Multi-Drug Resistant Pathogenic Escherichia coli Isolated from Wild Birds, Chicken, and the Environment in Malaysia
Abstract
1. Introduction
2. Results
2.1. Multidrug Resistant E. coli Isolates
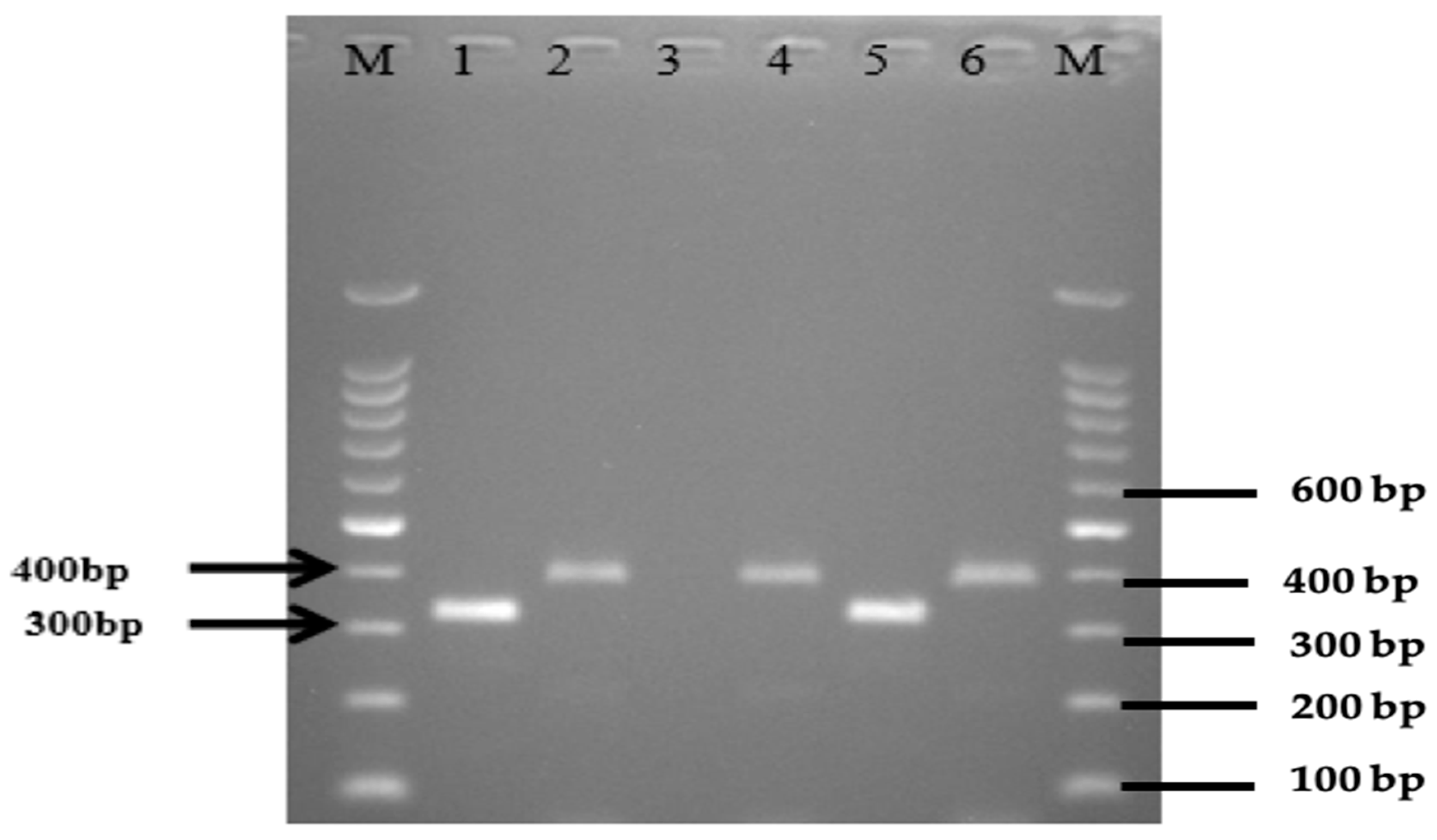
2.2. Occurrence of E. coli Virulence Genes in Wild Birds, Chickens, and Environment in Villages
2.3. Statistical Analysis
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Confirmation of E. coli Isolates
4.3. Detection of eaeA, EPEC, and EIEC Using Monoplex and Modified Multiplex PCR Assay
4.3.1. Genomic DNA Extraction
4.3.2. Primer and PCR Cycling Conditions Modified Multiplex PCR to Detect EPEC and EIEC
4.4. Antibiotic Susceptibility Test
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sonola, V.S.; Katakweba, A.S.; Misinzo, G.; Matee, M.I. Occurrence of Multi-Drug-Resistant Escherichia coli in chickens, humans, rodents and household soil in Karatu, Northern Tanzania. Antibiotics 2021, 9, 1137. [Google Scholar] [CrossRef] [PubMed]
- Bujňáková, D.; Puvača, N.; Ćirković, I. Virulence Factors and Antibiotic Resistance of Enterobacterales. Microorganisms 2022, 10, 1588. [Google Scholar] [CrossRef] [PubMed]
- Harb, A.; Abraham, S.; Rusdi, B.; Laird, T.; O’Dea, M.; Habib, I. Molecular Detection and Epidemiological Features of Selected Bacterial, Viral, and Parasitic Enteropathogens in Stool Specimens from Children with Acute Diarrhea in Thi-Qar Governorate, Iraq. Int. J. Environ. Res. Public Health 2019, 16, 1573. [Google Scholar] [CrossRef] [PubMed]
- Puvača, N.; de Llanos Frutos, R. Antimicrobial Resistance in Escherichia coli Strains Isolated from Humans and Pet Animals. Antibiotics 2021, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Habib, I.; Mohamed, M.-Y.I.; Khan, M. Current State of Salmonella, Campylobacter and Listeria in the Food Chain across the Arab Countries: A Descriptive Review. Foods 2021, 10, 2369. [Google Scholar] [CrossRef]
- Noster, J.; Thelen, P.; Hamprecht, A. Detection of Multidrug-Resistant Enterobacterales—From ESBLs to Carbapenemases. Antibiotics 2021, 9, 1140. [Google Scholar] [CrossRef]
- Mohamed, M.-Y.I.; Abu, J.; Aziz, S.A.; Zakaria, Z.; Khan, A.R.; Habib, I. Occurrence of antibiotic resistant C. jejuni and E. coli in wild birds, chickens, humans, and the environment in Malay villages, Kedah, Malaysia. Vet. Med.-Czech 2019, 67, 298–308. [Google Scholar] [CrossRef]
- Mohamed, M.-Y.I.; Aziz, S.A.; Abu, J.; Khairani-Bejo, S.; Puan, C.P.; Bitrus, A.A.; Aliyu, A.B.; Elmutaz, A.A. Occurrence of antibiotic resistant Campylobacter in wild birds and poultry. Malays. J. Microbiol. 2019, 15, 143–151. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Hu, H.-W.; Gou, M.; Wang, J.-T.; Chen, D.; He, J.-Z. Temporal succession of soil antibiotic resistance genes following application of swine, cattle and poultry manures spiked with or without antibiotics. Environ. Pollut. 2017, 231, 1621–1632. [Google Scholar] [CrossRef]
- Sanderson, H.; Brown, R.S.; Hania, P.; McAllister, T.A.; Majury, A.; Liss, S.N. Antimicrobial resistant genes and organisms as environmental contaminants of emerging concern: Addressing global public health risks. In Management of Emerging Public Health Issues and Risks; Elsevier: Amsterdam, The Netherlands, 2019; pp. 147–187. [Google Scholar]
- Touati, M.; Hadjadj, L.; Berrazeg, M.; Baron, S.A.; Rolain, J.M. Emergence of Escherichia coli harbouring mcr-1 and mcr-3 genes in Northwest Algerian farmlands. J. Glob. Antimicrob. Resist. 2020, 21, 132–137. [Google Scholar] [CrossRef]
- Ramey, A.M.; Hernandez, J.; Tyrlöv, V.; Uher-Koch, B.D.; Schmutz, J.A.; Atterby, C.; Järhult, J.D.; Bonnedahl, J. Antibiotic-resistant Escherichia coli in migratory birds inhabiting remote Alaska. EcoHealth 2018, 1, 72–81. [Google Scholar] [CrossRef]
- Ong, K.H.; Khor, W.C.; Quek, J.Y.; Low, Z.X.; Arivalan, S.; Humaidi, M.; Chua, C.; Seow, K.L.; Guo, S.; Tay, M.Y.; et al. Occurrence, and antimicrobial resistance traits of Escherichia coli from wild birds and rodents in Singapore. Int. J. Environ. Res. Public Health 2020, 17, 5606. [Google Scholar] [CrossRef]
- Santos, A.C.; Santos, F.F.; Silva, R.M.; Gomes, T.A. Diversity of hybrid-and hetero-pathogenic Escherichia coli and their potential implication in more severe diseases. Front. Cell. Infect. Microbiol. 2020, 10, 339. [Google Scholar] [CrossRef]
- Johnson, J.R.; Russo, T.A. Molecular epidemiology of extraintestinal pathogenic Escherichia coli. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef]
- Baumann, D.; Salia, H.; Greune, L.; Norkowski, S.; Körner, B.; Uckeley, Z.M.; Frankel, G.; Guenot, M.; Rüter, C.; Schmidt, M.A. Multitalented EspB of enteropathogenic Escherichia coli (EPEC) enters cells autonomously and induces programmed cell death in human monocytic THP-1 cells. Int. J. Med. Microbiol. 2018, 308, 387–404. [Google Scholar] [CrossRef]
- von Mentzer, A.; Connor, T.R.; Wieler, L.H.; Semmler, T.; Iguchi, A.; Thomson, N.R.; Rasko, D.A.; Joffre, E.; Corander, J.; Pickard, D.; et al. Identification of enterotoxigenic Escherichia coli (ETEC) clades with long-term global distribution. Nat. Genet. 2014, 46, 1321–1326. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Zheng, D.; Fujihara, S.; Wakabayashi, A.; Okahata, K.; Hara-Kudo, Y. Prevalence of Diarrheagenic Escherichia coli in Foods and Fecal Specimens Obtained from Cattle, Pigs, Chickens, Asymptomatic Carriers, and Patients in Osaka and Hyogo, Japan. Jpn. J. Infect. Dis. 2017, 70, 464–469. [Google Scholar] [CrossRef]
- Trabulsi, L.R.; Keller, R.; Gomes, T.A.T. Typical and atypical enteropathogenic Escherichia coli. Emerg. Infect. Dis. 2002, 8, 508–513. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123. [Google Scholar] [CrossRef]
- Gomes, T.A.T.; Elias, W.P.; Scaletsky, I.C.A.; Guth, B.E.C.; Rodrigues, J.F.; Piazza, R.M.F.; Martinez, M.B. Diarrheagenic Escherichia coli. Braz. J. Microbiol. 2016, 47, 3–30. [Google Scholar] [CrossRef]
- Sanches, L.A.; Gomes, M.; Teixeira, R.H.; Cunha, M.P.; Oliveira, M.G.; Vieira, M.A.; Gomes, T.A.; Knobl, T. Captive wild birds as reservoirs of enteropathogenic E. coli (EPEC) and Shiga-toxin producing E. coli (STEC). Braz. J. Microbiol. 2017, 48, 760–763. [Google Scholar] [CrossRef]
- Hughes, D.T.; Clarke, M.B.; Yamamoto, K.; Rasko, D.A.; Sperandio, V. The QseC adrenergic signaling cascade in enterohemorrhagic E. coli (EHEC). PLoS Pathog. 2009, 5, e1000553. [Google Scholar] [CrossRef]
- Yahia, H.B.; Sallem, R.B.; Tayh, G.; Klibi, N.; Amor, I.B.; Gharsa, H.; Boudabbous, A.; Slama, K.B. Detection of CTX-M-15 harboring Escherichia coli isolated from wild birds in Tunisia. BMC Microbiol. 2018, 18, 1–8. [Google Scholar]
- Kobayashi, H.; Kanazaki, M.; Hata, E.; Kubo, M. Prevalence, and characteristics of eae-and stx-positive strains of Escherichia coli from wild birds in the immediate environment of Tokyo Bay. Appl. Environ. Microbiol. 2009, 75, 292–295. [Google Scholar] [CrossRef]
- Sharma, P.; Maherchandani, S.; Shringi, B.N.; Kashyap, S.K.; Sundar, K.G. Temporal variations in patterns of Escherichia coli strain diversity and antimicrobial resistance in the migrant Egyptian vulture. Infect. Ecol. Epidemiol. 2018, 8, 1450590. [Google Scholar] [PubMed]
- Calero-Cáceres, W.; Méndez, J.; Martín-Díaz, J.; Muniesa, M. The Occurrence of Antibiotic Resistance Genes in a Mediterranean River and Their Persistence in the Riverbed Sediment. Environ. Pollut. 2017, 223, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Harnisz, M.; Korzeniewska, E.; Gołaś, I. The Impact of a Freshwater Fish Farm on the Community of Tetracycline-resistant Bacteria and the Structure of Tetracycline Resistance Genes in River Water. Chemosphere 2015, 128, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.Z.; Krüger, A.; Sanz, M.E.; Padola, N.L.; Lucchesi, P.M. Serotypes, virulence profiles and stx subtypes of Shigatoxigenic Escherichia coli isolated from chicken derived products. Rev. Argent. Microbiol. 2016, 48, 325–328. [Google Scholar] [CrossRef]
- Vittecoq, M.; Laurens, C.; Brazier, L.; Durand, P.; Elguero, E.; Arnal, A.; Thomas, F.; Aberkane, S.; Renaud, N.; Prugnolle, F.; et al. VIM-1 carbapenemase-producing Escherichia coli in gulls from southern France. Ecol. Evol. 2017, 7, 1224–1232. [Google Scholar] [CrossRef]
- Mohamed-Yousif, I.M.; Abu, J.; Abdul-Aziz, S.; Zakaria, Z.; Rashid, A.; Awad, E.A. Occurrence of antibiotic resistant C. jejuni and E. coli in wild birds, chickens, environment and humans from Orang Asli villages in Sungai Siput, Perak, Malaysia. Am. J. Anim. Vet. Sci. 2019, 14, 158–169. [Google Scholar] [CrossRef]
- Kamaruzzaman, E.A.; Abdul-Aziz, S.; Bitrus, A.A.; Zakaria, Z.; Hassan, L. Occurrence, and characteristics of extended-spectrum β-lactamase-producing Escherichia coli from dairy cattle, milk, and farm environments in Peninsular Malaysia. Pathogens 2020, 12, 1007. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Le Van, P.; Le Huy, C.; Gia, K.N.; Weintraub, A. Detection and characterization of diarrheagenic Escherichia coli from young children in Hanoi, Vietnam. J. Clin. Microbiol. 2005, 43, 755–760. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 2010. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 1 August 2022).


| Villages | No. of Isolates | No. (%) Resistant Isolates | No. of Antibiotics Resistant to * | No. (%) MDR |
|---|---|---|---|---|
| Wild birds | ||||
| A | 9 | 9 (100%) | 4–8 | 9 (100%) |
| B | 21 | 21 (100%) | 4–7 | 21 (100%) |
| C | 10 | 10 (100%) | 4–8 | 10 (100%) |
| D | 9 | 9 (100%) | 1–4 | 6 (66.7%) |
| E | 3 | 3 (100%) | 1 | 0 (0%) |
| F | 6 | 6 (100%) | 1–3 | 2 (33.3%) |
| 58 | 5 (100%) | 1–8 | 48 (82.8%) | |
| Chickens | ||||
| A | 10 | 10 (100%) | 2–9 | 8 (80%) |
| B | 10 | 10 (100%) | 1–8 | 8 (80%) |
| C | 20 | 20 (100%) | 1–6 | 3 (15%) |
| D | 16 | 16 (100%) | 1–5 | 10 (62.5%) |
| E | 15 | 15 (100%) | 1–5 | 8 (53.3%) |
| F | 13 | 13 (100%) | 3–8 | 13 (100%) |
| 84 | 84 (100%) | 1–9 | 50 (59.5%) | |
| Village | Wild Bird Species | No. of Isolates | No. eaeA Gene Positive (%) |
|---|---|---|---|
| A | Oriental Magpie Robin | 2 | 0 (0) |
| White-rumped Shama | 4 | 0 (0) | |
| Little Spiderhunter | 3 | 0 (0) | |
| B | Oriental Magpie Robin | 13 | 0 (0) |
| White-rumped Shama | 8 | 0 (0) | |
| C | Oriental Magpie Robin | 9 | 0 (0) |
| Little Spiderhunter | 1 | 0 (0) | |
| D | Eurasian Tree Sparrow | 8 | 5 (62.5) |
| White-Vented Myna | 1 | 1 (100) | |
| E | Eurasian Tree Sparrow | 2 | 0 (0) |
| Jungle Myna | 1 | 0 (0) | |
| F | Eurasian Tree Sparrow | 4 | 1 (25) |
| White-Vented Myna | 0 | 0 (0) | |
| Jungle Myna | 2 | 0(0) | |
| Total | 58 | 7 (12.1%) |
| Village | Chicken Isolates | eaeA Gene | EPEC | EIEC |
|---|---|---|---|---|
| A * | 10 | 1 (10%) | 0 (0%) | 0 (0%) |
| B * | 10 | 3 (30%) | 0 (0%) | 0 (0%) |
| C * | 20 | 18 (90%) | 1 (5%) | 1 (5%) |
| Total no. | 40 | 22 (55%) | 1 (2.5%) | 1 (2.5%) |
| D # | 16 | 5 (31.3%) | 1 (6.3%) | 0 (0%) |
| E # | 15 | 8 (53.3%) | 2 (13.3%) | 1 (6.7%) |
| F # | 13 | 3 (23.1%) | 2 (15.4%) | 0 (0%) |
| Total no. | 44 | 16 (36.4%) | 5 (11.4%) | 1 (2.3%) |
| Total | 84 | 38 (45.2%) | 6 (7.1%) | 2 (2.4%) |
| Village | Flies (Three Isolates Per Village) | Water (Three Isolates Per Village) | Soil (Three Isolates Per Village) | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eaeA Gene (%) | EPEC (%) | EIEC (%) | eaeA Gene (%) | EPEC (%) | EIEC (%) | eaeA gene (%) | EPEC (%) | EIEC (%) | eaeA Gene (%) | EPEC (%) | EIEC (%) | |
| A | 0 (0) | 0 (0) | 0 (0) | 2 (66.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (22.2) | 0 (0) | 0 (0) |
| B | 1 (33.3) | 0 (0) | 0 (0) | 2 (66.7) | 1 (33.3) | 0 (0) | 2 (66.7) | 0 (0) | 0 (0) | 5 (55.6) | 1 (11.1) | 0 (0) |
| C | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (66.7) | 0 (0) | 0 (0) | 3 (33.3) | 0 (0) | 0 (0) |
| Total * | 2 (22.2) | 0 (0) | 0 (0) | 4 (44.4) | 1 (11.1) | 0 (0) | 4 (44.4) | 0 (0) | 0 (0) | 10 (37) | 1 (3.7) | 0 (0) |
| D | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 2 (66.7) | 0 (0) | 0 (0) | 3 (33.3) | 0 (0) | 0 (0) |
| E | 1 (33.3) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 1 (33.3) | 3 (33.3) | 0 (0) | 1 (11.1) |
| F | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total # | 1 (11.1) | 0 (0) | 0 (0) | 2 (22.2) | 0 (0) | 0 (0) | 3 (33.3) | 0 (0) | 0 (0) | 6 (22.2) | 0 (0) | 1 (3.7) |
| Total | 3 (16.7) | 0 (0) | 0 (0) | 6 (33.3) | 1 (5.6) | 0 (0) | 7 (38.8) | 0 (0) | 0 (0) | 16 (29.6) | 1 (1.9) | 1 (1.9) |
| Sample ID | Antibiotype | No. Ab | eaeA Gene | EPEC | EIEC | ||
|---|---|---|---|---|---|---|---|
| AS4 *, FW2 | ** ESxtCipCpdSEnrTeCnSamNa | 10 | - | - | - | ||
| AC7 | ESxtCpdSEnrTeCnSamNa | 9 | |||||
| AF13 | ESxtCipSEnrTeCnSamNa | ||||||
| FF5, FW18 | ESxtCipCpdSEnrTeCnNa | ||||||
| FS8 | ESxtCipCpdSEnrTeSamNa | ||||||
| AWb6 | ESxtCpdSEnrTeCnNa | 8 | EW15 | - | - | ||
| CWb36 | ECipCpdSEnrTeSamNa | ||||||
| BC7 | ESxtCpdSEnrTeSamNa | ||||||
| FC20, EW15, FW8 | ESxtCipSEnrTeCnNa | ||||||
| AF8, EW2, EW17, FS3, FS9 | ESxtCipCpdSEnrTeNa | ||||||
| AF9 | ESxtCipSEnrTeSamNa | ||||||
| AWb1 | ECipCpdSEnrTeNa | 7 | FC11, FC14, ES1 | FC14 | ES1 | ||
| AWb9, BWb10, BWb29 | ESxtSEnrTeSamNa | ||||||
| FC11, FC14, FC25, ES13 | ESxtCipSEnrTeNa | ||||||
| FC15, FF4, FF6 | ESxtCipEnrTeCnNa | ||||||
| FC28 | ESxtSEnrTeCnSam | ||||||
| AS6 | ESxtCpdSTeSamNa | ||||||
| AS10, ES1, ES5 | ESxtCpdSEnrTeNa | ||||||
| AWb5, CWb31, CWb35 | ECipSEnrTeNa | 6 | CC12, FC1, BF7, BW2, BW6, BS4, CS11 | FC1, BW6 | |||
| BWb13 | ECpdSEnrTeNa | ||||||
| BWb15, BWb16, BWb21, BWb30, BF7, BW2, BW6, BS4 | ESxtSEnrTeSam | ||||||
| BWb27, CWb34, FC1, DF28 | ESxtSEnrTeNa | ||||||
| AC4 | ESxtCpdSTeSam | ||||||
| CC12 | ESxtCipEnrTeNa | ||||||
| CS11 | ESxtCipSTeNa | ||||||
| AWb3 | ECipSTeNa | 5 | EC8, BC4, BC2, DC6, DC12, BS2 - | ||||
| AWb4, DF15, BW7 | ESxtSEnrTe | ||||||
| BWb11 | ECipSEnrTe | ||||||
| BWb14, BWb24, BWb25, FC21 | ESxtEnrTeSam | ||||||
| BWb20, BWb26, BWb28, CWb37, CWb38, FC18, BS2 | ESxtSTeSam | ||||||
| CWb33, BC8, BF9 | ESEnrTeNa | ||||||
| AC2, AC10 | ESxtCpdSTe | ||||||
| BC2, EC8 | ECipEnrTeNa | ||||||
| BC4 | ESxtCpdTeSam | ||||||
| DC6, DC12, EC7 | ESxtEnrTeNa | ||||||
| FC9, DW9 | ESxtSTeNa | ||||||
| EF1 | ESxtSTeCn | ||||||
| CW1 | ESxtCpdSSam | ||||||
| BS9 | EEnrTeSamNa | ||||||
| AWb2, AWb8, BWb12, BWb18, EC11 | ESxtEnrTe | 4 | DWb2, DWb3, CC13, EC11, CF11 | ||||
| AWb7, BWb17, CWb32, CWb39, CWb40, DWb2, DWb3, BC5, BC9, CC13, DC1, DC3, DC5, DC9, CF11, DF2 | ESxtSTe | ||||||
| BWb18 | ESEnrTe | ||||||
| BWb22, BWb23 | ESxtTeSam | ||||||
| AC6 | ECpdSTe | ||||||
| BC6, BF2 | EEnrTeNa | ||||||
| DC7, FC8 | ESxtTeNa | ||||||
| FWb32, BC1, EC18, FC29, CF5, DS19 | ESxtTe | 3 | DWb1, DWb4, DWb5, BC1, EC18, CC14, EC12, DS19, EF2, DW11, CS13 | ||||
| DWb4, DWbK5, DWb6, DC2, DC11, DC13, EC3, EC5, EF2, EF3, DW11, DW12, CS12, CS13 | ESTe | ||||||
| DWb1, FWb22, CF4 | ETeSam | ||||||
| AC3 | ECpdTe | ||||||
| AC5 | ECpdS | ||||||
| AC8 | ESxtS | ||||||
| CC14, EC12, FC13 | ETeNa | ||||||
| EC14 | SxtCpdS | ||||||
| FWb21, FWb29, AC1, BC11, CC2, CC3, CC5, CC6, CC11, CC15, CC16, CC20, DC14, DC15, DC18, EC1, EC2, EC4, EC6, AW4, AW6, CW4, CW5, DS2, DS15 | ETe | 2 | CC4, AC9, CC3, CC5, CC6, CC11, CC15, CC16, CC20, DC15, EC1, EC2, EC6, AW4, AW6, DS15 | CC4, EC1, EC2, | CC6, EC6, | ||
| AC9 | ECpd | ||||||
| CC4 | ENa | ||||||
| DWb11, DWb12, DWb13, EWb14, EWb19, EWb20, FWb25, FWb35, BC3, CC1, CC7, CC8, CC9, CC10, CC17, CC18, CC19, DC17, DC19, DC21, EC9, EC13, EC17, AW1 | E | 1 | DWb12, FWb35, CC1, CC7, CC8, CC9, CC10, CC17, CC18, DC17, DC19, EC9 | DC17 | |||
| Village | Wild Birds | Chickens |
|---|---|---|
| A * | 100 | 80 |
| B * | 100 | 80 |
| C * | 100 | 15 |
| D # | 66.7 | 62.5 |
| E # | 0 | 53.3 |
| F # | 33.3 | 100 |
| SEM | 10.20621 | 31.78283 |
| p. values | <0.0001 + | 0.0423 + |
| Primer | Target Gene | Oligonucleotide Sequence | Amplicon Size (bp) | Reference Strain | Category of Pathogenic E. coli |
|---|---|---|---|---|---|
| eae | eaeA | *FW: 5′CACACGAATAAACTGACTAAAATG-3′ RV: 5′AAAAACGCTGACCCGCACCTAAAT-3′ | 376 | ATCC43887 | eaeA |
| SHIG | ial | FW: 5′-CTGGTAGGTATGGTGAGG-3′ RV: 5′-CCAGGCCAACAATTATTTCC-3′ | 320 | ATCC43893 | EIEC |
| bfpA | bfpA | FW: 5′-TTCTTGGTGCTTGCGTGTCTTTT-3′ RV: 5′-TTTTGTTTGTTGTATCTTTGTAA-3′ | 367 | ATCC43887 | EPEC |
| ATCC11775 | Negative control |
| Antibiotic Class | Antimicrobial Agents | Disc Concentration (µg) | Clinical Break Points of Antimicrobial Agents (mm) | ||
|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistance | |||
| Aminoglycosides | Streptomycin | 10 | ≥15 | 12–14 | ≤11 |
| Gentamicin | 10 | ≥15 | 13–15 | ≤12 | |
| Penicillin-combination | Ampicillin-sulfabactams | 10 | ≥17 | 14–16 | ≤13 |
| Tetracyclines | Tetracycline | 30 | ≥19 | 15–18 | ≤14 |
| Macrolides | Erythromycin | 15 | ≥23 | 14–22 | ≤13 |
| Quinolones | Nalidixic acid | 30 | ≥19 | 14–18 | ≤13 |
| Flouroquinolones | Enrofloxacin | 5 | ≥21 | 18–20 | ≤17 |
| Ciprofloxacin | 5 | ≥21 | 16–20 | ≤15 | |
| Cephalosporin/cephamycins | Cefpodoxime | 10 | ≥21 | 18–20 | ≤17 |
| Sulphamethoxazole-Trimethoprim | Sulpamethoxazole-trimethoprim | 25 | ≥16 | 11–15 | ≤10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, M.-Y.I.; Abu, J.; Zakaria, Z.; Khan, A.R.; Abdul Aziz, S.; Bitrus, A.A.; Habib, I. Multi-Drug Resistant Pathogenic Escherichia coli Isolated from Wild Birds, Chicken, and the Environment in Malaysia. Antibiotics 2022, 11, 1275. https://doi.org/10.3390/antibiotics11101275
Mohamed M-YI, Abu J, Zakaria Z, Khan AR, Abdul Aziz S, Bitrus AA, Habib I. Multi-Drug Resistant Pathogenic Escherichia coli Isolated from Wild Birds, Chicken, and the Environment in Malaysia. Antibiotics. 2022; 11(10):1275. https://doi.org/10.3390/antibiotics11101275
Chicago/Turabian StyleMohamed, Mohamed-Yousif Ibrahim, Jalila Abu, Zunita Zakaria, Abdul Rashid Khan, Saleha Abdul Aziz, Asinamai Athliamai Bitrus, and Ihab Habib. 2022. "Multi-Drug Resistant Pathogenic Escherichia coli Isolated from Wild Birds, Chicken, and the Environment in Malaysia" Antibiotics 11, no. 10: 1275. https://doi.org/10.3390/antibiotics11101275
APA StyleMohamed, M.-Y. I., Abu, J., Zakaria, Z., Khan, A. R., Abdul Aziz, S., Bitrus, A. A., & Habib, I. (2022). Multi-Drug Resistant Pathogenic Escherichia coli Isolated from Wild Birds, Chicken, and the Environment in Malaysia. Antibiotics, 11(10), 1275. https://doi.org/10.3390/antibiotics11101275








