Synthetic Amphipathic β-Sheet Temporin-Derived Peptide with Dual Antibacterial and Anti-Inflammatory Activities
Abstract
1. Introduction
2. Results
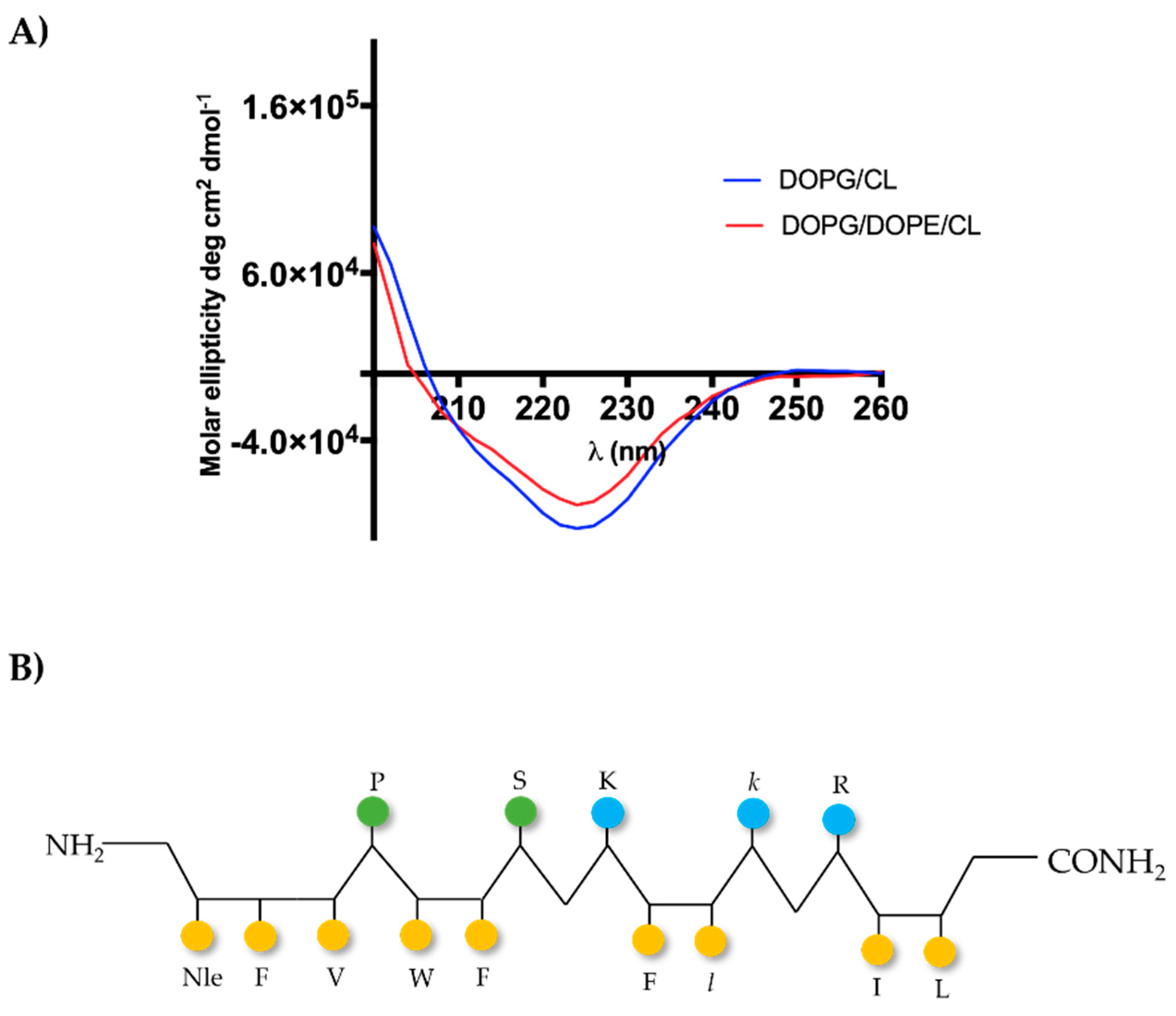
2.1. Peptide Design
2.2. Physicochemical Properties
2.3. Broad-Spectrum Antimicrobial Activity of [Nle1, dLeu9, dLys10]TL
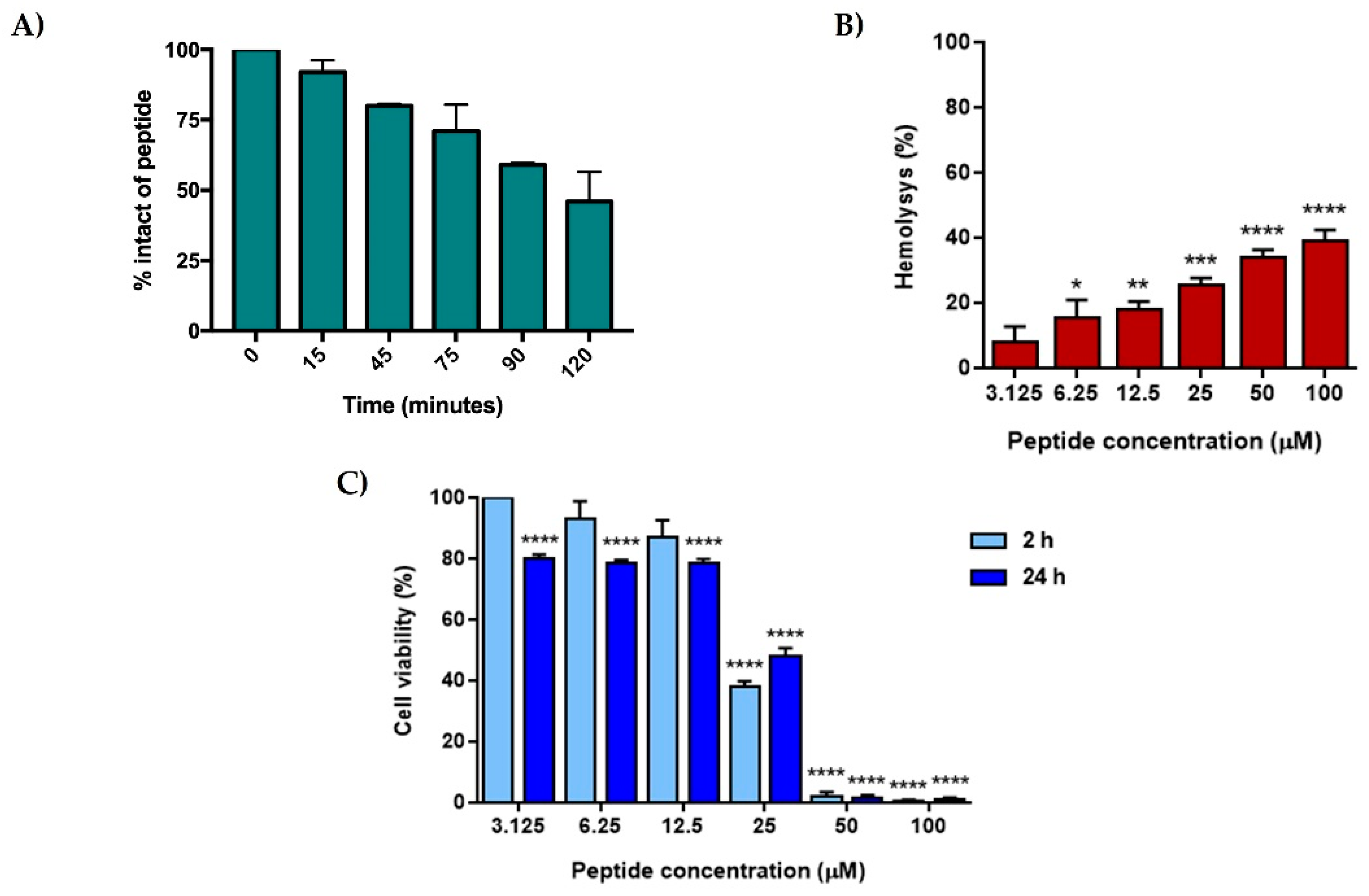
2.4. [Nle1, dLeu9, dLys10]TL Was Stable and Did Not Cause Cytotoxicity on Human Cells
2.5. Aggregation Propensity of [Nle1, dLeu9, dLys10]TL in Large Unilamellar Vesicles (LUVs)
2.6. [Nle1, dLeu9, dLys10]TL Forms β-Aggregates in LUVs
2.7. [Nle1, dLeu9, dLys10]TL Changes Significantly the Membrane Fluidity
2.8. [Nle1, dLeu9, dLys10]TL Induces Leakage LUVs
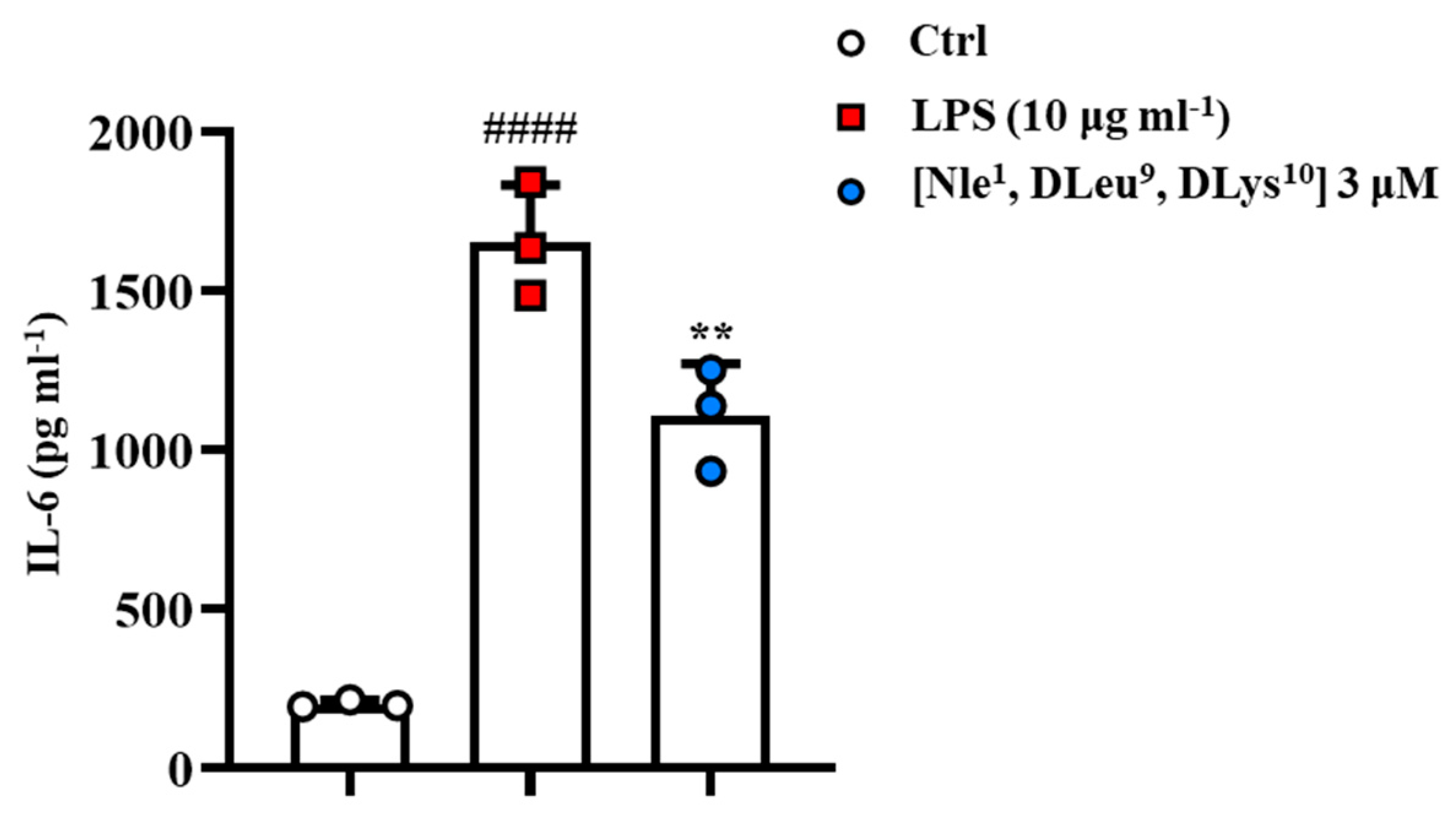
2.9. [Nle1, dLeu9, dLys10]TL Exhibited Anti-Inflammatory Activity In Vitro Model
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis
4.2. Antimicrobial Susceptibility Testing
4.3. Proteolytic Stability of [Nle1, dLeu9, dLys10]TL
4.4. Hemolytic Assay
4.5. Cytotoxicity on Human Keratinocytes
4.6. Peptide Aggregation in Solution and in LUVs
4.7. Circular Dichroism in SUVs
4.8. Membrane Fluidity
4.9. ANTS/DPX Leakage Assay
4.10. Peptide Cytotoxicity on Murine Macrophage J774A.1 Cells
4.11. Anti-Inflammatory Activity on Murine Macrophages
4.12. Data and Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic resistance: One health one world outlook. Front. Cell. Infect. Microbiol. 2021, 11, 771510. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Zeng, L.; Xu, T.; Sun, S.; Yan, S.; Yang, L.; Huang, Y.; Jia, J.; Dou, T. Antibiotic resistance genes in bacteria: Occurrence, spread, and control. J. Basic. Microbiol. 2021, 61, 1049–1070. [Google Scholar] [CrossRef] [PubMed]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.M.; Bechinger, B.; Naas, T. Antimicrobial peptides: A potent alternative to antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Kim, C.; Seo, C.H.; Park, Y. The therapeutic applications of antimicrobial peptides (AMPs): A patent review. J. Microbiol. 2017, 55, 1–12. [Google Scholar] [CrossRef]
- Mwangi, J.; Hao, X.; Lai, R.; Zhang, Z.Y. Antimicrobial peptides: New hope in the war against multidrug resistance. Zool. Res. 2019, 40, 488–505. [Google Scholar] [CrossRef]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug. Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dou, X.; Song, J.; Lyu, Y.; Zhu, X.; Xu, L.; Li, W.; Shan, A. Antimicrobial peptides: Promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2019, 39, 831–859. [Google Scholar] [CrossRef]
- Drayton, M.; Deisinger, J.P.; Ludwig, K.C.; Raheem, N.; Müller, A.; Schneider, T.; Straus, S.K. Host defense peptides: Dual antimicrobial and immunomodulatory action. Int. J. Mol. Sci. 2021, 22, 11172. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of antimicrobial peptides: Antimicrobial, anti-inflammatory and antibiofilm activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef]
- Maione, A.; Bellavita, R.; de Alteriis, E.; Galdiero, S.; Albarano, L.; La Pietra, A.; Guida, M.; Parrilli, E.; D’Angelo, C.; Galdiero, E.; et al. WMR peptide as antifungal and antibiofilm against albicans and non-albicans Candida species: Shreds of evidence on the mechanism of action. Int. J. Mol. Sci. 2022, 23, 2151. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Maione, A.; La Pietra, A.; de Alteriis, E.; Vitale, S.; Bellavita, R.; Carotenuto, R.; Turrà, D.; Galdiero, S.; Galdiero, E.; et al. Competitiveness during dual-species biofilm formation of Fusarium oxysporum and Candida albicans and a novel treatment strategy. Pharmaceutics 2022, 14, 1167. [Google Scholar] [CrossRef] [PubMed]
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The multifaceted nature of antimicrobial peptides: Current synthetic chemistry approaches and future directions. Chem. Soc. Rev. 2021, 50, 7820–7880. [Google Scholar] [CrossRef] [PubMed]
- Benfield, A.H.; Henriques, S.T. Mode-of-action of antimicrobial peptides: Membrane disruption vs. intracellular mechanisms. Front. Med. Technol. 2020, 2, 610997. [Google Scholar] [CrossRef] [PubMed]
- Pirtskhalava, M.; Vishnepolsky, B.; Grigolava, M.; Managadze, G. Physicochemical features and peculiarities of interaction of AMP with the membrane. Pharmaceuticals 2021, 14, 471. [Google Scholar] [CrossRef] [PubMed]
- Travkova, O.G.; Moehwald, H.; Brezesinski, G. The interaction of antimicrobial peptides with membranes. Adv. Colloid. Interface. Sci. 2017, 247, 521–532. [Google Scholar] [CrossRef]
- Falcigno, L.; D’Auria, G.; Palmieri, G.; Gogliettino, M.; Agrillo, B.; Tatè, R.; Dardano, P.; Nicolais, L.; Balestrieri, M. Key physicochemical determinants in the antimicrobial peptide RiLK1 promote amphipathic structures. Int. J. Mol. Sci. 2021, 22, 10011. [Google Scholar] [CrossRef]
- He, S.; Stone, T.A.; Deber, C.M. Uncoupling amphipathicity and hydrophobicity: Role of charge clustering in membrane interactions of cationic antimicrobial peptides. Biochemistry 2021, 60, 2586–2592. [Google Scholar] [CrossRef]
- Wieprecht, T.; Dathe, M.; Beyermann, M.; Krause, E.; Maloy, W.L.; MacDonald, D.L.; Bienert, M. Peptide hydrophobicity controls the activity and selectivity of magainin 2 amide in interaction with membranes. Biochemistry 1997, 36, 6124–6132. [Google Scholar] [CrossRef]
- Lee, D.G.; Kim, H.N.; Park, Y.; Kim, H.K.; Choi, B.H.; Choi, C.H.; Hahm, K.S. Design of novel analogue peptides with potent antibiotic activity based on the antimicrobial peptide, HP (2−20), derived from n-terminus of Helicobacter pylori ribosomal protein L1. Biochim. Biophys. Acta 2002, 1598, 185–194. [Google Scholar] [CrossRef]
- Hollmann, A.; Martínez, M.; Noguera, M.E.; Augusto, M.T.; Disalvo, A.; Santos, N.C.; Semorile, L.; Maffía, P.C. Role of amphipathicity and hydrophobicity in the balance between hemolysis and peptide–membrane interactions of three related antimicrobial peptides. Colloids. Surf. B. Biointerfaces 2016, 141, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Simonson, A.W.; Aronson, M.R.; Medina, S.H. Supramolecular peptide assemblies as antimicrobial scaffolds. Molecules 2020, 25, 2751. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Sun, F.; Zhou, X.R.; Luo, S.Z.; Chen, L. Role of peptide self-assembly in antimicrobial peptides. J. Pept. Sci. 2015, 21, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.M.; Edwards, M.A.; Li, J.; Yip, C.M.; Deber, C.M. Roles of hydrophobicity and charge distribution of cationic antimicrobial peptides in peptide-membrane interactions. J. Biol. Chem. 2012, 287, 7738–7745. [Google Scholar] [CrossRef]
- Bowerman, C.J.; Liyanage, W.; Federation, A.J.; Nilsson, B.L. Tuning β-sheet peptide self-assembly and hydrogelation behavior by modification of sequence hydrophobicity and aromaticity. Biomacromolecules 2011, 12, 2735–2745. [Google Scholar] [CrossRef]
- Lombardi, L.; Shi, Y.; Falanga, A.; Galdiero, E.; de Alteriis, E.; Franci, G.; Chourpa, I.; Azevedo, H.S.; Galdiero, S. Enhancing the potency of antimicrobial peptides through molecular engineering and self-assembly. Biomacromolecules 2019, 20, 1362–1374. [Google Scholar] [CrossRef]
- Bellavita, R.; Falanga, A.; Buommino, E.; Merlino, F.; Casciaro, B.; Cappiello, F.; Mangoni, M.L.; Novellino, E.; Catania, M.R.; Paolillo, R.; et al. Novel temporin L antimicrobial peptides: Promoting self-assembling by lipidic tags to tackle superbugs. J. Enzyme Inhib. Med. Chem. 2020, 35, 1751–1764. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, M.; Lai, R.; Zhang, Z. Chemical modifications to increase the therapeutic potential of antimicrobial peptides. Peptides 2021, 146, 170666. [Google Scholar] [CrossRef]
- Kundu, R. Cationic amphiphilic peptides: Synthetic antimicrobial agents inspired by nature. ChemMedChem 2020, 15, 1887–1896. [Google Scholar] [CrossRef]
- de Alteriis, E.; Maione, A.; Falanga, A.; Bellavita, R.; Galdiero, S.; Albarano, L.; Salvatore, M.M.; Galdiero, E.; Guida, M. Activity of free and liposome-encapsulated essential oil from Lavandula angustifolia against persister-derived biofilm of Candida auris. Antibiotics 2021, 11, 26. [Google Scholar] [CrossRef]
- Pandit, G.; Sarkar, T.S.R.V.; Debnath, S.; Satpati, P.; Chatterjee, S. Delineating the mechanism of action of a protease resistant and salt tolerant synthetic antimicrobial peptide against Pseudomonas aeruginosa. ACS Omega 2022, 7, 15951–15968. [Google Scholar] [CrossRef] [PubMed]
- Del Genio, V.; Bellavita, R.; Falanga, A.; Hervé-Aubert, K.; Chourpa, I.; Galdiero, S. Peptides to overcome the limitations of current anticancer and antimicrobial nanotherapies. Pharmaceutics 2022, 14, 1235. [Google Scholar] [CrossRef]
- Romero, S.M.; Cardillo, A.B.; Martínez Ceron, M.C.; Camperi, S.A.; Giudicessi, S.L. Temporins: An approach of potential pharmaceutic candidates. Surg. Infect. 2020, 21, 309–322. [Google Scholar] [CrossRef]
- Rinaldi, A.C.; Di Giulio, A.; Liberi, M.; Gualtieri, G.; Oratore, A.; Bozzi, A.; Schininà, M.E.; Simmaco, M. Effects of temporins on molecular dynamics and membrane permeabilization in lipid vesicles. J. Pept. Res. 2001, 58, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.C.; Mangoni, M.L.; Rufo, A.; Luzi, C.; Barra, D.; Zhao, H.; Kinnunen, P.K.; Bozzi, A.; Di Giulio, A.; Simmaco, M. Temporin L: Antimicrobial, haemolytic and cytotoxic activities, and effects on membrane permeabilization in lipid vesicles. Biochem. J. 2002, 368, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Buommino, E.; Carotenuto, A.; Antignano, I.; Bellavita, R.; Casciaro, B.; Loffredo, M.R.; Merlino, F.; Novellino, E.; Mangoni, M.L.; Nocera, F.P.; et al. The Outcomes of decorated prolines in the discovery of antimicrobial peptides from Temporin-L. ChemMedChem 2019, 14, 1283–1290. [Google Scholar] [CrossRef]
- Roscetto, E.; Bellavita, R.; Paolillo, R.; Merlino, F.; Molfetta, N.; Grieco, P.; Buommino, E.; Catania, M.R. Antimicrobial activity of a lipidated Temporin L analogue against carbapenemase-producing Klebsiella pneumoniae clinical isolates. Antibiotics 2021, 10, 1312. [Google Scholar] [CrossRef]
- Bellavita, R.; Casciaro, B.; Di Maro, S.; Brancaccio, D.; Carotenuto, A.; Falanga, A.; Cappiello, F.; Buommino, E.; Galdiero, S.; Novellino, E.; et al. First-in-class cyclic Temporin L analogue: Design, synthesis, and antimicrobial assessment. J. Med. Chem. 2021, 64, 11675–11694. [Google Scholar] [CrossRef]
- Bellavita, R.; Maione, A.; Merlino, F.; Siciliano, A.; Dardano, P.; De Stefano, L.; Galdiero, S.; Galdiero, E.; Grieco, P.; Falanga, A. Antifungal and antibiofilm activity of cyclic Temporin L peptide analogues against albicans and non-albicans Candida species. Pharmaceutics 2022, 14, 454. [Google Scholar] [CrossRef]
- Zannella, C.; Chianese, A.; Palomba, L.; Marcocci, M.E.; Bellavita, R.; Merlino, F.; Grieco, P.; Folliero, V.; De Filippis, A.; Mangoni, M.; et al. Broad-spectrum antiviral activity of the amphibian antimicrobial peptide Temporin L and its analogs. Int. J. Mol. Sci. 2022, 23, 2060. [Google Scholar] [CrossRef]
- Merlino, F.; Carotenuto, A.; Casciaro, B.; Martora, F.; Loffredo, M.R.; Di Grazia, A.; Yousif, A.M.; Brancaccio, D.; Palomba, L.; Novellino, E.; et al. Glycine-replaced derivatives of [Pro3,DLeu9]TL, a temporin L analogue: Evaluation of antimicrobial, cytotoxic and hemolytic activities. Eur. J. Med. Chem. 2017, 139, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Bellavita, R.; Vollaro, A.; Catania, M.R.; Merlino, F.; De Martino, L.; Nocera, F.P.; DellaGreca, M.; Lembo, F.; Grieco, P.; Buommino, E. Novel antimicrobial peptide from Temporin L in the treatment of Staphylococcus pseudintermedius and Malassezia pachydermatis in polymicrobial inter-kingdom infection. Antibiotics 2020, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Bellavita, R.; Raucci, F.; Merlino, F.; Piccolo, M.; Ferraro, M.G.; Irace, C.; Santamaria, R.; Iqbal, A.J.; Novellino, E.; Grieco, P.; et al. Temporin L-derived peptide as a regulator of the acute inflammatory response in zymosan-induced peritonitis. Biomed. Pharmacother. 2020, 123, 109788. [Google Scholar] [CrossRef] [PubMed]
- Tews, J.K.; Repa, J.J.; Harper, A.E. Norleucine: A branched-chain amino acid analog affecting feeding behavior of rats. Pharmacol. Biochem. Behav. 1990, 35, 911–921. [Google Scholar] [CrossRef]
- Haernvall, K.; Fladischer, P.; Schoeffmann, H.; Zitzenbacher, S.; Pavkov-Keller, T.; Gruber, K.; Schick, M.; Yamamoto, M.; Kuenkel, A.; Ribitsch, D.; et al. Residue-specific incorporation of the non-canonical amino acid norleucine improves lipase activity on synthetic polyesters. Front. Bioeng. Biotechnol. 2022, 10, 769830. [Google Scholar] [CrossRef]
- Dathe, M.; Wieprecht, T.; Nikolenko, H.; Handel, L.; Maloy, W.L.; MacDonald, D.L.; Beyermann, M.; Bienert, M. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett. 1997, 403, 208–212. [Google Scholar] [CrossRef]
- Porto, W.F.; Ferreira, K.C.V.; Ribeiro, S.M.; Franco, O.L. Sense the moment: A highly sensitive antimicrobial activity predictor based on hydrophobic moment. Biochim. Biophys. Acta. Gen. Subj. 2022, 1866, 130070. [Google Scholar] [CrossRef]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- White, S.H.; Wimley, W.C. Hydrophobic interactions of peptides with membrane interfaces. Biochim. Biophys. Acta 1998, 1376, 339–352. [Google Scholar] [CrossRef]
- Wimley, W.C.; White, S.H. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat. Struct. Biol. 1996, 3, 842–848. [Google Scholar] [CrossRef]
- Kurniasih, I.N.; Liang, H.; Mohr, P.C.; Khot, G.; Rabe, J.P.; Mohr, A. Nile red dye in aqueous surfactant and micellar solution. Langmuir 2015, 31, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Amaro, M.; Reina, F.; Hof, M.; Eggeling, C.; Sezgin, E. Laurdan and Di-4-ANEPPDHQ probe different properties of the membrane. Phys. D. Appl. Phys. 2017, 50, 134004. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Kim, D.H.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial peptides: Their physicochemical properties and therapeutic application. Arch. Pharm. Res. 2012, 35, 409–413. [Google Scholar] [CrossRef]
- Edwards, I.A.; Elliott, A.G.; Kavanagh, A.M.; Zuegg, J.; Blaskovich, M.A.; Cooper, M.A. Contribution of amphipathicity and hydrophobicity to the antimicrobial activity and cytotoxicity of β-Hairpin peptides. ACS Infect. Dis. 2016, 2, 442–450. [Google Scholar] [CrossRef]
- Li, W.; Separovic, F.; O’Brien-Simpson, N.M.; Wade, J.D. Chemically modified and conjugated antimicrobial peptides against superbugs. Chem. Soc. Rev. 2021, 50, 4932–4973. [Google Scholar] [CrossRef]
- Shai, Y. Mode of action of membrane active antimicrobial peptides. Biopolymers 2002, 66, 236–248. [Google Scholar] [CrossRef]
- Le, C.F.; Fang, C.M.; Sekaran, S.D. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob. Agents. Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef]
- Li, J.; Koh, J.J.; Liu, S.; Lakshminarayanan, R.; Verma, C.S.; Beuerman, R.W. Membrane active antimicrobial peptides: Translating mechanistic insights to design. Front. Neurosci. 2017, 11, 73. [Google Scholar] [CrossRef]
- Del Genio, V.; Falanga, A.; Allard-Vannier, E.; Hervé-Aubert, K.; Leone, M.; Bellavita, R.; Uzbekov, R.; Chourpa, I.; Galdiero, S. Design and validation of nanofibers made of self-assembled peptides to become multifunctional stimuli-sensitive nanovectors of anticancer drug doxorubicin. Pharmaceutics 2022, 14, 1544. [Google Scholar] [CrossRef]
- Hudson, S.A.; Ecroyd, H.; Kee, T.W.; Carver, J.A. The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS J. 2009, 276, 5960–5972. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, C.F.; Calori, I.R.; Tessaro, A.L.; Caetano, W.; Hioka, N. Rapid formation of Small Unilamellar Vesicles (SUV) through low-frequency sonication: An innovative approach. Colloids Surf. B. Biointerfaces 2019, 181, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Ladokhin, A.S.; Wimley, W.C.; White, S.H. Leakage of membrane vesicle contents: Determination of mechanism using fluorescence requenching. Biophys. J. 1995, 69, 1964–1971. [Google Scholar] [CrossRef]
- Curtis, M.J.; Alexander, S.; Cirino, G.; Docherty, J.R.; George, C.H.; Giembycz, M.A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. Br. J. Pharmacol. 2018, 175, 987–993. [Google Scholar] [CrossRef] [PubMed]
- George, C.H.; Stanford, S.C.; Alexander, S.; Cirino, G.; Docherty, J.R.; Giembycz, M.A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. Updating the guidelines for data transparency in the British Journal of Pharmacology—Data sharing and the use of scatter plots instead of bar charts. Br. J. Pharmacol. 2017, 174, 2801–2804. [Google Scholar] [CrossRef]



| Peptide | GRAVY | Aliphatic Index | Hydrophobicity * | Hydrophobic Moment (μH) |
|---|---|---|---|---|
| [dLeu9, dLys10]TL | 0.700 | 112.31 | +4.15 Kcal∙mol−1 | 0.670 |
| [Nle1, dLeu9, Lys10]TL | 0.921 | 132.14 | +2.90 Kcal∙mol−1 | 0.601 |
| Strains | MIC Values (μM) | |
|---|---|---|
| [Nle1, dLeu9, dLys10]TL | [dLeu9, dLys10]TL * | |
| E. coli ATCC 25922 | 6.25 | 12.5 |
| P. aeruginosa ATCC 27853 | 12.5 | 12.5 |
| A. baumannii ATCC 19606 | 3.12 | 6.25 |
| K. pneumoniae ATCC BAA-1705 | 12.5 | 12.5 |
| S. aureus ATCC 25923 | 3.12 | 6.25 |
| S. epidermidis ATCC 12228 | 3.12 | 3.12 |
| B. megaterium Bm11 | 0.78 | 3.12 |
| GP Value | |||
|---|---|---|---|
| LUVs: DOPG/CL | |||
| cmpd | Unloaded LUVs | LUVs + 5 μM cmpd | LUVs + 30 μM cmpd |
| [dLeu9, dLys10]TL | −0.18 ± 0.01 | −0.06 ± 0.01 | 0.26 ± 0.02 |
| [Nle1, dLeu9, dLys10]TL | −0.18 ± 0.01 | 0.03 ± 0.01 | 0.29 ± 0.03 |
| LUVs: DOPG/DOPE/CL | |||
| cmpd | Unloaded LUVs | LUVs + 5 μM cmpd | LUVs + 30 μM cmpd |
| [dLeu9, dLys10]TL | −0.07 ± 0.01 | 0.09 ± 0.02 | 0.11 ± 0.02 |
| [Nle1, dLeu9, dLys10]TL | −0.07 ± 0.01 | 0.05 ± 0.01 | 0.13 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellavita, R.; Buommino, E.; Casciaro, B.; Merlino, F.; Cappiello, F.; Marigliano, N.; Saviano, A.; Maione, F.; Santangelo, R.; Mangoni, M.L.; et al. Synthetic Amphipathic β-Sheet Temporin-Derived Peptide with Dual Antibacterial and Anti-Inflammatory Activities. Antibiotics 2022, 11, 1285. https://doi.org/10.3390/antibiotics11101285
Bellavita R, Buommino E, Casciaro B, Merlino F, Cappiello F, Marigliano N, Saviano A, Maione F, Santangelo R, Mangoni ML, et al. Synthetic Amphipathic β-Sheet Temporin-Derived Peptide with Dual Antibacterial and Anti-Inflammatory Activities. Antibiotics. 2022; 11(10):1285. https://doi.org/10.3390/antibiotics11101285
Chicago/Turabian StyleBellavita, Rosa, Elisabetta Buommino, Bruno Casciaro, Francesco Merlino, Floriana Cappiello, Noemi Marigliano, Anella Saviano, Francesco Maione, Rosaria Santangelo, Maria Luisa Mangoni, and et al. 2022. "Synthetic Amphipathic β-Sheet Temporin-Derived Peptide with Dual Antibacterial and Anti-Inflammatory Activities" Antibiotics 11, no. 10: 1285. https://doi.org/10.3390/antibiotics11101285
APA StyleBellavita, R., Buommino, E., Casciaro, B., Merlino, F., Cappiello, F., Marigliano, N., Saviano, A., Maione, F., Santangelo, R., Mangoni, M. L., Galdiero, S., Grieco, P., & Falanga, A. (2022). Synthetic Amphipathic β-Sheet Temporin-Derived Peptide with Dual Antibacterial and Anti-Inflammatory Activities. Antibiotics, 11(10), 1285. https://doi.org/10.3390/antibiotics11101285














