Antibiotic Susceptibility and Minimum Inhibitory Concentration for Stenotrophomonas maltophilia Ocular Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibiotic Susceptibility Testing
- -
- A suspension from a single colony of an overnight growth blood agar plate was prepared.
- -
- The turbidity of inoculum was adjusted to match 0.5 McFarland standard and was inoculated on MHA within 15 min.
- -
- A sterile cotton swab was dipped into the solution and was rotated inside the tube to remove excess liquid.
- -
- The swab was inoculated over the entire surface of the plate.
- -
- The E-test strip was applied to the plate within 15 min, and the whole plate was put in the incubator within 15 min.
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacob, A.; Iyadurai, R.; Punitha, J.V.; Chacko, B.; Jasmine, S.; Bharathy, M.; Mathew, D.; Veeraraghavan, B. Stenotrophomonas isolates in a tertiary care centre in South India. Indian J. Med. Microbiol. 2021, 40, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Zöllner, S.K.; Kampmeier, S.; Froböse, N.J.; Herbrüggen, H.; Masjosthusmann, K.; van den Heuvel, A.; Reicherts, C.; Ranft, A.; Groll, A.H. Stenotrophomonas maltophilia Infections in Pediatric Patients-Experience at a European Center for Pediatric Hematology and Oncology. Front. Oncol. 2021, 11, 752037. [Google Scholar] [CrossRef] [PubMed]
- Kogler, W.; Davison, N.; Richardson, A.; Rollini, F.; Isache, C. Endocarditis caused by Stenotrophomonas maltophilia—A rare presentation of an emerging opportunistic pathogen. IDCases 2019, 17, e00556. [Google Scholar] [CrossRef] [PubMed]
- Brooke, J.S. Stenotrophomonas maltophilia: An emerging global opportunistic pathogen. Clin. Microbiol. Rev. 2012, 25, 2–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.C.; Lim, H.R.; Park, S.J.; Koh, J.W. Clinical Features and Management of Stenotrophomonas maltophilia Keratitis. Ophthalmol. Ther. 2021, 10, 525–533. [Google Scholar] [CrossRef]
- Penland, R.L.; Wilhelmus, K.R. Stenotrophomonas maltophilia ocular infections. Arch. Ophthalmol. 1996, 114, 433–436. [Google Scholar] [CrossRef]
- Ramos-Esteban, J.C.; Jeng, B.H. Posttraumatic Stenotrophomonas maltophilia infectious scleritis. Cornea 2008, 27, 232–235. [Google Scholar] [CrossRef]
- Ji, Y.; Jiang, C.; Ji, J.; Luo, Y.; Jiang, Y.; Lu, Y. Post-cataract endophthalmitis caused by multidrug-resistant Stenotrophomonas maltophilia: Clinical features and risk factors. BMC Ophthalmol. 2015, 15, 14. [Google Scholar] [CrossRef] [Green Version]
- Bostanghadiri, N.; Ardebili, A.; Ghalavand, Z.; Teymouri, S.; Mirzarazi, M.; Goudarzi, M.; Ghasemi, E.; Hashemi, A. Antibiotic resistance, biofilm formation, and biofilm-associated genes among Stenotrophomonas maltophilia clinical isolates. BMC Res. Notes 2021, 14, 151. [Google Scholar] [CrossRef]
- Govender, R.; Amoah, I.D.; Kumari, S.; Bux, F.; Stenström, T.A. Detection of multidrug resistant environmental isolates of acinetobacter and Stenotrophomonas maltophilia: A possible threat for community acquired infections? J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2021, 56, 213–225. [Google Scholar] [CrossRef]
- Baseri, Z.; Dehghan, A.; Yaghoubi, S.; Razavi, S. Prevalence of resistance genes and antibiotic resistance profile among Stenotrophomonas maltophilia isolates from hospitalized patients in Iran. New Microbes New Infect. 2021, 44, 100943. [Google Scholar] [CrossRef] [PubMed]
- Çıkman, A.; Parlak, M.; Bayram, Y.; Güdücüoğlu, H.; Berktaş, M. Antibiotics resistance of Stenotrophomonas maltophilia strains isolated from various clinical specimens. Afr. Health Sci. 2016, 16, 149–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamanlıoğlu, D.; Dizbay, M. In vitro combination of tigecycline with other antibiotics in Stenotrophomonas maltophilia isolates. Turk. J. Med. Sci. 2019, 49, 683–686. [Google Scholar] [CrossRef]
- Gil-Gil, T.; Martínez, J.L.; Blanco, P. Mechanisms of antimicrobial resistance in Stenotrophomonas maltophilia: A review of current knowledge. Expert Rev. Anti-Infect. Ther. 2020, 18, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Brooke, J.S. Advances in the Microbiology of Stenotrophomonas maltophilia. Clin. Microbiol. Rev. 2021, 34, e0003019. [Google Scholar] [CrossRef] [PubMed]
- Nicodemo, A.C.; Paez, J.I.G. Antimicrobial therapy for Stenotrophomonas maltophilia infections. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 229–237. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Ho, M.C.; Hsiao, C.H.; Sun, M.H.; Hwang, Y.S.; Lai, C.C.; Wu, W.C.; Chen, K.J. Antimicrobial Susceptibility, Minimum Inhibitory Concentrations, and Clinical Profiles of Stenotrophomonas maltophilia Endophthalmitis. Microorganisms 2021, 9, 1840. [Google Scholar] [CrossRef]
- Watanabe, K.; Zhu, H.; Willcox, M. Susceptibility of Stenotrophomonas maltophilia clinical isolates to antibiotics and contact lens multipurpose disinfecting solutions. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8475–8479. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Yao, J.D.; Louie, M.; Louie, L.; Goodfellow, J.; Simor, A.E. Comparison of E test and agar dilution for antimicrobial susceptibility testing of Stenotrophomonas (Xanthomonas) maltophilia. J. Clin. Microbiol. 1995, 33, 1428–1430. [Google Scholar] [CrossRef]
- Gülmez, D.; Cakar, A.; Sener, B.; Karakaya, J.; Hasçelik, G. Comparison of different antimicrobial susceptibility testing methods for Stenotrophomonas maltophilia and results of synergy testing. J. Infect. Chemother. 2010, 16, 322–328. [Google Scholar] [CrossRef]
- Kiehlbauch, J.A.; Hannett, G.E.; Salfinger, M.; Archinal, W.; Monserrat, C.; Carlyn, C. Use of the National Committee for Clinical Laboratory Standards guidelines for disk diffusion susceptibility testing in New York state laboratories. J. Clin. Microbiol. 2000, 38, 3341–3348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, J.H.; Ferraro, M.J. Antimicrobial susceptibility testing: General principles and contemporary practices. Clin. Infect. Dis. 1998, 26, 973–980. [Google Scholar] [CrossRef] [Green Version]
- Nicodemo, A.C.; Araujo, M.R.E.; Ruiz, A.S.; Gales, A.C. In vitro susceptibility of Stenotrophomonas maltophilia isolates: Comparison of disc diffusion, Etest and agar dilution methods. J. Antimicrob. Chemother. 2004, 53, 604–608. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Pettaway, C.; Bard, J.D.; Arias, C.A.; Bhatti, M.M.; Humphries, R.M. Evaluation of the Performance of Manual Antimicrobial Susceptibility Testing Methods and Disk Breakpoints for Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2021, 65, e02631-20. [Google Scholar] [CrossRef] [PubMed]
- Gales, A.C.; Seifert, H.; Gur, D.; Castanheira, M.; Jones, R.N.; Sader, H.S. Antimicrobial Susceptibility of Acinetobacter calcoaceticus–Acinetobacter baumannii Complex and Stenotrophomonas maltophilia Clinical Isolates: Results from the SENTRY Antimicrobial Surveillance Program (1997–2016). Open Forum Infect. Dis. 2019, 6, S34–S46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palioura, S.; Gibbons, A.; Miller, D.; O’Brien, T.P.; Alfonso, E.C.; Spierer, O. Clinical Features, Antibiotic Susceptibility Profile, and Outcomes of Infectious Keratitis Caused by Stenotrophomonas maltophilia. Cornea 2018, 37, 326–330. [Google Scholar] [CrossRef]
- Wu, A.L.; Yeh, L.K.; Ma, D.H.; Chen, P.Y.; Lin, H.C.; Sun, C.C.; Tan, H.Y.; Chen, H.C.; Chen, S.Y.; Hsiao, C.H. Clinical Characteristics of Stenotrophomonas maltophilia Keratitis. Cornea 2016, 35, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Plésiat, P.; Nikaido, H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef] [Green Version]
- Farrell, D.J.; Sader, H.S.; Jones, R.N. Antimicrobial Susceptibilities of a Worldwide Collection of Stenotrophomonas maltophilia Isolates Tested against Tigecycline and Agents Commonly Used for S. maltophilia Infections. Antimicrob. Agents Chemother. 2010, 54, 2735–2737. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.-T.; Shiau, Y.-R.; Wang, H.-Y.; Yang Lauderdale, T.-L.; Chang, S.-C. A multicenter surveillance of antimicrobial resistance on Stenotrophomonas maltophilia in Taiwan. J. Microbiol. Immunol. Infect. 2012, 45, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Lin, C.Y.; Chen, Y.H.; Hsueh, P.R. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front. Microbiol. 2015, 6, 893. [Google Scholar] [CrossRef] [PubMed]
- Felix, J.P.F.; Lira, R.P.C.; Grupenmacher, A.T.; Filho, H.L.G.A.; Cosimo, A.B.; Nascimento, M.A.; Arieta, C.E.L. Long-term Results of Trimethoprim-Sulfamethoxazole Versus Placebo to Reduce the Risk of Recurrent Toxoplasma gondii Retinochoroiditis. Am. J. Ophthalmol. 2020, 213, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Tatman-Otkun, M.; Gürcan, S.; Ozer, B.; Aydoslu, B.; Bukavaz, S. The antimicrobial susceptibility of Stenotrophomonas maltophilia isolates using three different methods and their genetic relatedness. BMC Microbiol. 2005, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Arias, C.A.; Abbott, A.; Bard, J.D.; Bhatti, M.M.; Humphries, R.M. Evaluation of the Vitek 2, Phoenix, and MicroScan for Antimicrobial Susceptibility Testing of Stenotrophomonas maltophilia. J. Clin. Microbiol. 2021, 59, e0065421. [Google Scholar] [CrossRef]
- Huang, H.-H.; Lin, Y.-T.; Chen, P.-Y.; Li, L.-H.; Ning, H.-C.; Yang, T.-C. ClpA and HtpX Proteases Are Involved in Intrinsic Aminoglycoside Resistance of Stenotrophomonas maltophilia and Are Potential Aminoglycoside Adjuvant Targets. Antimicrob. Agents Chemother. 2018, 62, e00554-18. [Google Scholar] [CrossRef]
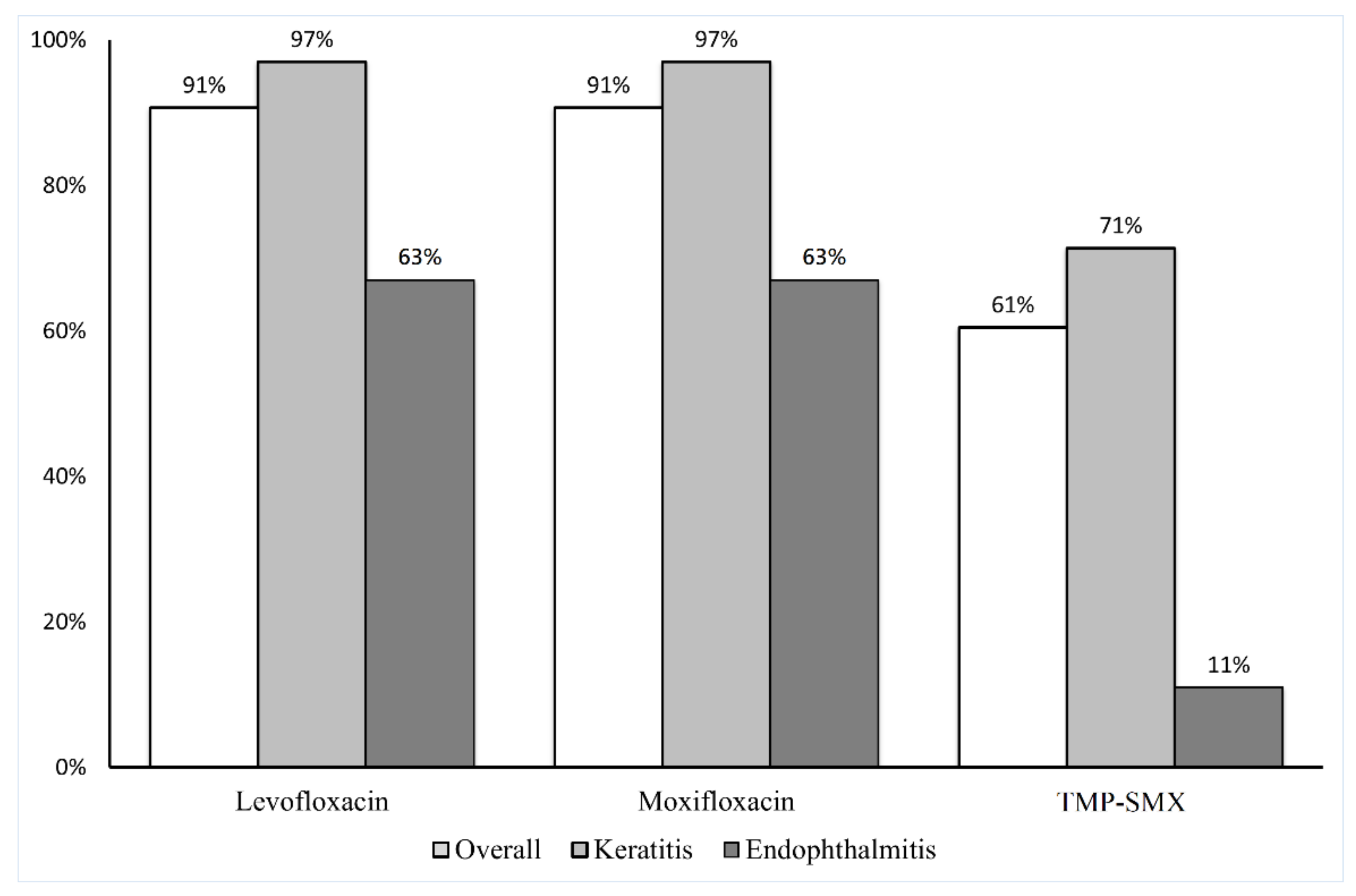

| Antibiotics | n | MIC (µg/mL) | Susceptibility (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC50 a | MIC90 b | GM MIC | Mode * | MIC Range | S | I | R | ||
| Amikacin | 43 | 12 | >256 | 16.732 | >256 | 2–>256 | - | - | - |
| Ceftazidime | 43 | 48 | >256 | 21.562 | >256 | 0.064–>256 | 40 | 7 | 53 |
| Cefuroxime | 43 | >256 | >256 | 242.288 | >256 | 24–>256 | 0 | 2 | 98 |
| Tigecycline | 43 | 1 | 3 | 1.249 | 1 | 0.38–8 | 100 | 0 | 0 |
| TMP-SMX | 43 | 0.19 | >32 | 0.668 | >32 | 0.047–>32 | 72 | 0 | 28 |
| Levofloxacin | 43 | 0.5 | 1 | 0.575 | 0.5 | 0.064–>32 | 91 | 7 | 2 |
| Gatifloxacin | 43 | 0.38 | 2 | 0.424 | 0.38 | 0.094–12 | 91 | 2 | 7 |
| Moxifloxacin | 43 | 0.125 | 0.75 | 0.172 | 0.125 | 0.032–8 | 93 | 7 | 2 |
| Antibiotics | Keratitis MIC (µg/mL) | Endophthalmitis MIC (µg/mL) | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC50 a | MIC90 b | GM MIC † | MIC Range | MIC50 | MIC90 | GM MIC † | MIC Range | ||
| Amikacin | 16 | >256 | 18.817 | 2–>256 | 4 | >256 | 10.212 | 2–>256 | 0.0690 |
| Ceftazidime | 24 | >256 | 14.931 | 0.064–>256 | >256 | >256 | 174.181 | 8–>256 | 0.0047 * |
| Cefuroxime | >256 | >256 | 239.259 | 24–>256 | >256 | >256 | 256.000 | all > 256 | 0.6121 |
| Tigecycline | 1 | 2 | 1.127 | 0.38–4 | 2 | 8 | 2.476 | 0.5–8 | 0.0535 |
| TMP-SMX | 0.19 | >32 | 0.350 | 0.047–>32 | 12 | 12 | 5.992 | 0.5–12 | 0.0030 * |
| Levofloxacin | 0.5 | 1 | 0.447 | 0.064–1 | 1 | 12 | 1.901 | 0.5–12 | 0.0029 * |
| Gatifloxacin | 0.25 | 0.75 | 0.326 | 0.094–3 | 0.5 | 12 | 1.904 | 0.25–12 | 0.0003 * |
| Moxifloxacin | 0.125 | 0.38 | 0.128 | 0.032–1.5 | 0.25 | 8 | 0.939 | 0.125–8 | 0.0004 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, M.M.-C.; Sun, M.-H.; Wu, W.-C.; Lai, C.-C.; Yeh, L.-K.; Hwang, Y.-S.; Hsiao, C.-H.; Chen, K.-J. Antibiotic Susceptibility and Minimum Inhibitory Concentration for Stenotrophomonas maltophilia Ocular Infections. Antibiotics 2022, 11, 1457. https://doi.org/10.3390/antibiotics11111457
Ho MM-C, Sun M-H, Wu W-C, Lai C-C, Yeh L-K, Hwang Y-S, Hsiao C-H, Chen K-J. Antibiotic Susceptibility and Minimum Inhibitory Concentration for Stenotrophomonas maltophilia Ocular Infections. Antibiotics. 2022; 11(11):1457. https://doi.org/10.3390/antibiotics11111457
Chicago/Turabian StyleHo, Margaret Ming-Chih, Ming-Hui Sun, Wei-Chi Wu, Chi-Chun Lai, Lung-Kun Yeh, Yih-Shiou Hwang, Ching-Hsi Hsiao, and Kuan-Jen Chen. 2022. "Antibiotic Susceptibility and Minimum Inhibitory Concentration for Stenotrophomonas maltophilia Ocular Infections" Antibiotics 11, no. 11: 1457. https://doi.org/10.3390/antibiotics11111457
APA StyleHo, M. M.-C., Sun, M.-H., Wu, W.-C., Lai, C.-C., Yeh, L.-K., Hwang, Y.-S., Hsiao, C.-H., & Chen, K.-J. (2022). Antibiotic Susceptibility and Minimum Inhibitory Concentration for Stenotrophomonas maltophilia Ocular Infections. Antibiotics, 11(11), 1457. https://doi.org/10.3390/antibiotics11111457







