Antimicrobial Peptides Epinecidin-1 and Beta-Defesin-3 Are Effective against a Broad Spectrum of Antibiotic-Resistant Bacterial Isolates and Increase Survival Rate in Experimental Sepsis
Abstract
:1. Introduction
2. Results
2.1. Epi-1 Is Effective against MRSA and Carbapenem-Resistant Gram-Negative Bacteria In Vitro
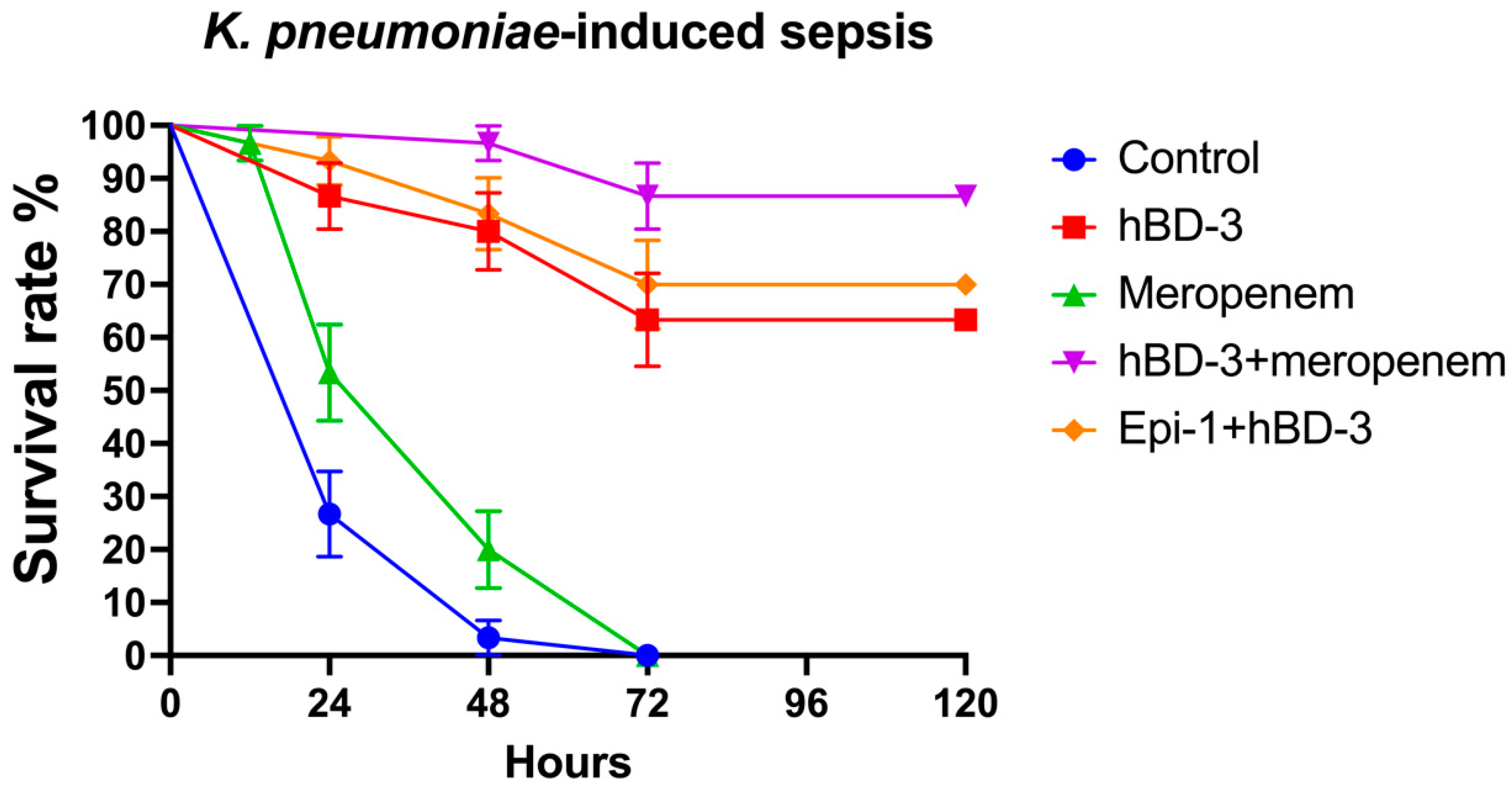
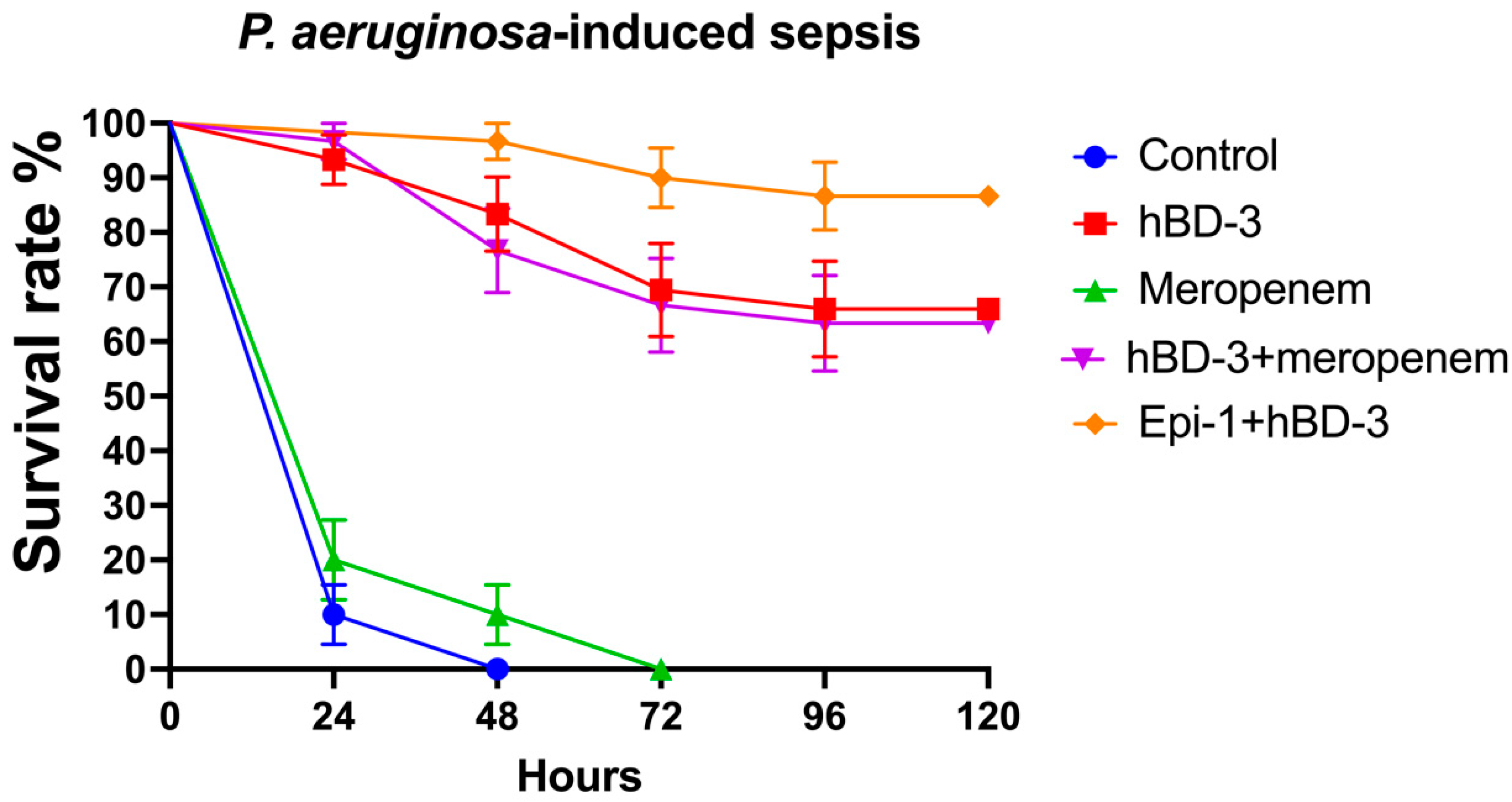
2.2. hBD-3 and Epi-1 Decrease Mortality in Experimental Sepsis
3. Discussion
4. Materials and Methods
4.1. Peptides
4.2. Bacterial Isolates
4.3. Study of the Minimum Inhibitory Concentrations and Combined Antimicrobial Effect of Epi-1 + Vancomycin and Epi-1 + hBD-3
4.4. Study of the Effect of hBD-3 and Epi-1 in an Experimental Model of Sepsis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, S.C.; Zembower, T.R. Global Increases in Antibiotic Consumption: A Concerning Trend for WHO Targets. Lancet Infect. Dis. 2021, 21, 10–11. [Google Scholar] [CrossRef]
- O’Neill, J. Review on Antimicrobial Resistance. Tackling a Global Health Crisis: Rapid Diagnostics: Stopping Unnecessary Use of Antibiotics. Indep. Rev. AMR 2015, 1–36. Available online: https://amr-review.org/ (accessed on 21 December 2021).
- US CDC. Antibiotic Resistance Threats in the United States; Centers for Disease Control and Prevention: Atlanta, USA, 2019. [CrossRef] [Green Version]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial Peptides: Mechanism of Action, Activity and Clinical Potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The Antimicrobial Peptides and Their Potential Clinical Applications. Am. J. Transl. Res. 2019, 11, 3919. [Google Scholar] [PubMed]
- Barbosa, F.; Pinto, E.; Kijjoa, A.; Pinto, M.; Sousa, E. Targeting Antimicrobial Drug Resistance with Marine Natural Products. Int. J. Antimicrob. Agents 2020, 56, 106005. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [Green Version]
- Rathinakumar, R.; Walkenhorst, W.F.; Wimley, W.C. Broad-Spectrum Antimicrobial Peptides by Rational Combinatorial Design and High-Throughput Screening: The Importance of Interfacial Activity. J. Am. Chem. Soc. 2009, 131, 7609. [Google Scholar] [CrossRef] [Green Version]
- Bolatchiev, A. Antibacterial Activity of Human Defensins against Staphylococcus Aureus and Escherichia Coli. PeerJ 2020, 8, e10455. [Google Scholar] [CrossRef]
- Zharkova, M.S.; Orlov, D.S.; Golubeva, O.Y.; Chakchir, O.B.; Eliseev, I.E.; Grinchuk, T.M.; Shamova, O.V. Application of Antimicrobial Peptides of the Innate Immune System in Combination with Conventional Antibiotics-a Novel Way to Combat Antibiotic Resistance? Front. Cell. Infect. Microbiol. 2019, 9, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.X.; He, W.; Chen, W.J.; Yan, J.H.; Yang, J.N.; Chan, S.M.; He, J.G. Cloning, Expression and Antimicrobial Activity of an Antimicrobial Peptide, Epinecidin-1, from the Orange-Spotted Grouper, Epinephelus Coioides. Aquaculture 2006, 253, 204–211. [Google Scholar] [CrossRef]
- Neshani, A.; Zare, H.; Eidgahi, M.R.A.; Khaledi, A.; Ghazvini, K. Epinecidin-1, a Highly Potent Marine Antimicrobial Peptide with Anticancer and Immunomodulatory Activities. BMC Pharmacol. Toxicol. 2019, 20, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chee, P.Y.; Mang, M.; Lau, E.S.; Tan, L.T.H.; He, Y.W.; Lee, W.L.; Pusparajah, P.; Chan, K.G.; Lee, L.H.; Goh, B.H. Epinecidin-1, an Antimicrobial Peptide Derived From Grouper (Epinephelus Coioides): Pharmacological Activities and Applications. Front. Microbiol. 2019, 10, 2631. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.N.; Pan, C.Y.; Su, B.C.; Wu, H.Y.; Chen, J.Y. Epinecidin-1 Protects against Methicillin Resistant Staphylococcus Aureus Infection and Sepsis in Pyemia Pigs. Mar. Drugs 2019, 17, 693. [Google Scholar] [CrossRef] [Green Version]
- White, R.L.; Burgess, D.S.; Manduru, M.; Bosso, J.A. Comparison of Three Different in Vitro Methods of Detecting Synergy: Time-Kill, Checkerboard, and E Test. Antimicrob. Agents Chemother. 1996, 40, 1914–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orhan, G.; Bayram, A.; Zer, Y.; Balci, I. Synergy Tests by E Test and Checkerboard Methods of Antimicrobial Combinations against Brucella Melitensis. J. Clin. Microbiol. 2005, 43, 140–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Espinel-Ingroff, A.; Boyken, L.; Hollis, R.J.; Kroeger, J.; Messer, S.A.; Tendolkar, S.; Diekema, D.J. Comparison of the Broth Microdilution (BMD) Method of the European Committee on Antimicrobial Susceptibility Testing with the 24-Hour CLSI BMD Method for Testing Susceptibility of Candida Species to Fluconazole, Posaconazole, and Voriconazole by Use of Ep. J. Clin. Microbiol. 2011, 49, 845–850. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.C.; Hui, C.F.; Chen, J.Y.; Wu, J.L. Truncated Antimicrobial Peptides from Marine Organisms Retain Anticancer Activity and Antibacterial Activity against Multidrug-Resistant Staphylococcus Aureus. Peptides 2013, 44, 139–148. [Google Scholar] [CrossRef]
- Germovsek, E.; Barker, C.I.; Sharland, M. What Do I Need to Know about Aminoglycoside Antibiotics? Arch. Dis. Child.-Educ. Pract. 2017, 102, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Preuss, C.V.; Bernice, F. Vancomycin. In StatPearls; 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459263/ (accessed on 21 December 2021).
- Giacometti, A.; Cirioni, O.; Barchiesi, F.; Scalise, G. In-Vitro Activity and Killing Effect of Polycationic Peptides on Methicillin-Resistant Staphylococcus Aureus and Interactions with Clinically Used Antibiotics. Diagn. Microbiol. Infect. Dis. 2000, 38, 115–118. [Google Scholar] [CrossRef]
- Shin, S.Y.; Yang, S.T.; Park, E.J.; Eom, S.H.; Song, W.K.; Kim, J.I.; Kim, Y.; Hahm, K.S. Salt Resistance and Synergistic Effect with Vancomycin of α-Helical Antimicrobial Peptide P18. Biochem. Biophys. Res. Commun. 2002, 290, 558–562. [Google Scholar] [CrossRef]
- Feng, Q.; Huang, Y.; Chen, M.; Li, G.; Chen, Y. Functional Synergy of α-Helical Antimicrobial Peptides and Traditional Antibiotics against Gram-Negative and Gram-Positive Bacteria in Vitro and in Vivo. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Z.; Li, X.; Tian, Y.; Fan, Y.; Yu, C.; Zhou, B.; Liu, Y.; Xiang, R.; Yang, L. Synergistic Effects of Antimicrobial Peptide DP7 Combined with Antibiotics against Multidrug-Resistant Bacteria. Drug Des. Dev. Ther. 2017, 11, 939–946. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.Y.; Chen, J.Y.; Cheng, Y.S.E.; Chen, C.Y.; Ni, I.H.; Sheen, J.F.; Pan, Y.L.; Kuo, C.M. Gene Expression and Localization of the Epinecidin-1 Antimicrobial Peptide in the Grouper (Epinephelus Coioides), and Its Role in Protecting Fish Against Pathogenic Infection. DNA Cell Biol. 2007, 26, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.M.; Bechinger, B.; Naas, T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef]
- Pan, C.Y.; Chen, J.C.; Sheen, J.F.; Lin, T.L.; Chen, J.Y. Epinecidin-1 Has Immunomodulatory Effects, Facilitating Its Therapeutic Use in a Mouse Model of Pseudomonas Aeruginosa Sepsis. Antimicrob. Agents Chemother. 2014, 58, 4264–4274. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.C.; Pan, C.Y.; Chen, J.Y. The Antimicrobial Peptide, Epinecidin-1, Mediates Secretion of Cytokines in the Immune Response to Bacterial Infection in Mice. Peptides 2012, 36, 100–108. [Google Scholar] [CrossRef]
- Pollini, S.; Brunetti, J.; Sennati, S.; Rossolini, G.M.; Bracci, L.; Pini, A.; Falciani, C. Synergistic Activity Profile of an Antimicrobial Peptide against Multidrug-Resistant and Extensively Drug-Resistant Strains of Gram-Negative Bacterial Pathogens. J. Pept. Sci. 2017, 23, 329–333. [Google Scholar] [CrossRef]
- Sánchez-Gómez, S.; Japelj, B.; Jerala, R.; Moriyón, I.; Alonso, M.F.; Leiva, J.; Blondelle, S.E.; Andrä, J.; Brandenburg, K.; Lohner, K.; et al. Structural Features Governing the Activity of Lactoferricin-Derived Peptides That Act in Synergy with Antibiotics against Pseudomonas Aeruginosa in Vitro and in Vivo. Antimicrob. Agents Chemother. 2011, 55, 218–228. [Google Scholar] [CrossRef] [Green Version]
- Giacometti, A.; Cirioni, O.; del Prete, M.S.; Paggi, A.M.; D’Errico, M.M.; Scalise, G. Combination Studies between Polycationic Peptides and Clinically Used Antibiotics against Gram-Positive and Gram-Negative Bacteria. Peptides 2000, 21, 1155–1160. [Google Scholar] [CrossRef]
- Dhople, V.; Krukemeyer, A.; Ramamoorthy, A. The Human Beta-Defensin-3, an Antibacterial Peptide with Multiple Biological Functions. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1499–1512. [Google Scholar] [CrossRef] [Green Version]
- Schibli, D.J.; Hunter, H.N.; Aseyev, V.; Starner, T.D.; Wiencek, J.M.; McCray, P.B.; Tack, B.F.; Vogel, H.J. The Solution Structures of the Human β-Defensins Lead to a Better Understanding of the Potent Bactericidal Activity of HBD3 against Staphylococcus Aureus. J. Biol. Chem. 2002, 277, 8279–8289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 11.0. 2021. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf (accessed on 20 September 2021).
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of Minimum Inhibitory Concentrations (MICs) of Antibacterial Agents by Broth Dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [Google Scholar] [CrossRef] [Green Version]
- Ruden, S.; Hilpert, K.; Berditsch, M.; Wadhwani, P.; Ulrich, A.S. Synergistic Interaction between Silver Nanoparticles and Membrane-Permeabilizing Antimicrobial Peptides. Antimicrob. Agents Chemother. 2009, 53, 3538–3540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, D.; Leontiadou, H.; Mark, A.E.; Marrink, S.J. Toroidal Pores Formed by Antimicrobial Peptides Show Significant Disorder. Biochim. Et Biophys. Acta-Biomembr. 2008, 1778, 2308–2317. [Google Scholar] [CrossRef] [Green Version]
- Du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Van der Weide, H.; ten Kate, M.T.; Vermeulen-De Jongh, D.M.C.; van der Meijden, A.; Wijma, R.A.; Boers, S.A.; van Westreenen, M.; Hays, J.P.; Goessens, W.H.F.; Bakker-Woudenberg, I.A.J.M. Successful High-Dosage Monotherapy of Tigecycline in a Multidrug-Resistant Klebsiella Pneumoniae Pneumonia–Septicemia Model in Rats. Antibiotics 2020, 9, 109. [Google Scholar] [CrossRef] [Green Version]
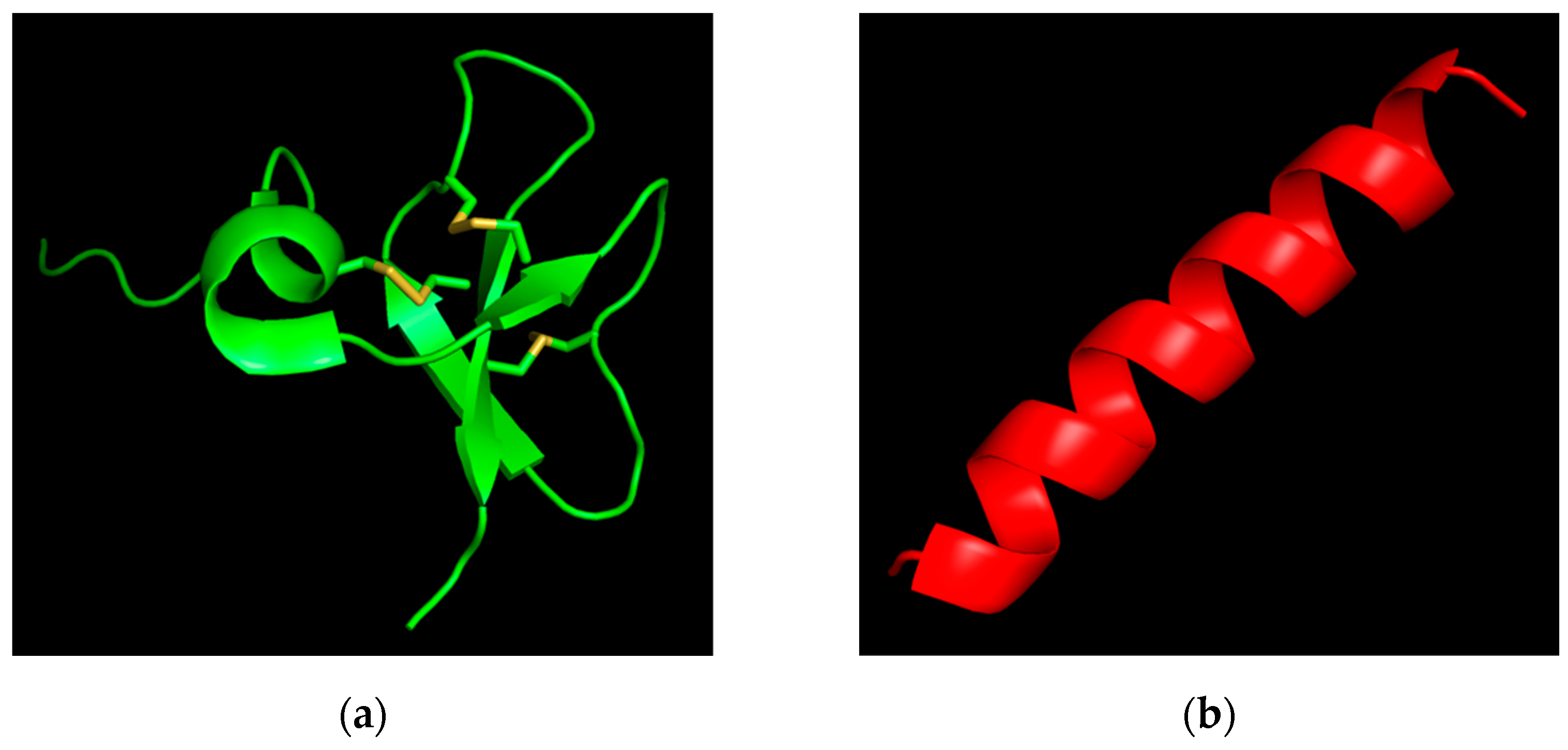
| Peptide | Amino Acid Sequence | Length | Molecular Weight | Charge | Hydrophobic Residues |
|---|---|---|---|---|---|
| hBD-3 | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK | 45 | 5.17 kDa | +11 | 33% |
| Epi-122–42 | GFIFHIIKGLFHAGKMIHGLV | 21 | 2.34 kDa | +3 | 57% |
| Bacterial Isolates | Epi-1 MIC | Vancomycin MIC | FICI |
|---|---|---|---|
| MRSA_1 | 16 (6.8) | 1 (0.69) | 1.25 |
| MRSA_2 | 16 (6.8) | 2 (1.38) | 0.5 * |
| MRSA_3 | 32 (13.7) | 2 (1.38) | 0.375 * |
| MRSA_4 | 8 (3.4) | 2 (1.38) | 0.75 |
| MRSA_5 | 16 (6.8) | 2 (1.38) | 0.5 * |
| MRSA_6 | 8 (3.4) | 1 (0.69) | 1.125 |
| MRSA_7 | 8 (3.4) | 2 (1.38) | 0.75 |
| MRSA_8 | 16 (6.8) | 2 (1.38) | 0.5 * |
| MRSA_9 | 32 (13.7) | 2 (1.38) | 0.5 * |
| MRSA_10 | 16 (6.8) | 2 (1.38) | 0.75 |
| MRSA_11 | 16 (6.8) | 4 (2.76) | 0.75 |
| MRSA_12 | 16 (6.8) | 2 (1.38) | 0.5 * |
| MRSA_13 | 16 (6.8) | 2 (1.38) | 1 |
| MRSA_14 | 16 (6.8) | 2 (1.38) | 0.75 |
| MRSA_15 | 16 (6.8) | 2 (1.38) | 0.5 * |
| MRSA_16 | 16 (6.8) | 4 (2.76) | 0.3125 * |
| MRSA_17 | 8 (3.4) | 4 (2.76) | 0.5 * |
| MRSA_18 | 16 (6.8) | 4 (2.76) | 0.75 |
| MRSA_19 | 16 (6.8) | 1 (0.69) | 0.75 |
| MRSA_20 | 16 (6.8) | 2 (1.38) | 1 |
| MRSA_21 | 16 (6.8) | 2 (1.38) | 0.5 * |
| MRSA_22 | 8 (3.4) | 1 (0.69) | 0.75 |
| Bacterial Isolates | Epi-1 MIC | hBD-3 MIC | FICI |
|---|---|---|---|
| CRKP_1 | 8 (3.4) | 4 (0.8) | 1 |
| CRKP_2 | 16 (6.8) | 4 (0.8) | 0.75 |
| CRKP_3 | 8 (3.4) | 4 (0.8) | 0.5 * |
| CRKP_4 | 8 (3.4) | 8 (1.6) | 0.375 * |
| CRKP_5 | 8 (3.4) | 4 (0.8) | 0.5 * |
| CRKP_6 | 16 (6.8) | 2 (0.4) | 1.25 |
| CRKP_7 | 8 (3.4) | 4 (0.8) | 0.5 * |
| CRKP_8 | 8 (3.4) | 4 (0.8) | 1 |
| CRKP_9 | 8 (3.4) | 4 (0.8) | 1 |
| CRKP_10 | 4 (1.7) | 8 (1.6) | 1 |
| CRKP_11 | 4 (1.7) | 8 (1.6) | 1.25 |
| CRKP_12 | 16 (6.8) | 16 (3.1) | 1.5 |
| CRKP_13 | 16 (6.8) | 8 (1.6) | 1.5 |
| CRKP_14 | 8 (3.4) | 8 (1.6) | 0.375 * |
| CRKP_15 | 4 (1.7) | 8 (1.6) | 0.625 |
| CRKP_16 | 8 (3.4) | 2 (0.4) | 1 |
| CRKP_17 | 8 (3.4) | 4 (0.8) | 1.5 |
| CRKP_18 | 8 (3.4) | 8 (1.6) | 0.75 |
| CRKP_19 | 8 (3.4) | 8 (1.6) | 1.5 |
| CRKP_20 | 8 (3.4) | 8 (1.6) | 1 |
| CRKP_21 | 8 (3.4) | 4 (0.8) | 0.75 |
| CRKP_22 | 16 (6.8) | 4 (0.8) | 0.75 |
| CRKP_23 | 16 (6.8) | 4 (0.8) | 1 |
| Bacterial Isolates | Epi-1 MIC | hBD-3 MIC | FICI |
|---|---|---|---|
| CRKA_1 | 16 (6.8) | 2 (0.4) | 2 |
| CRKA_2 | 8 (3.4) | 4 (0.8) | 0.75 |
| CRKA_3 | 4 (1.7) | 2 (0.4) | 0.75 |
| CRKA_4 | 16 (6.8) | 4 (0.8) | 0.5 * |
| CRKA_5 | 4 (1.7) | 4 (0.8) | 0.75 |
| CRKA_6 | 16 (6.8) | 2 (0.4) | 0.75 |
| CRKA_7 | 4 (1.7) | 2 (0.4) | 0.75 |
| CRKA_8 | 8 (3.4) | 2 (0.4) | 0.75 |
| CRKA_9 | 16 (6.8) | 2 (0.4) | 1 |
| CRKA_10 | 8 (3.4) | 4 (0.8) | 0.5 * |
| CRKA_11 | 4 (1.7) | 4 (0.8) | 0.5 * |
| CRKA_12 | 8 (3.4) | 2 (0.4) | 0.75 |
| CRKA_13 | 16 (6.8) | 4 (0.8) | 0.375 * |
| CRKA_14 | 8 (3.4) | 4 (0.8) | 0.75 |
| CRKA_15 | 8 (3.4) | 2 (0.4) | 0.625 |
| CRKA_16 | 8 (3.4) | 4 (0.8) | 0.375 * |
| CRKA_17 | 4 (1.7) | 4 (0.8) | 0.75 |
| Bacterial Isolates | Epi-1 MIC | hBD-3 MIC | FICI |
|---|---|---|---|
| CRPA_1 | 8 (3.4) | 4 (0.8) | 0.5 * |
| CRPA_2 | 16 (6.8) | 8 (1.6) | 0.5 * |
| CRPA_3 | 8 (3.4) | 4 (0.8) | 1 |
| CRPA_4 | 16 (6.8) | 4 (0.8) | 1 |
| CRPA_5 | 16 (6.8) | 16 (3.1) | 0.5 * |
| CRPA_6 | 8 (3.4) | 8 (1.6) | 0.75 |
| CRPA_7 | 4 (1.7) | 4 (0.8) | 0.75 |
| CRPA_8 | 16 (6.8) | 2 (0.4) | 1.5 |
| CRPA_9 | 8 (3.4) | 2 (0.4) | 1.5 |
| CRPA_10 | 16 (6.8) | 4 (0.8) | 1.5 |
| CRPA_11 | 16 (6.8) | 8 (1.6) | 0.5 * |
| CRPA_12 | 16 (6.8) | 8 (1.6) | 0.75 |
| CRPA_13 | 32 (13.7) | 16 (3.1) | 0.5 * |
| Bacterial Isolates | Epi-1 MIC | hBD-3 MIC | FICI |
|---|---|---|---|
| CRAB_1 | 32 (13.7) | 16 (3.1) | 0.5 * |
| CRAB_2 | 32 (13.7) | 8 (1.6) | 0.5 * |
| CRAB_3 | 4 (1.7) | 8 (1.6) | 0.5 * |
| CRAB_4 | 16 (6.8) | 4 (0.8) | 0.75 |
| CRAB_5 | 32 (13.7) | 4 (0.8) | 1.25 |
| CRAB_6 | 16 (6.8) | 4 (0.8) | 0.75 |
| CRAB_7 | 32 (13.7) | 8 (1.6) | 0.5 * |
| CRAB_8 | 8 (3.4) | 16 (3.1) | 0.5 * |
| CRAB_9 | 16 (6.8) | 4 (0.8) | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolatchiev, A. Antimicrobial Peptides Epinecidin-1 and Beta-Defesin-3 Are Effective against a Broad Spectrum of Antibiotic-Resistant Bacterial Isolates and Increase Survival Rate in Experimental Sepsis. Antibiotics 2022, 11, 76. https://doi.org/10.3390/antibiotics11010076
Bolatchiev A. Antimicrobial Peptides Epinecidin-1 and Beta-Defesin-3 Are Effective against a Broad Spectrum of Antibiotic-Resistant Bacterial Isolates and Increase Survival Rate in Experimental Sepsis. Antibiotics. 2022; 11(1):76. https://doi.org/10.3390/antibiotics11010076
Chicago/Turabian StyleBolatchiev, Albert. 2022. "Antimicrobial Peptides Epinecidin-1 and Beta-Defesin-3 Are Effective against a Broad Spectrum of Antibiotic-Resistant Bacterial Isolates and Increase Survival Rate in Experimental Sepsis" Antibiotics 11, no. 1: 76. https://doi.org/10.3390/antibiotics11010076
APA StyleBolatchiev, A. (2022). Antimicrobial Peptides Epinecidin-1 and Beta-Defesin-3 Are Effective against a Broad Spectrum of Antibiotic-Resistant Bacterial Isolates and Increase Survival Rate in Experimental Sepsis. Antibiotics, 11(1), 76. https://doi.org/10.3390/antibiotics11010076






