, it is recommended that future studies study the concentrations of antibiotics in sediments at different depths.
- The widespread use of antibiotics and the lack of advanced sewage treatment systems in developing countries have caused the pollution of water sources, necessitating more detection of antibiotic contamination and the improvement of treatment systems to remove pollutants.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Anh, H.Q.; Le, T.P.Q.; Da Le, N.; Lu, X.X.; Duong, T.T.; Garnier, J.; Rochelle-Newall, E.; Zhang, S.; Oh, N.-H.; Oeurng, C. Antibiotics in surface water of East and Southeast Asian countries: A focused review on contamination status, pollution sources, potential risks, and future perspectives. Sci. Total Environ. 2021, 764, 142865. [Google Scholar] [CrossRef]
- Page, M.G. Beta-Lactam Antibiotics. In Antibiotic Discovery and Development; Springer: Berlin/Heidelberg, Germany, 2012; pp. 79–117. [Google Scholar]
- Välitalo, P.; Kruglova, A.; Mikola, A.; Vahala, R. Toxicological impacts of antibiotics on aquatic micro-organisms: A mini-review. Int. J. Hyg. Environ. Health 2017, 220, 558–569. [Google Scholar] [CrossRef]
- Giedraitienė, A.; Vitkauskienė, A.; Naginienė, R.; Pavilonis, A. Antibiotic resistance mechanisms of clinically important bacteria. Medicina 2011, 47, 19. [Google Scholar] [CrossRef]
- Heuer, H.; Krögerrecklenfort, E.; Wellington, E.; Egan, S.; Van Elsas, J.; Van Overbeek, L.; Collard, J.-M.; Guillaume, G.; Karagouni, A.; Nikolakopoulou, T. Gentamicin resistance genes in environmental bacteria: Prevalence and transfer. FEMS Microbiol. Ecol. 2002, 42, 289–302. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, X.; Hua, G.; Li, G.; Feng, R.; Liu, X. Migration and degradation of swine farm tetracyclines at the river catchment scale: Can the multi-pond system mitigate pollution risk to receiving rivers? Environ. Pollut. 2017, 220, 1301–1310. [Google Scholar] [CrossRef]
- Ohore, O.E.; Addo, F.G.; Han, N.; Li, X.; Zhang, S. Profiles of ARGs and their relationships with antibiotics, metals and environmental parameters in vertical sediment layers of three lakes in China. J. Environ. Manag. 2020, 255, 109583. [Google Scholar] [CrossRef]
- Liu, X.; Lu, S.; Guo, W.; Xi, B.; Wang, W. Antibiotics in the aquatic environments: A review of lakes, China. Sci. Total Environ. 2018, 627, 1195–1208. [Google Scholar] [CrossRef]
- Wu, Q.; Pan, C.-G.; Wang, Y.-H.; Xiao, S.-K.; Yu, K.-F. Antibiotics in a subtropical food web from the Beibu Gulf, South China: Occurrence, bioaccumulation and trophic transfer. Sci. Total Environ. 2021, 751, 141718. [Google Scholar] [CrossRef]
- Elmahdi, S.; Da Silva, L.V.; Parveen, S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: A review. Food Microbiol. 2016, 57, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Hoa, P.T.P.; Managaki, S.; Nakada, N.; Takada, H.; Shimizu, A.; Anh, D.H.; Viet, P.H.; Suzuki, S. Antibiotic contamination and occurrence of antibiotic-resistant bacteria in aquatic environments of northern Vietnam. Sci. Total Environ. 2011, 409, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, J.; Khan, S.; Su, J.Q.; Hesham, A.E.-L.; Ditta, A.; Nawab, J.; Ali, A. Antibiotics in poultry manure and their associated health issues: A systematic review. J. Soils Sedim. 2020, 20, 486–497. [Google Scholar] [CrossRef]
- Chow, L.K.; Ghaly, T.M.; Gillings, M.R. A survey of sub-inhibitory concentrations of antibiotics in the environment. J. Environ. Sci. 2021, 99, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jing, L.; Teng, Y.; Wang, J. Characterization of antibiotics in a large-scale river system of China: Occurrence pattern, spatiotemporal distribution and environmental risks. Sci. Total Environ. 2018, 618, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Zhou, C.; Guo, C.; Wang, D.; Du, P.; Luo, Y.; Wan, J.; Meng, W. Distribution, sources and composition of antibiotics in sediment, overlying water and pore water from Taihu Lake, China. Sci. Total Environ. 2014, 497, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Pharmaceuticals and personal care products in the aquatic environment in China: A review. J. Hazard. Mater. 2013, 262, 189–211. [Google Scholar] [CrossRef]
- Li, S.; Shi, W.; Liu, W.; Li, H.; Zhang, W.; Hu, J.; Ke, Y.; Sun, W.; Ni, J. A duodecennial national synthesis of antibiotics in China’s major rivers and seas (2005–2016). Sci. Total Environ. 2018, 615, 906–917. [Google Scholar] [CrossRef]
- Lyu, J.; Yang, L.; Zhang, L.; Ye, B.; Wang, L. Antibiotics in soil and water in China—A systematic review and source analysis. Environ. Pollut. 2020, 266, 115147. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Zhang, Z.; Li, P.; Zang, Y.; Liu, X. Antibiotics in aquatic environments of China: A review and meta-analysis. Ecotoxicol. Environ. Saf. 2020, 199, 110668. [Google Scholar] [CrossRef]
- Wu, S.; Hua, P.; Gui, D.; Zhang, J.; Ying, G.; Krebs, P. Occurrences, transport drivers, and risk assessments of antibiotics in typical oasis surface and groundwater. Water Res. 2022, 225, 119138. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.-Y.; Zhang, Q.-Q.; Yan, X.-T.; Zhai, Y.-Q.; Guo, Z.; Li, N.; Ying, G.-G. Antibiotic pollution in lakes in China: Emission estimation and fate modeling using a temperature-dependent multimedia model. Sci. Total Environ. 2022, 842, 156633. [Google Scholar] [CrossRef] [PubMed]
- Lü, D.-Y.; Yu, C.; Zhuo, Z.-J.; Meng, S.-R.; Liu, S.-B. The distribution and ecological risks of antibiotics in surface water in key cities along the lower reaches of the Yellow River: A case study of Kaifeng City, China. China Geol. 2022, 5, 411–420. [Google Scholar] [CrossRef]
- Li, M.; Yang, L.; Yen, H.; Zhao, F.; Wang, X.; Zhou, T.; Feng, Q.; Chen, L. Occurrence, spatial distribution and ecological risks of antibiotics in soil in urban agglomeration. J. Environ. Sci. 2023, 125, 678–690. [Google Scholar] [CrossRef]
- Shen, W.; Chen, Y.; Wang, N.; Wan, P.; Peng, Z.; Zhao, H.; Wang, W.; Xiong, L.; Zhang, S.; Liu, R. Seasonal variability of the correlation network of antibiotics, antibiotic resistance determinants, and bacteria in a wastewater treatment plant and receiving water. J. Environ. Manag. 2022, 317, 115362. [Google Scholar] [CrossRef]
- Leonard, A.F.C.; Morris, D.; Schmitt, H.; Gaze, W.H. Natural recreational waters and the risk that exposure to antibiotic resistant bacteria poses to human health. Curr. Opin. Microbiol. 2022, 65, 40–46. [Google Scholar] [CrossRef]
- Díaz-Cruz, M.S.; Barceló, D. Recent advances in LC-MS residue analysis of veterinary medicines in the terrestrial environment. TrAC Trends Anal. Chem. 2007, 26, 637–646. [Google Scholar] [CrossRef]
- Aydin, E.; Talinli, I. Analysis, occurrence and fate of commonly used pharmaceuticals and hormones in the Buyukcekmece Watershed, Turkey. Chemosphere 2013, 90, 2004–2012. [Google Scholar] [CrossRef]
- Shurbaji, S.; Huong, P.T.; Altahtamouni, T.M. Review on the visible light photocatalysis for the decomposition of ciprofloxacin, norfloxacin, tetracyclines, and sulfonamides antibiotics in wastewater. Catalysts 2021, 11, 437. [Google Scholar] [CrossRef]
- Roose-Amsaleg, C.; Laverman, A.M. Do antibiotics have environmental side-effects? Impact of synthetic antibiotics on biogeochemical processes. Environ. Sci. Pollut. Res. 2016, 23, 4000–4012. [Google Scholar] [CrossRef]
- Zhang, K.; Ruan, R.; Zhang, Z.; Zhi, S. An exhaustive investigation on antibiotics contamination from livestock farms within sensitive reservoir water area: Spatial density, source apportionment and risk assessment. Sci. Total Environ. 2022, 847, 157688. [Google Scholar] [CrossRef] [PubMed]
- Zainab, S.M.; Junaid, M.; Rehman, M.Y.A.; Lv, M.; Yue, L.; Xu, N.; Malik, R.N. First insight into the occurrence, spatial distribution, sources, and risks assessment of antibiotics in groundwater from major urban-rural settings of Pakistan. Sci. Total Environ. 2021, 791, 148298. [Google Scholar] [CrossRef] [PubMed]
- Jafari Ozumchelouei, E.; Hamidian, A.H.; Zhang, Y.; Yang, M. Physicochemical properties of antibiotics: A review with an emphasis on detection in the aquatic environment. Water Environ. Res. 2020, 92, 177–188. [Google Scholar] [CrossRef] [PubMed]
- O’Flynn, D.; Lawler, J.; Yusuf, A.; Parle-McDermott, A.; Harold, D.; Mc Cloughlin, T.; Holland, L.; Regan, F.; White, B. A review of pharmaceutical occurrence and pathways in the aquatic environment in the context of a changing climate and the COVID-19 pandemic. Anal. Methods 2021, 13, 575–594. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Liu, X.; Wang, J.; Li, S. Accumulation and risk assessment of antibiotics in edible plants grown in contaminated farmlands: A review. Sci. Total Environ. 2022, 853, 158616. [Google Scholar] [CrossRef] [PubMed]
- Imwene, K.O.; Ngumba, E.; Kairigo, P.K. Emerging technologies for enhanced removal of residual antibiotics from source-separated urine and wastewaters: A review. J. Environ. Manag. 2022, 322, 116065. [Google Scholar] [CrossRef]
- Brisson-Noël, A.; Trieu-Cuot, P.; Courvalin, P. Mechanism of action of spiramycin and other macrolides. J. Antimicrob. Chemother. 1988, 22, 13–23. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Dueck, M.; Hoban, D.J.; Vercaigne, L.M.; Embil, J.M.; Gin, A.S.; Karlowsky, J.A. Review of macrolides and ketolides. Drugs 2001, 61, 443–498. [Google Scholar] [CrossRef]
- Holten, K.B.; Onusko, E.M. Appropriate prescribing of oral beta-lactam antibiotics. Am. Fam. Phys. 2000, 62, 611–620. [Google Scholar]
- Deshpande, A.; Baheti, K.; Chatterjee, N. Degradation of β-lactam antibiotics. Curr. Sci. 2004, 1684–1695. [Google Scholar]
- Kumar, M.; Jaiswal, S.; Sodhi, K.K.; Shree, P.; Singh, D.K.; Agrawal, P.K.; Shukla, P. Antibiotics bioremediation: Perspectives on its ecotoxicity and resistance. Environ. Int. 2019, 124, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Hoff, R.; Pizzolato, T.M.; Diaz-Cruz, M.S. Trends in sulfonamides and their by-products analysis in environmental samples using mass spectrometry techniques. Trends Environ. Anal. Chem. 2016, 9, 24–36. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Jjemba, P.K. Excretion and ecotoxicity of pharmaceutical and personal care products in the environment. Ecotoxicol. Environ. Saf. 2006, 63, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Riaz, L.; Mahmood, T.; Khalid, A.; Rashid, A.; Siddique, M.B.A.; Kamal, A.; Coyne, M.S. Fluoroquinolones (FQs) in the environment: A review on their abundance, sorption and toxicity in soil. Chemosphere 2018, 191, 704–720. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Xu, X.-R.; Liu, S.-S.; Zhou, G.-J.; Sun, K.-F.; Zhao, J.-L.; Ying, G.-G. Antibiotics in typical marine aquaculture farms surrounding Hailing Island, South China: Occurrence, bioaccumulation and human dietary exposure. Mar. Pollut. Bull. 2015, 90, 181–187. [Google Scholar] [CrossRef]
- Shao, S.; Hu, Y.; Cheng, J.; Chen, Y. Research progress on distribution, migration, transformation of antibiotics and antibiotic resistance genes (ARGs) in aquatic environment. Crit. Rev. Biotechnol. 2018, 38, 1195–1208. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Kafaei, R.; Papari, F.; Seyedabadi, M.; Sahebi, S.; Tahmasebi, R.; Ahmadi, M.; Sorial, G.A.; Asgari, G.; Ramavandi, B. Occurrence, distribution, and potential sources of antibiotics pollution in the water-sediment of the northern coastline of the Persian Gulf, Iran. Sci. Total Environ. 2018, 627, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zhao, S.; Zhang, X.; Wang, X.; Song, C.; Wang, S. Distribution, combined pollution and risk assessment of antibiotics in typical marine aquaculture farms surrounding the Yellow Sea, North China. Environ. Int. 2020, 138, 105551. [Google Scholar] [CrossRef] [PubMed]
- Managaki, S.; Murata, A.; Takada, H.; Tuyen, B.C.; Chiem, N.H. Distribution of macrolides, sulfonamides, and trimethoprim in tropical waters: Ubiquitous occurrence of veterinary antibiotics in the Mekong Delta. Environ. Sci. Technol. 2007, 41, 8004–8010. [Google Scholar] [CrossRef] [PubMed]
- Chau, H.; Kadokami, K.; Duong, H.; Kong, L.; Nguyen, T.; Nguyen, T.; Ito, Y. Occurrence of 1153 organic micropollutants in the aquatic environment of Vietnam. Environ. Sci. Pollut. Res. 2018, 25, 7147–7156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kang, Y.; Zhang, R.; Han, M.; Zeng, W.; Wang, Y.; Yu, K.; Yang, Y. Occurrence, source, and the fate of antibiotics in mariculture ponds near the Maowei Sea, South China: Storm caused the increase of antibiotics usage. Sci. Total Environ. 2021, 752, 141882. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, R.; Wang, Y.; Pan, X.; Tang, J.; Zhang, G. Occurrence and distribution of antibiotics in the Beibu Gulf, China: Impacts of river discharge and aquaculture activities. Mar. Environ. Res. 2012, 78, 26–33. [Google Scholar] [CrossRef]
- Cheng, D.; Xie, Y.; Yu, Y.; Liu, X.; Zhao, S.; Cui, B.; Bai, J. Occurrence and partitioning of antibiotics in the water column and bottom sediments from the intertidal zone in the Bohai Bay, China. Wetlands 2016, 36, 167–179. [Google Scholar] [CrossRef]
- Su, H.; Xu, W.; Hu, X.; Xu, Y.; Wen, G.; Cao, Y. Spatiotemporal variations and source tracking of antibiotics in an ecological aquaculture farm in Southern China. Sci. Total Environ. 2021, 763, 143022. [Google Scholar] [CrossRef] [PubMed]
- de García, S.O.; Pinto, G.P.; Encina, P.G.; Mata, R.I. Consumption and occurrence of pharmaceutical and personal care products in the aquatic environment in Spain. Sci. Total Environ. 2013, 444, 451–465. [Google Scholar] [CrossRef]
- Xu, W.H.; Zhang, G.; Wai, O.W.; Zou, S.C.; Li, X.D. Transport and adsorption of antibiotics by marine sediments in a dynamic environment. J. Soils Sedim. 2009, 9, 364–373. [Google Scholar] [CrossRef]
- Siedlewicz, G.; Białk-Bielińska, A.; Borecka, M.; Winogradow, A.; Stepnowski, P.; Pazdro, K. Presence, concentrations and risk assessment of selected antibiotic residues in sediments and near-bottom waters collected from the polish coastal zone in the Southern Baltic Sea—Summary of 3 years of studies. Mar. Pollut. Bull. 2018, 129, 787–801. [Google Scholar] [CrossRef]
- Du, J.; Zhao, H.; Wang, Y.; Xie, H.; Zhu, M.; Chen, J. Presence and environmental risk assessment of selected antibiotics in coastal water adjacent to mariculture areas in the Bohai Sea. Ecotoxicol. Environ. Saf. 2019, 177, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Xu, X.-R.; Zhou, G.-J.; Liu, S.-S.; Yue, W.-Z.; Sun, K.-F.; Ying, G.-G. Antibiotics in the coastal environment of the Hailing Bay region, South China Sea: Spatial distribution, source analysis and ecological risks. Mar. Pollut. Bull. 2015, 95, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Xu, B.; Chen, H.; Zhao, X.; Li, G.; Zheng, Y.; Qiu, W.; Zheng, C.; Duan, L.; Wang, W. Occurrence and distribution of antibiotics in groundwater, surface water, and sediment in Xiong’an New Area, China, and their relationship with antibiotic resistance genes. Sci. Total Environ. 2022, 807, 151011. [Google Scholar] [CrossRef] [PubMed]
- Al Aukidy, M.; Verlicchi, P.; Jelic, A.; Petrovic, M.; Barcelò, D. Monitoring release of pharmaceutical compounds: Occurrence and environmental risk assessment of two WWTP effluents and their receiving bodies in the Po Valley, Italy. Sci. Total Environ. 2012, 438, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Xu, W.; Zhang, R.; Tang, J.; Chen, Y.; Zhang, G. Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: Impacts of river discharge and aquaculture activities. Environ. Pollut. 2011, 159, 2913–2920. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, L.; Chen, W.; Bao, Y.; Zheng, Y.; Huang, B.; Mu, Q.; Wen, D.; Feng, C. Antibiotics in coastal water and sediments of the East China Sea: Distribution, ecological risk assessment and indicators screening. Mar. Pollut. Bull. 2020, 151, 110810. [Google Scholar] [CrossRef]
- Zhou, L.-J.; Ying, G.-G.; Zhao, J.-L.; Yang, J.-F.; Wang, L.; Yang, B.; Liu, S. Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in northern China. Environ. Pollut. 2011, 159, 1877–1885. [Google Scholar] [CrossRef]
- Larsson, D.J. Antibiotics in the environment. Upsala J. Med. Sci. 2014, 119, 108–112. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Gao, L.; Liu, J.; Cai, Y. Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China. Chemosphere 2012, 89, 1307–1315. [Google Scholar] [CrossRef]
- Gbadegesin, L.A.; Tang, X.; Liu, C.; Cheng, J. Transport of veterinary antibiotics in farmland soil: Effects of dissolved organic matter. Int. J. Environ. Res. Public Health 2022, 19, 1702. [Google Scholar] [CrossRef]
- Kay, P.; Blackwell, P.A.; Boxall, A.B. Column studies to investigate the fate of veterinary antibiotics in clay soils following slurry application to agricultural land. Chemosphere 2005, 60, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, X.; Cao, Z.; Zhan, Y.; Shi, X.; Yang, Y.; Zhou, J.; Xu, J. Adsorption behavior and mechanism of chloramphenicols, sulfonamides, and non-antibiotic pharmaceuticals on multi-walled carbon nanotubes. J. Hazard. Mater. 2016, 310, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cao, X.; Lin, H.; Wang, J. Antibiotics and antibiotic resistance genes in sediment of Honghu Lake and East Dongting Lake, China. Microb. Ecol. 2016, 72, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Liu, X.; Wang, L.; Gong, W.; Liu, G.; Fu, W.; Cheng, M. Seasonal variation and sediment–water exchange of antibiotics in a shallower large lake in North China. Sci. Total Environ. 2014, 476, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Jing, L.; Teng, Y. Ecotoxicological risk assessment and source apportionment of antibiotics in the waters and sediments of a peri-urban river. Sci. Total Environ. 2020, 731, 139128. [Google Scholar] [CrossRef]
- Arikan, O.A.; Rice, C.; Codling, E. Occurrence of antibiotics and hormones in a major agricultural watershed. Desalination 2008, 226, 121–133. [Google Scholar] [CrossRef]
- Tamtam, F.; Mercier, F.; Le Bot, B.; Eurin, J.; Dinh, Q.T.; Clément, M.; Chevreuil, M. Occurrence and fate of antibiotics in the Seine River in various hydrological conditions. Sci. Total Environ. 2008, 393, 84–95. [Google Scholar] [CrossRef]
- Murray, K.E.; Thomas, S.M.; Bodour, A.A. Prioritizing research for trace pollutants and emerging contaminants in the freshwater environment. Environ. Pollut. 2010, 158, 3462–3471. [Google Scholar] [CrossRef]
- Vanneste, J.; Cornish, D.; Yu, J.; Boyd, R.; Morris, C. Isolation of copper and streptomycin resistant phytopathogenic Pseudomonas syringae from lakes and rivers in the central North Island of New Zealand. N. Zeal. Plant Prot. 2008, 61, 80–85. [Google Scholar] [CrossRef][Green Version]
- Liang, X.; Chen, B.; Nie, X.; Shi, Z.; Huang, X.; Li, X. The distribution and partitioning of common antibiotics in water and sediment of the Pearl River Estuary, South China. Chemosphere 2013, 92, 1410–1416. [Google Scholar] [CrossRef]
- Gao, L.; Shi, Y.; Li, W.; Niu, H.; Liu, J.; Cai, Y. Occurrence of antibiotics in eight sewage treatment plants in Beijing, China. Chemosphere 2012, 86, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Mesdaghinia, A.; Hoseini, S.S.; Yunesian, M. Antibiotics in urban wastewater and rivers of Tehran, Iran: Consumption, mass load, occurrence, and ecological risk. Chemosphere 2019, 221, 55–66. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Q.; Zhang, J.; Dong, J.; Yan, H.; Chen, C.; Feng, R. Characterization and source identification of tetracycline antibiotics in the drinking water sources of the lower Yangtze River. J. Environ. Manag. 2019, 244, 13–22. [Google Scholar] [CrossRef]
- Spataro, F.; Ademollo, N.; Pescatore, T.; Rauseo, J.; Patrolecco, L. Antibiotic residues and endocrine disrupting compounds in municipal wastewater treatment plants in Rome, Italy. Microchem. J. 2019, 148, 634–642. [Google Scholar] [CrossRef]
- Gao, L.; Shi, Y.; Li, W.; Liu, J.; Cai, Y. Occurrence, distribution and bioaccumulation of antibiotics in the Haihe River in China. J. Environ. Monit. 2012, 14, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Meng, W.; Xu, J.; Zhang, Y.; Guo, C. Occurrence, distribution and bioaccumulation of antibiotics in the Liao River Basin in China. Environ. Sci. Process. Impacts 2014, 16, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Qiu, X.; Chen, B.; Yu, X.; Liu, Z.; Zhong, G.; Li, H.; Chen, M.; Sun, G.; Huang, H. Antibiotics pollution in Jiulong River estuary: Source, distribution and bacterial resistance. Chemosphere 2011, 84, 1677–1685. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, J. Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China. Chemosphere 2014, 95, 604–612. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, X.; Yin, D.; Zhang, H.; Yu, Z. Occurrence, distribution and seasonal variation of antibiotics in the Huangpu River, Shanghai, China. Chemosphere 2011, 82, 822–828. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, G.; Zou, S.; Ling, Z.; Wang, G.; Yan, W. A preliminary investigation on the occurrence and distribution of antibiotics in the Yellow River and its tributaries, China. Water Environ. Res. 2009, 81, 248–254. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, L.; Rysz, M.; Wang, Y.; Zhang, H.; Alvarez, P.J. Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River Basin, China. Environ. Sci. Technol. 2011, 45, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Huang, X.; Witter, J.D.; Spongberg, A.L.; Wang, K.; Wang, D.; Liu, J. Occurrence of pharmaceuticals and personal care products and associated environmental risks in the central and lower Yangtze river, China. Ecotoxicol. Environ. Saf. 2014, 106, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.V.; Zanetti, M.O.B.; Pitondo-Silva, A.; Stehling, E.G. Aquatic environments polluted with antibiotics and heavy metals: A human health hazard. Environ. Sci. Pollut. Res. 2014, 21, 5873–5878. [Google Scholar] [CrossRef] [PubMed]
- Praveena, S.M.; Shaifuddin, S.N.M.; Sukiman, S.; Nasir, F.A.M.; Hanafi, Z.; Kamarudin, N.; Ismail, T.H.T.; Aris, A.Z. Pharmaceuticals residues in selected tropical surface water bodies from Selangor (Malaysia): Occurrence and potential risk assessments. Sci. Total Environ. 2018, 642, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Nakamichi, S.; Habibullah-Al-Mamun, M.; Tani, K.; Masunaga, S.; Matsuda, H. Occurrence, distribution, ecological and resistance risks of antibiotics in surface water of finfish and shellfish aquaculture in Bangladesh. Chemosphere 2017, 188, 329–336. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, X.; Shen, Y.; Di, M.; Wang, J. Antibiotics in surface water and sediments from Hanjiang River, Central China: Occurrence, behavior and risk assessment. Ecotoxicol. Environ. Saf. 2018, 157, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cui, M.; Zhang, H. Spatial and temporal variations of antibiotics in a tidal river. Environ. Monit. Assess. 2020, 192, 1–14. [Google Scholar] [CrossRef]
- Hossain, A.; Nakamichi, S.; Habibullah-Al-Mamun, M.; Tani, K.; Masunaga, S.; Matsuda, H. Occurrence and ecological risk of pharmaceuticals in river surface water of Bangladesh. Environ. Res. 2018, 165, 258–266. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Zhang, H.; Luo, Z.; Yan, C. Occurrence, distribution, and seasonal variation of estrogenic compounds and antibiotic residues in Jiulongjiang River, South China. Environ. Sci. Pollut. Res. 2012, 19, 1392–1404. [Google Scholar] [CrossRef]
- Hanamoto, S.; Nakada, N.; Yamashita, N.; Tanaka, H. Source estimation of pharmaceuticals based on catchment population and in-stream attenuation in Yodo River watershed, Japan. Sci. Total Environ. 2018, 615, 964–971. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, S.; Wang, Y.; Liu, X.; Liu, Y.; Xu, J.; Zhang, T.; Wang, Z.; Yang, Y. Occurrence of antibiotics and antibiotic resistance genes and their correlations in lower Yangtze River, China. Environ. Pollut. 2020, 257, 113365. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Zhang, R.; Wang, Y.; Liu, X.; Li, J.; Zhang, G. Antibiotic contamination in a typical developing city in south China: Occurrence and ecological risks in the Yongjiang River impacted by tributary discharge and anthropogenic activities. Ecotoxicol. Environ. Saf. 2013, 92, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Mandaric, L.; Diamantini, E.; Stella, E.; Cano-Paoli, K.; Valle-Sistac, J.; Molins-Delgado, D.; Bellin, A.; Chiogna, G.; Majone, B.; Diaz-Cruz, M.S. Contamination sources and distribution patterns of pharmaceuticals and personal care products in Alpine rivers strongly affected by tourism. Sci. Total Environ. 2017, 590, 484–494. [Google Scholar] [CrossRef]
- Xu, W.; Yan, W.; Li, X.; Zou, Y.; Chen, X.; Huang, W.; Miao, L.; Zhang, R.; Zhang, G.; Zou, S. Antibiotics in riverine runoff of the Pearl River Delta and Pearl River Estuary, China: Concentrations, mass loading and ecological risks. Environ. Pollut. 2013, 182, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Omar, T.F.T.; Aris, A.Z.; Yusoff, F.M.; Mustafa, S. Risk assessment of pharmaceutically active compounds (PhACs) in the Klang River estuary, Malaysia. Environ. Geochem. Health 2019, 41, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Feng, M.; Chen, J.; Shen, W.; Zhang, S. Occurrence of antibiotics and antibiotic resistance genes and their correlations in river-type drinking water source, China. Environ. Sci. Pollut. Res. 2021, 28, 42339–42352. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, L.; Liu, S.; Guo, Z.; Hua, X. Antibiotics in water and sediments from Liao River in Jilin Province, China: Occurrence, distribution, and risk assessment. Environ. Earth Sci. 2016, 75, 1–10. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, G.; Zheng, Q.; Tang, J.; Chen, Y.; Xu, W.; Zou, Y.; Chen, X. Occurrence and risks of antibiotics in the Laizhou Bay, China: Impacts of river discharge. Ecotoxicol. Environ. Saf. 2012, 80, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Feitosa-Felizzola, J.; Chiron, S. Occurrence and distribution of selected antibiotics in a small Mediterranean stream (Arc River, Southern France). J. Hydrol. 2009, 364, 50–57. [Google Scholar] [CrossRef]
- Kim, S.-C.; Carlson, K. Temporal and spatial trends in the occurrence of human and veterinary antibiotics in aqueous and river sediment matrices. Environ. Sci. Technol. 2007, 41, 50–57. [Google Scholar] [CrossRef]
- Challis, J.K.; Cuscito, L.D.; Joudan, S.; Luong, K.H.; Knapp, C.W.; Hanson, M.L.; Wong, C.S. Inputs, source apportionment, and transboundary transport of pesticides and other polar organic contaminants along the lower Red River, Manitoba, Canada. Sci. Total Environ. 2018, 635, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Azanu, D.; Styrishave, B.; Darko, G.; Weisser, J.J.; Abaidoo, R.C. Occurrence and risk assessment of antibiotics in water and lettuce in Ghana. Sci. Total Environ. 2018, 622, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Vieno, N.M.; Härkki, H.; Tuhkanen, T.; Kronberg, L. Occurrence of pharmaceuticals in river water and their elimination in a pilot-scale drinking water treatment plant. Environ. Sci. Technol. 2007, 41, 5077–5084. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Yunesian, M.; Nasseri, S.; Gholami, M.; Jalilzadeh, E.; Shoeibi, S.; Mesdaghinia, A. Occurrence and fate of most prescribed antibiotics in different water environments of Tehran, Iran. Sci. Total Environ. 2018, 619, 446–459. [Google Scholar] [CrossRef]
- Boy-Roura, M.; Mas-Pla, J.; Petrovic, M.; Gros, M.; Soler, D.; Brusi, D.; Menció, A. Towards the understanding of antibiotic occurrence and transport in groundwater: Findings from the Baix Fluvià alluvial aquifer (NE Catalonia, Spain). Sci. Total Environ. 2018, 612, 1387–1406. [Google Scholar] [CrossRef]
- White, D.; Lapworth, D.J.; Civil, W.; Williams, P. Tracking changes in the occurrence and source of pharmaceuticals within the River Thames, UK.; from source to sea. Environ. Pollut. 2019, 249, 257–266. [Google Scholar] [CrossRef]
- Rico, A.; Arenas-Sánchez, A.; Alonso-Alonso, C.; López-Heras, I.; Nozal, L.; Rivas-Tabares, D.; Vighi, M. Identification of contaminants of concern in the upper Tagus river basin (central Spain). Part 1: Screening, quantitative analysis and comparison of sampling methods. Sci. Total Environ. 2019, 666, 1058–1070. [Google Scholar] [CrossRef]
- Miossec, C.; Lanceleur, L.; Monperrus, M. Multi-residue analysis of 44 pharmaceutical compounds in environmental water samples by solid-phase extraction coupled to liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2019, 42, 1853–1866. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Xu, C.; Wei, B.; Wang, J. Antibiotic resistance genes in lakes from middle and lower reaches of the Yangtze River, China: Effect of land use and sediment characteristics. Chemosphere 2017, 178, 19–25. [Google Scholar] [CrossRef]
- Blair, B.D.; Crago, J.P.; Hedman, C.J.; Klaper, R.D. Pharmaceuticals and personal care products found in the Great Lakes above concentrations of environmental concern. Chemosphere 2013, 93, 2116–2123. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Xu, J.; Guo, C.; Li, L.; Fan, W. Simultaneous quantification of several classes of antibiotics in water, sediments, and fish muscles by liquid chromatography-tandem mass spectrometry. Front. Environ. Sci. Eng. 2014, 8, 357–371. [Google Scholar] [CrossRef]
- Binaeian, E.; Seghatoleslami, N.; Chaichi, M.J.; Tayebi, H.-a. Preparation of titanium dioxide nanoparticles supported on hexagonal mesoporous silicate (HMS) modified by oak gall tannin and its photocatalytic performance in degradation of azo dye. Adv. Powder Technol. 2016, 27, 1047–1055. [Google Scholar] [CrossRef]
- Tang, J.; Shi, T.; Wu, X.; Cao, H.; Li, X.; Hua, R.; Tang, F.; Yue, Y. The occurrence and distribution of antibiotics in Lake Chaohu, China: Seasonal variation, potential source and risk assessment. Chemosphere 2015, 122, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wu, Y.; Zhang, W.; Zhong, J.; Lou, Q.; Yang, P.; Fang, Y. Occurrence, distribution, and risk assessment of antibiotics in the surface water of Poyang Lake, the largest freshwater lake in China. Chemosphere 2017, 184, 137–147. [Google Scholar] [CrossRef]
- Lu, G.; Yang, X.; Li, Z.; Zhao, H.; Wang, C. Contamination by metals and pharmaceuticals in northern Taihu Lake (China) and its relation to integrated biomarker response in fish. Ecotoxicology 2013, 22, 50–59. [Google Scholar] [CrossRef]
- Lei, X. Distribution and pollution levels of antibiotics from typical lakes in Xinjiang. Shihezi Univ. 2014. [Google Scholar]
- Archundia, D.; Duwig, C.; Lehembre, F.; Chiron, S.; Morel, M.; Prado, B.; Bourdat-Deschamps, M.; Vince, E.; Aviles, G.F.; Martins, J. Antibiotic pollution in the Katari subcatchment of the Titicaca Lake: Major transformation products and occurrence of resistance genes. Sci. Total Environ. 2017, 576, 671–682. [Google Scholar] [CrossRef]
- Dalahmeh, S.; Björnberg, E.; Elenström, A.-K.; Niwagaba, C.B.; Komakech, A.J. Pharmaceutical pollution of water resources in Nakivubo wetlands and Lake Victoria, Kampala, Uganda. Sci. Total Environ. 2020, 710, 136347. [Google Scholar] [CrossRef]
- Wang, H.; Long, W.; Chadwick, D.; Zhang, X.; Zhang, S.; Piao, X.; Hou, Y. Dietary acidifiers as an alternative to antibiotics for promoting pig growth performance: A systematic review and meta-analysis. Anim. Feed Sci. Technol. 2022, 289, 115320. [Google Scholar] [CrossRef]
- Prabhukarthikeyan, S.R.; Keerthana, U.; Raguchander, T. Antibiotic-producing Pseudomonas fluorescens mediates rhizome rot disease resistance and promotes plant growth in turmeric plants. Microbiol. Res. 2018, 210, 65–73. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.M.; Youssef, G.B.A.; Taha, A.E.; Soliman, S.M.; Ahmed, A.E.; El-kott, A.F.; et al. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird’s health and production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment—A review–Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Kairigo, P.; Ngumba, E.; Sundberg, L.-R.; Gachanja, A.; Tuhkanen, T. Contamination of surface water and river sediments by antibiotic and antiretroviral drug cocktails in low and middle-income countries: Occurrence, risk and mitigation strategies. Water 2020, 12, 1376. [Google Scholar] [CrossRef]
- Barani, A.; Fallah, A.A. Occurrence of tetracyclines, sulfonamides, fluoroquinolones and florfenicol in farmed rainbow trout in Iran. Food Agric. Immunol. 2015, 26, 420–429. [Google Scholar] [CrossRef]
- Adel, M.; Dadar, M.; Oliveri Conti, G. Antibiotics and malachite green residues in farmed rainbow trout (Oncorhynchus mykiss) from the Iranian markets: A risk assessment. Int. J. Food Prop. 2017, 20, 402–408. [Google Scholar] [CrossRef]
- Liu, X.; Lu, S.; Meng, W.; Zheng, B. Residues and health risk assessment of typical antibiotics in aquatic products from the Dongting Lake, China—“Did you eat “Antibiotics” today?”. Environ. Sci. Pollut. Res. 2018, 25, 3913–3921. [Google Scholar] [CrossRef]


| Class | Antibiotics | Symbol | Molecular Formula | Molecular Weight | Solubility in Water (mg/L) | Main Use | Ref. |
|---|---|---|---|---|---|---|---|
| Tetracyclines (TCs) | Chlortetracycline Doxycycline Oxytetracycline Tetracycline | CTC DXC OTC TET | C22H23ClN2O8 C22H24N2O8 C22H24N2O9 C22H24N2O8 | 478.88 444.43 460.43 444.43 | 259 (25 °C) 50,000 (25 °C, pH = 2.16) 313 (25 °C) 231 (25 °C) | Veterinary Human, Veterinary Human, Veterinary, Plants Human, Veterinary | [31,32] |
| Sulfonamides (SFs) | Sulfadiazine Sulfamerazine Sulfamethazine Sulfamethizole SulfamethoxazoleSulfapyridine Sulfathiazole | SDZ SMR SMT SMZ SMX SPY STZ | C10H10N4O2S C11H12N4O2S C12H14N4O2S C9H10N4O2S2 C10H11N3O3S C11H11N3O2S C9H9N3O2S2 | 250.28 264.31 278.33 270.33 253.28 249.29 255.32 | 77 (25 °C) 202 (20 °C) 1500 (29 °C) 1050 (37 °C) 610 (37 °C) 268 (25 °C) 373 (25 °C) | Veterinary - Human, Veterinary Human Human - Veterinary | [33,34] |
| Macrolides (MLs) | Azithromycin Clarithromycin Erythromycin Roxithromycin Tylosin | AZI CLA ERY ROX TYL | C38H72N2O12 C38H69NO13 C37H67NO13 C41H76N2O15 C46H77NO17 | 748.98 747.95 733.93 837.05 916.10 | 2.37 (25 °C) 1.69 (25 °C) 4.2 (25 °C) 0.0189 (25 °C) 211 | Human Human Human, Veterinary Human - | [8,34] |
| Fluoroquinolones (FQs) | Ciprofloxacin Enrofloxacin Levofloxacin Lomefloxacin Norfloxacin Ofloxacin | CIP ENR LEV LOM NOR OFL | C17H18FN3O3 C19H22FN3O3 C18H20FN3O4 C17H19F2N3O3 C16H18FN3O3 C18H20FN3O4 | 331.34 359.39 361.37 351.35 319.33 361.37 | 30,000 (20 °C) 612 1440 27,200 280 (25 °C) 10,800 (25 °C) | Human Veterinary - - - Human | [8,34,35] |
| β-Lactams | Amoxicillin Ampicillin Cephalexin Cefazolin Penicillin | AMO AMP CEP CEZ PEN | C16H19N3O5S C16H19N3O4S C16H17N3O4S C14H14N8O4S3 C16H18N2O4S | 365.40 349.40 347.39 454.51 334.39 | 3430 (25 °C) 10,100 (21 °C) 10,000 210 (25 °C) 210 | Veterinary Veterinary Human Human Veterinary | [8,34] |
| Other classes | Chloramphenicol Lincomycin Trimethoprim | CHP LIN TMP | C11H12Cl2N2O5 C18H34N2O6S C14H18N4O3 | 323.13 406.54 290.32 | 2500 (25 °C) 927 (25 °C) 400 (25 °C) | Human Human Human | [8,36] |
| Sea (Country) | Geographical Coordinates | Sediments (ng/g) | Seawater (ng/L) | Number of Stations | Ref. |
|---|---|---|---|---|---|
| Beibu Gulf (China) | 21°30′36.00′′ N, 108°07′07.60′′ E | - | SMX: 15.9, TMP: 4.11, and ERY: 2.59–47.6 | 4 | [55] |
| Bohai Bay (China) | 38°53′18.37′′ N, 119°48′40.09′′ E | TCs: 7.71–130.36 | TCs: 41.53–222.43 | 16 | [56] |
| Maowei Sea (China) | 21°50′49.05′′ N, 108°29′54.87′′ E | - | DETM: 276 ± 71.6, NOX: 1.56 ± 1.46, and ENX: 0.85 ± 0.65 | 7 | [54] |
| Beibu Gulf (China) | 22°48′45.26′′ N, 108°22′18.46′′ E | FQs: 9.69–15.43 | MLs: 52.9477.76 | 3 | [57] |
| Hailing Island (south coast of England) | 21°37′41.12′′ N, 111°55′00.56′′ E | ERY: 0.8–4.8 | OTC: 16,000 and TMP: 20 | 6 | [46] |
| North coast of the Persian Gulf (Iran) | 28°55′09.27′′ N, 50°55′16.92′′ E | NOR: 1.40–25.32 | NOR: 1.21–51.50 | 3 | [50] |
| Spain | 40°27′49.20.′′ N, 3°44′57.18′′ W | - | AMO: 326.70 | - | [58] |
| Victoria Harbour (South China) | 22°45′40.74′′ N, 51°24′56.21′′ E | OFL: 17.5, ERY: 17.5, and SMX: 0 | SMX: 1.036–629 | 1 | [59] |
| Baltic Sea (Northern Europe) | 53°56′13.58′′ N, 14°11′59.69′′ E | TMP: 35.7 and OTC: 15.5 | SMX: 311 and TMP: 279 | 29 | [60] |
| Bohai Sea (China) | 38°56′14.71′′ N, 121°12′22.67′′ E | - | ENR:139, SMX: 17.7, and TMP: 5.0 | 22 | [61] |
| Hailing Bay (China) | 50°46′37.34′′ N, 0°58′41.76′′ E | CIP: 184 and ERY < 1.95 | ERY: 1318, CLA: 15.16, TMP: 4.24, and OTC: 2.12 | 5 | [62] |
| Yellow Sea (China) | 36°46′43.11′′ N, 117°53′14.95′′ E | OTC: 895.32–1478.29 | ENR: 0.56–125.96 and CIP: 14.94–48.26 | 11 | [51] |
| Xiong’an New Area (China) | 38°43′ and 39°10′ N, 115°38′ and 116°20′ E | FQs: 38.03–406.31 | AMO: 12.71–260.56 in surface water AMO: ND-196.12 in groundwater | - | [63] |
| Po Valley (Italy) | 45°00′00.06′′ N, 10°30′00.79′′ E | - | CLA: 128,103 and CIP: 124 | 2 | [64] |
| Jiaozhou Bay (China) | 36°11′24.11′′ N, 120°18′02.07′′ E | OFL: ND −3.337 | - | 2 | [15] |
| Bohai Bay(China) | 37°41′04.55′′ N, 120°17′24.23′′ E | - | NOR: 460, OFL: 390, and CIP: 110 | 28 | [65] |
| East China Sea | 30°26′36.19′′ N, 125°57′35.29′′ E | FQs: 7.3, MLs: 5.2, and SFs: 2.6 | β-lactams: 215.6, FQs: 54.2 and SFS: 39.3 | 3 | [66] |
| River (Country) | Geographical Coordinates | Sediments (ng/g) | Water (ng/L) | Number of Stations | Ref. |
|---|---|---|---|---|---|
| Haihe River (China) | 39°02′04.57′′ N, 117°27′55.19′′ E | NOR: 63.5, ENR: 50.8 | SMX: 68 | 5 | [77] |
| Liao River (China) | 41°56′46.28′′ N, 122°51′06.01′′ E | MLs: 375,130, TCs: 404.82 | MLs: 3,162.22 | 50 | [78] |
| Jiulong River (China) | 25°00′12.37′′ N, 117°32′02.54′′ E | - | SFs: 81.07 | 35 | [79] |
| Huangpu River (China) | 31°08′02.87′′ N, 121°27′17.68′′ E | TCs: 18,000, MLs: 12,000 | SFs: 34–859 | 30 | [80] |
| Huangpu River (China) | 31°08′02.87′′ N, 121°27′17.68′′ E | - | SMT: 468.13 and TCs: 75.29 | 19 | [81] |
| Yellow River (China) | 36°31′08.14′′ N, 116°36′38.16′′ E | - | NOR: 327, OFL: 119, and MLs: 91 | 24 | [82] |
| Pearl River Estuary (china) | 22°46′10.36′′ N, 113°37′17.53′′ E | NOR: 7.62, OFL: 3.63, and: MLs: 2.69 | NOR: 68.06, OFL: 6.93, and MLs: 21.7 | 14 | [83] |
| Haihe River Basin (China) | 39°02′12.42′′ N, 117°27′57.71′′ E | SFs: 210–385 | OTC: (4.0 ± 0) × 10, ERY: (3.8 ± 0.6) × 10 | 15 | [84] |
| Yangtze River (China) | 29°43′.11.37′′ N, 112°39′01.61′′ E | - | ERY: 296 | 4 | [85] |
| Pardo River (Brazil) | 23°33′56.23′′ S, 46°45′26.60′′ w | - | TCs: ND a | - | [86] |
| Lui River (Malaysia) | 3°05′24.86′′ N, 102°25′55.60′′ E | - | AMO: ND- 4.44, 7.11–7.81, and 1.75–6.08, CIP: 52.50–138.17, 225.18–299.88, and 143.75–258.53, and SMX: 19.26–75.48, 96.81–109.34, and 84.31–114.24 | 3 | [87] |
| Hai River (China) | 15°29′17.12′′ N, 114°24′17.10′′ E | TCs: 2.76 × 102 | SMX: 1.57 × 102 and TCs: 6.82 × 103 | 5 | [47] |
| Chaobai Rive (China) | 40°01′43.21′′ N, 116°46′39.58′′ E | FQs: 12.0 and TCs: 11.8 | SFs: 4.71–95.3 and MLs: 0.41–85.3 | 3 | [75] |
| Rajshahi, Jessore and Mymensingh (Bangladesh) | 23°36′30.76′′ N, 90°20′02.93′′ E | - | SMX: ND -20.02, TMP: ND -41.67 and SDZ: 17.97 | 6 | [88] |
| Hanjiang River (China) | 24°03′44.05′′ N, 116°30′10.98′′ E | SFs: 5.4, TCs: 9.6 and FQs: 5.4 | SFs: 24, TCs: 10, and FQs: 5.5 | 14 | [89] |
| Xiaoqing River (China) | 37°16′34.19′′ N, 118°58′30.68′′ E | - | TMP: 1272, ERY: 97.36, and SMX: 76.84 | 10 | [90] |
| Brahmaputra River (Bangladesh) | 23°29′46.19′′ N, 90°22′22.79′′ E | - | TMP: 17.20 | [91] | |
| Jiulongjiang River (China) | 25°00′13.52′′ N, 117°32′01.91′′ E | - | Average SFs: 383, TCs: 424.25, and FQs: 3.91 | 19 | [92] |
| Yangtze River (China) | 29°43′12.47′′ N, 112°39′00.51′′ E | - | DXC: 56.09, OTC: 18.98, and TET: 11.16 | 28 | [93] |
| Beiyun River (China) | 39°47′36.23′′ N, 116°47′42.81′′ E | - | ERY: 319 | 34 | [88] |
| Yodo River (Japan) | 34°45′23.69′′ N, 135°33′44.37′′ E | - | CLA. | 4 | [94] |
| Yangtze River (China) | 29°43′12.47′′ N, 112°39′00.51′′ E | OFL: 8.4 | ERY: 0.29 | 29 | [95] |
| Yongjiang River (China) | 22°47′49.95′′ N, 108°22′55.48′′ E | SMX: 0.032, SDZ: 0.017 and TMP: 0.32 | SMX: 9.96, SDZ: 55.8, and TMP: 93.5 | 35 | [96] |
| Alpine rivers | 45°55′35.02′′ N, 11°34′45.04′′ E | - | TMP and SMX >100 | 12 | [97] |
| Pearl River Delta (China) | 21°30′N, 113°00′ E | - | Average concentrations OFL, SMX, and ERY: 1.2–127 | 8 | [98] |
| Zhuhai City (China) | 22°16′36.11′′ N, 113°35′06.26′′ E | AGs: 74.5–152 | AGS: 54.6–134 and FQs: 154–256 | 9 | [15] |
| Klang (Malaysia) | 3°04′33.74′′ N, 101°37′27.20′′ E | - | AMO: 102.31 | 12 | [99] |
| Nanjing (China) | 32°04′19.29′′ N, 118°47′32.17′′ E | - | SFs: 23.52–219.00 and NOR: 146.72–290.20 | 13 | [100] |
| Liao River in Jilin Province (China) | 41°57′16.22′′ N, 122°51′26.12′′ E | OFL: 152.2 ± 108.3, OTC: 149 ± 147.6, and NOR: 62.8 ± 83.3 | OTC: 266.9 ± 174.9, ERY: (103.2 ± 95.5, and OFL: 67.1 ± 77.3 | - | [101] |
| Rivers of Tehran (Iran) | 35°45′40.74′′ N, 51°24′56.21′′ E | - | AMO: 128,017,000,000 in 1000 people per day | 2 | [102] |
| Laizhou Bay (China) | 37°18′38.00′′ N, 119°21′47.54′′ E | - | ENR: 209, CIP: 66 and TMP: 1.3–330 | 10 | [103] |
| Seine River (Northern France) | 48°35′44.76′′ N, 2°27′16.16′′ W | - | SMZ: 544, NOR: 163, and TMP: 45 | 5 | [104] |
| Arc Rive (Southern France) | 43°30′37.36′′ N, 5°28′22.27′′ w | AZI: 130,660 and CLA: 1700 | CLA: 0.71 | 3 | [105] |
| Cache La Poudre (United States) | 40°25′11.32′′ N, 104°40′28.79′′ E | TCs: 6900–24,300, STZ: 4800, and RTM: 2100 | SMX: 110, SFs: 110, and TCs: 20–180 | 5 | [106] |
| Red River (Canada) | 48°22′52. 85′′ N, 97°05′21.9′′ W | - | SMX: 1.5–7.6 | - | [107] |
| Yellow River, Hai River, and Liao River (Northern China) | 36°31′08.14′′ N, 116°36′38.16′′ E 39°02′12.42′′ N, 117°27′57.71′′ E 41°56′46.28′′ N, 122°51′06.01′′ E | NOR: 7.76, OFL: 3.49, and ERY: 8.11 | - | 3 | [67] |
| Red River (Vietnam) | 21°01′41.29′′ N, 105°50′03.30′′ E | - | SFs, MLs, FQs, and TMP | 5 | [53] |
| Kumasi (Ghana) | 6°39′55.38′′ N, 1°36′58.58′′ W | - | SMX: 2861, ERY: 10.614–7944, | 7 | [108] |
| Finland | 60°18′55.84′′ N, 24°53′47.17′′ E | - | CIP: 20 | 1 | [109] |
| Mekong Delta (Vietnam) | 10°05′36.74′′ N, 105°23′15.78′′ E | - | SMT: 15–328, SMZ: 20–174, and TMP: 7–44 | 6 | [52] |
| Choptank River (USA) | 38°40′51.80′′ N, 75°57′05.30′′ W | CLA: 11–34 | CLA: 1–180 | 22 | [76] |
| Red River (Northern Vietnam) | 20°55′22.82′′ N, 105°58′11.95′′ E | - | SMT: 475–6662, SMX: 612–4330, ERY: 154–2246, and CLA: 2.8–778 | 10 | [12] |
| Kan River, Firozabad Ditch, and location of Ekbatan WWTP and south Tehran (Iran) | 35°45′10.60′′ N, 51°19′49.09′′ E | - | CIP: 552.6–796.2 in effluent, CIP: 127–248, and CEP: 523.3–977.7 | 22 | [110] |
| Baix Fluvià (northeastern Catalonia, Spain) | 41°32′35.50′′ N, 1°31′35.16′′ E | - | CIP: 211.8, SMX: 8.5 | 4 | [111] |
| Rome (Italy) | 41°54′20.31′′ N, 12°29′29.24′′ E | - | AMO: 1258 ± 7.6 | 4 | [112] |
| Thames (UK) | 51°33′58.29′′ N, 0°41′39.52′′ W | - | CLA: 5000, ERY: 790 and AZI: 7.3 | 33 | [113] |
| Tagus (Spain) | 39°42′20.03′′ N, 5°16′31.52′′ W | - | TMP: 4.42–99.89, SMX: 9.42–27.5, and AZI: 5.06–8.23 | 16 | [114] |
| Adour Estuary (France) | 43°37′01. 91′′ N, 1°26′43.21′′ W | - | NOR, OFL, and CIP | - | [115] |
| Lake (Country) | Geographical Coordinates | Sediments (ng/g) | Water (ng/L) | Number of Stations | Ref. |
|---|---|---|---|---|---|
| Honghu Lake (China) | 29°39′44.58′′ N, 113°20′52.91′′ E | TETs: 117.970, SAs: 77.730, and DC: 43,840 | - | 14 | [73] |
| Dongting Lake (China) | 28°59′37.73′′ N, 112°43′41.52′′ E | SAs: 151,940, TC: 126.270, and SMX: 57.320 | - | 14 | [73] |
| Michigan Lake (China) | 43°31′14.91′′ N, 87°13′19.25′′ W | AZI: 147.28, CLA: 67.66 | SMX: 10.22 | 7 | [116] |
| Dongting Lake (China) | 28°49′38.33′′ N, 112°41′44.29′′ E | - | TMP: ND a | 42 | [117] |
| Chaohu Lake (China) | 31°33′31. 16′′ N, 117°34′27.24′′ E | - | SMX: 95.6, OFL: 383.4 | 8 | [118] |
| Poyang Lake (China) | 29°08′20.44′′ N, 116°11′39.41′′ E | - | SDZ: 56.2, OTC: 48.7, and DXC: 39.7 | 4 | [119] |
| Dianchi Lake (China) | 24°48′46.92′′ N, 102°41′20.88′′ | CIP: 75.8, NOR: 55.2, and OFL: 108.9 | SMX: 17.6–499.2, and OFL: ND-713.6 | 27 | [120] |
| Taihu Lake (China) | 31°26′.56′′ N, 120°23′.46′′ E | - | SMX: 7.24–53.59, NOR: 15.83–56.22, and OFL: 7.45–17.01 | 8 | [121] |
| Turkey | 41°03′14. 63′′ N, 28°33 ′25.75′′ E | - | AMO: 1.1–1.15 | 6 | [28] |
| Taihu Lake (China) | 31°14′.43.36′′ N, 120°12′ 13.35′′ E | OTC: 52.8, TC: 47.9 | OTC: 47.8, SMT: 252.7 | 30 | [16] |
| Baiyangdian Lake (China) | 38°53′08.18′′ N, 116°00′48.19′′ E | NOR: 274.76, OFL: 39.73, TC: 25.71, and OTC: 15.66 | TC: 25.95–31.60, OTC: 18.86–23.80 | 6 | [74] |
| Baiyangdian Lake (China) | 38°53′08.18′′ N, 116°00′48.19′′ E | FQs: 65,500–1,166,000 | SFs: 0.86–1563 | 30 | [69] |
| Bosteng Lake (China) | 42°00′09.72′′ N, 87°01′40.97′′ E | CIP: 21.18–213.38, OFL: 18.39–94.1, and OTC: 4.61–20.67 | - | - | [122] |
| Ulungur Lake (China) | 47°17′53.10′′ N, 87°17′17.15′′ E | LOM: 6.34–53.85, CIP: 2.56–28.65, SAAM: 1.45–5.38, and SDZ: 1.03–3.68 | - | - | [122] |
| Titicaca Lake (South America) | 15°55′45.95′′ S, 69°20′07.44′′ W | TMP: 5000 SMX: 640 | TMP: 130 SMX: 159 | 4 | [123] |
| Hubei Province (China) | 30°35′04.61′′ N, 114°18′23.95′′ E | - | OFL in the pond: 1. 15.98 and OFL in the pond: 2. 21–127.40 | 2 | [15] |
| Nakivubo wetlands and Lake Victoria, Kampala (Uganda) | 00°18′ N, 32°38′32′ E | CIP and Metronidazole: ND | 8 | [124] | |
| Maoming City (China) | 21°39′44.91′′ N, 110°55′33.35′′ E | ERY7: 26–99.22 | In surface water ERY: 782–2634 and SDZ: 1.42–19.83; in the pond ERY: 19.02–2231 | 1 | [57] |
| Region (Country) | Geographical Coordinates | Antibiotics in Biota (ng/g) | Number of Stations | Ref. |
|---|---|---|---|---|
| Hailing Island (China) | 21°37′41.12′′ N, 111°55′00.56′′ E | ENR: 16.6–31.8 | 6 | [46] |
| Beibu Gulf (China) | 22°48′45.26′′ N, 108°22′18.46′′ E | FQs: 0.68–4.75 | 3 | [10] |
| Maowei Sea (China) | 21°50′49.05′′ N, 108°29′54.87′′ E | NOX and SMX | 7 | [54] |
| Central, northern, western, and north-western Iran | 32°25′40.46′′ N, 53°41′16.99′′ E | ENR:0.02–0.34 and FQs: 0.210 | 14 | [135] |
| Central, northern, western, and north-western Iran | 32°00′40.60′′ N, 53°41′16.99′′ E | FQs: 6.75–99.8 and SFs: 4.03–90.4 | 138 | [134] |
| Baiyangdian (China) | 38°53′08.18′′ N, 116°00′48.19′′ E | FQs:17.8–167, and MLs: 182 | 30 | [69] |
| Dianchi lake (China) | 24°48′46.92′′ N, 102°41′20.88′′ E | OFL: ND a-713.6, SMX: 17.6–499.2 | 27 | [120] |
| Liao River (China) | 41°56′46.28′′ N, 122°51′06.01′′ E | FQs: 286.6–1655 | 50 | [78] |
| Dongting Lake (China) | 27°49′.38.07′′ N, 113°41′41.68′′ E | SDX: 1.06, ENR: 1.05, SDZ: 0.68 and SMT: 0.57 | 3 | [136] |
| Hongze Lake (China) | 33°17′.15.36′′ N, 118°43′23.90′′ E | OTC: 74, SDZ: 47, and CIP: 24 | 30 | [137] |
| Haihe River (China) | 39°02′04.57′′ N, 117°27′55.19′′ E | NOR: 500–51,600, CIP: 1670–12,500 and SMZ: 540–6,8000 | 5 | [77] |
| Yellow Sea (China) | 36°46′43.11′′ N, 117°53 ′14.95′′ E | CIP: 9.26 and ENR: 24.75 | 11 | [51] |
| Cox’s Bazar, Shatkhira and Khulna (Bangladesh) | 23°43′ 50.76′′ N, 90°21′21.22′′ E | SMX: ND-16.67 and TMP: 11.39 | 6 | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maghsodian, Z.; Sanati, A.M.; Mashifana, T.; Sillanpää, M.; Feng, S.; Nhat, T.; Ramavandi, B. Occurrence and Distribution of Antibiotics in the Water, Sediment, and Biota of Freshwater and Marine Environments: A Review. Antibiotics 2022, 11, 1461. https://doi.org/10.3390/antibiotics11111461
Maghsodian Z, Sanati AM, Mashifana T, Sillanpää M, Feng S, Nhat T, Ramavandi B. Occurrence and Distribution of Antibiotics in the Water, Sediment, and Biota of Freshwater and Marine Environments: A Review. Antibiotics. 2022; 11(11):1461. https://doi.org/10.3390/antibiotics11111461
Chicago/Turabian StyleMaghsodian, Zeinab, Ali Mohammad Sanati, Tebogo Mashifana, Mika Sillanpää, Shengyu Feng, Tan Nhat, and Bahman Ramavandi. 2022. "Occurrence and Distribution of Antibiotics in the Water, Sediment, and Biota of Freshwater and Marine Environments: A Review" Antibiotics 11, no. 11: 1461. https://doi.org/10.3390/antibiotics11111461
APA StyleMaghsodian, Z., Sanati, A. M., Mashifana, T., Sillanpää, M., Feng, S., Nhat, T., & Ramavandi, B. (2022). Occurrence and Distribution of Antibiotics in the Water, Sediment, and Biota of Freshwater and Marine Environments: A Review. Antibiotics, 11(11), 1461. https://doi.org/10.3390/antibiotics11111461







