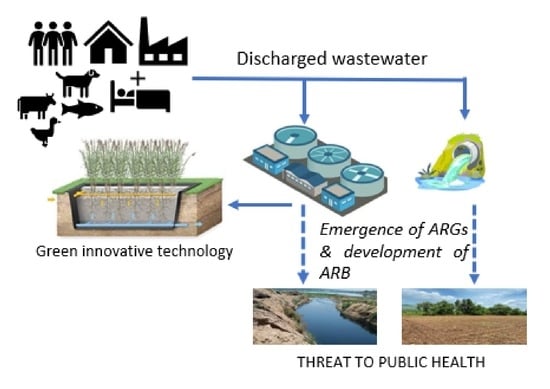
Performance Efficiency of Conventional Treatment Plants and Constructed Wetlands towards Reduction of Antibiotic Resistance
Abstract
:1. Introduction
2. Methods
2.1. General Approach
2.2. Bibliography Approach and Analysis
3. Review Findings
3.1. Main Drivers of Antibiotic Resistance in Wastewater
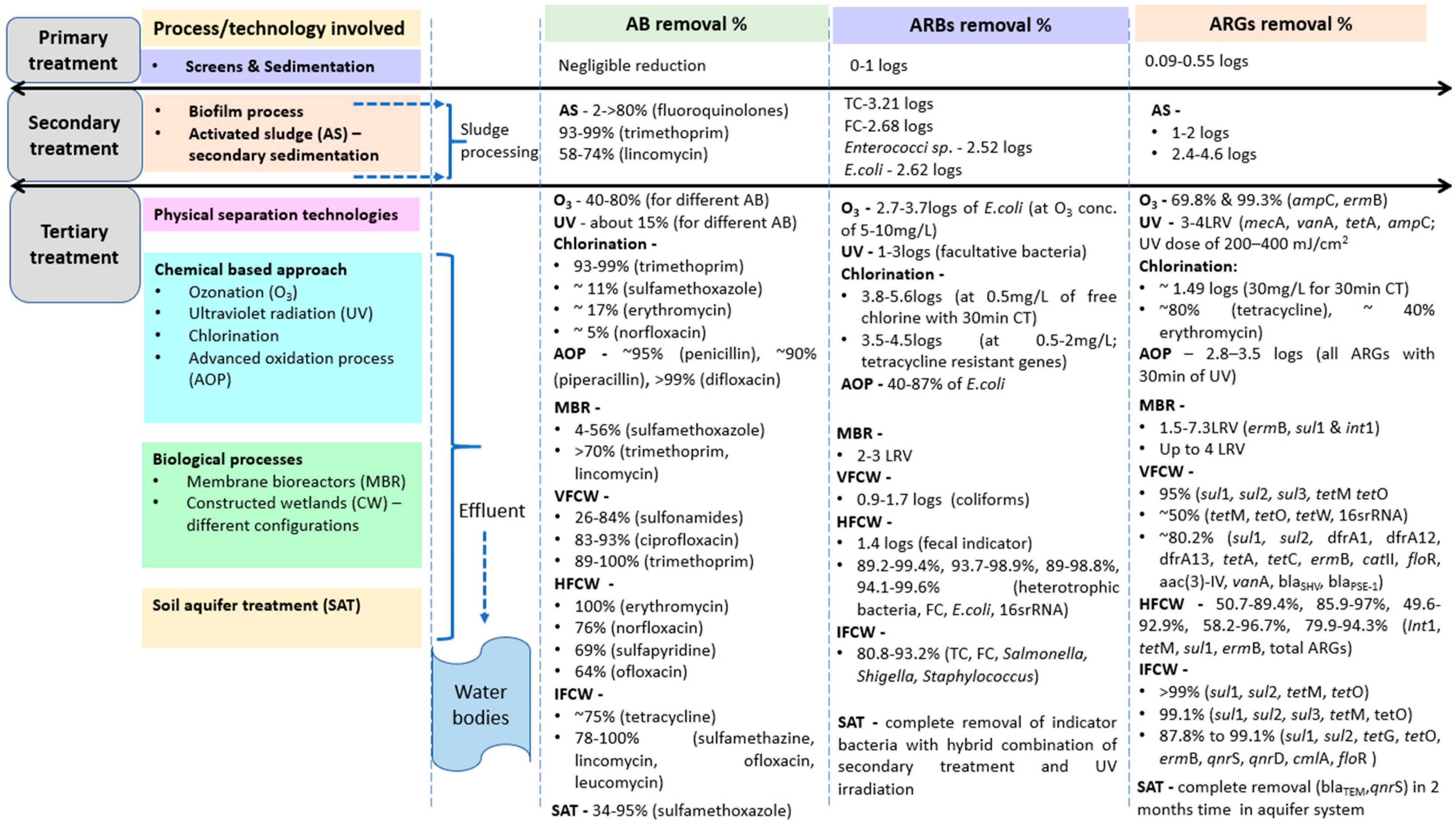
3.2. Efficiency of Conventional Treatment Plants for Reduction of Antibiotics, ARBs and ARGs
3.2.1. WWTPs and Antibiotic Reduction
Primary Treatment Data/Literature
Secondary Treatment Data/Literature
Tertiary Treatment Data/Literature
3.2.2. WWTPs and ARB Reduction
Primary Treatment Data/Literature
Secondary Treatment Data/Literature
Tertiary Treatment Data/Literature
3.2.3. WWTPs and ARG Reduction
Primary Treatment Data/Literature
Secondary Treatment Data/Literature
Tertiary Treatment Data/Literature
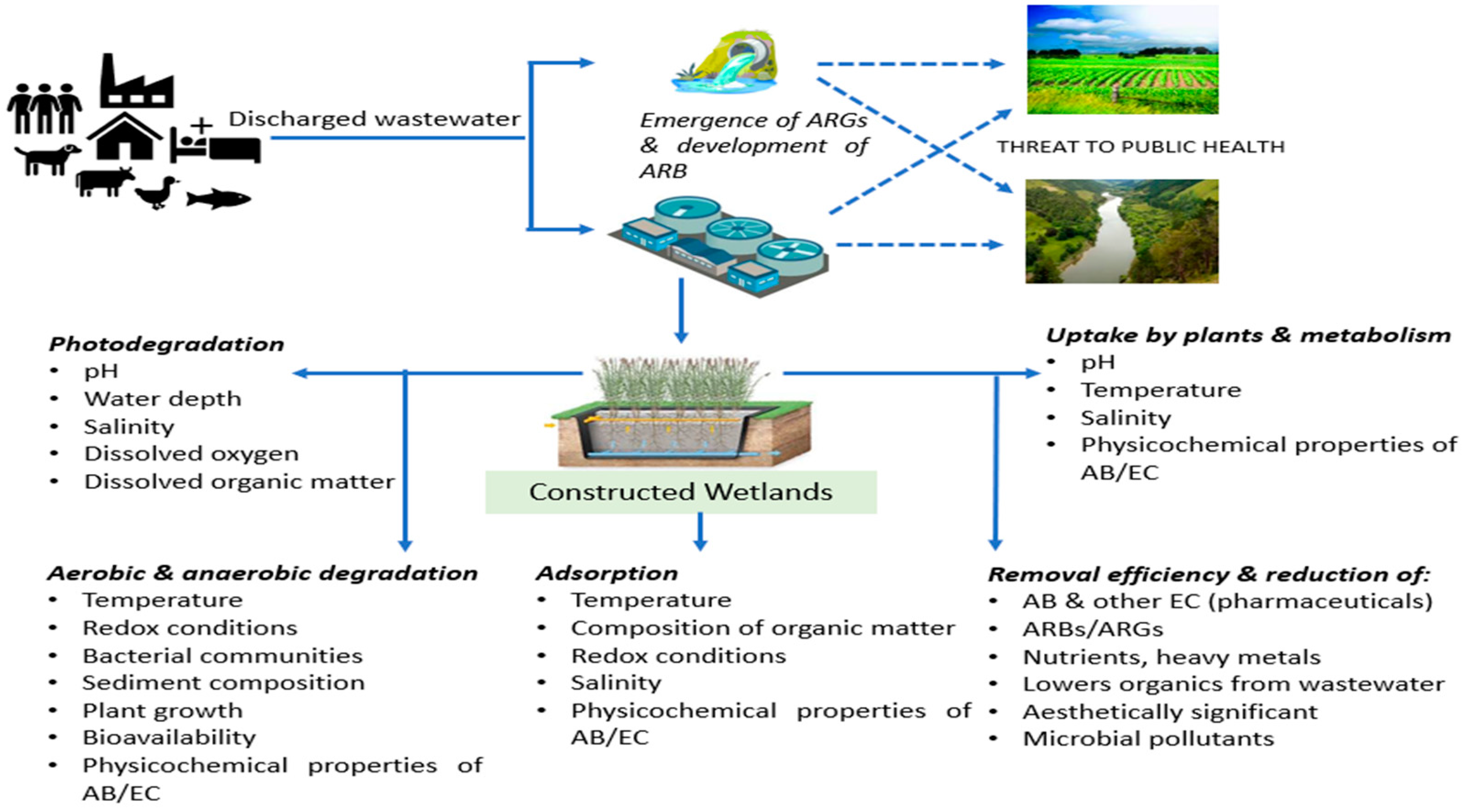
3.3. Performance of CW for Elimination of Antibiotics, Antibiotic-Resistant Bacteria and Genes
3.3.1. Efficiency of CWs for Removal of Antibiotics
3.3.2. Efficiency of CWs for Removal of ARBs and ARGs
3.4. Underlying Factors and Mechanisms Involved in the Reduction of Antibiotics, ARB and ARG in CW
3.5. Role of Plants in CWs
4. Conclusions and Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jäger, T.; Hembach, N.; Elpers, C.; Wieland, A.; Alexander, J.; Hiller, C.; Krauter, G.; Schwartz, T. Reduction of Antibiotic Resistant Bacteria During Conventional and Advanced Wastewater Treatment, and the Disseminated Loads Released to the Environment. Front. Microbiol. 2018, 9, 2599. [Google Scholar] [CrossRef] [Green Version]
- Anthony, E.T.; Ojemaye, M.O.; Okoh, O.O.; Okoh, A. A critical review on the occurrence of resistomes in the environment and their removal from wastewater using apposite treatment technologies: Limitations, successes and future improvement. Environ. Pollut. 2019, 263, 113791. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Zhang, T.; Fang, H.H.P. Antibiotic resistance genes in water environment. Appl. Microbiol. Biotechnol. 2009, 82, 397–414. [Google Scholar] [CrossRef]
- Nnadozie, C.F.; Kumari, S.; Bux, F. Status of pathogens, antibiotic resistance genes and antibiotic residues in wastewater treatment systems. Rev. Environ. Sci. Bio/Technol. 2017, 16, 491–515. [Google Scholar] [CrossRef]
- Kampouris, I.D.; Klümper, U.; Agrawal, S.; Orschler, L.; Cacace, D.; Kunze, S.; Berendonk, T.U. Treated wastewater irrigation promotes the spread of antibiotic resistance into subsoil pore-water. Environ. Int. 2020, 146, 106190. [Google Scholar] [CrossRef] [PubMed]
- Nappier, S.; Liguori, K.; Ichida, A.; Stewart, J.; Jones, K. Antibiotic Resistance in Recreational Waters: State of the Science. Int. J. Environ. Res. Public Health 2020, 17, 8034. [Google Scholar] [CrossRef]
- United Nations. UN-WATER Progress on Wastewater Treatment-Piloting the Monitoring Mehtodology and Intial Findings for SDG Indicator 6.3.1; United Nations: New York, NY, USA, 2018; ISBN 8228785852.
- O’Neill, J. Antimicrobials in Agriculture and the Environment: Reducing Unnecessary Use and Waste the Review on Antimicrobial Resistance. 2015. Available online: https://amr-review.org/sites/default/files/Antimicrobials%20in%20agriculture%20and%20the%20environment%20-%20Reducing%20unnecessary%20use%20and%20waste.pdf (accessed on 27 November 2021).
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2017, 42, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, K.J.; Patel, S.; Gibson, M.K.; Lauber, C.L.; Knight, R.; Fierer, N.; Dantas, G. Bacterial phylogeny structures soil resistomes across habitats. Nature 2014, 509, 612–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binh, C.T.T.; Heuer, H.; Kaupenjohann, M.; Smalla, K. Piggery manure used for soil fertilization is a reservoir for transferable antibiotic resistance plasmids. FEMS Microbiol. Ecol. 2008, 66, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Ewolters, B.; Kyselkovã¡, M.; Krãgerrecklenfort, E.; Ekreuzig, R.; Esmalla, K. Transferable antibiotic resistance plasmids from biogas plant digestates often belong to the IncP-1ε subgroup. Front. Microbiol. 2015, 5, 765. [Google Scholar] [CrossRef]
- Khan, F.A.; Söderquist, B.; Jass, J. Prevalence and Diversity of Antibiotic Resistance Genes in Swedish Aquatic Environments Impacted by Household and Hospital Wastewater. Front. Microbiol. 2019, 10, 688. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Zhao, W.; Xu, T.; Zheng, B.; Yin, D. Occurrence and distribution of antibiotic resistance genes in the water and sediments of Qingcaosha Reservoir, Shanghai, China. Environ. Sci. Eur. 2019, 31, 1–9. [Google Scholar] [CrossRef]
- D’Costa, V.M.; Griffiths, E.; Wright, G. Expanding the soil antibiotic resistome: Exploring environmental diversity. Curr. Opin. Microbiol. 2007, 10, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Nesme, J.; Simonet, P. The soil resistome: A critical review on antibiotic resistance origins, ecology and dissemination potential in telluric bacteria. Environ. Microbiol. 2014, 17, 913–930. [Google Scholar] [CrossRef]
- Segawa, T.; Takeuchi, N.; Rivera, A.; Yamada, A.; Yoshimura, Y.; Barcaza, G.; Shinbori, K.; Motoyama, H.; Kohshima, S.; Ushida, K. Distribution of antibiotic resistance genes in glacier environments. Environ. Microbiol. Rep. 2012, 5, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Stedtfeld, R.D.; Kim, O.-S.; Chai, B.; Yang, L.; Stedtfeld, T.M.; Hong, S.G.; Kim, D.; Lim, H.S.; Hashsham, S.A.; et al. Influence of Soil Characteristics and Proximity to Antarctic Research Stations on Abundance of Antibiotic Resistance Genes in Soils. Environ. Sci. Technol. 2016, 50, 12621–12629. [Google Scholar] [CrossRef]
- Van Goethem, M.W.; Pierneef, R.; Bezuidt, O.K.I.; Van De Peer, Y.; Cowan, D.A.; Makhalanyane, T.P. A reservoir of ‘historical’ antibiotic resistance genes in remote pristine Antarctic soils. Microbiome 2018, 6, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barancheshme, F.; Munir, M. Strategies to Combat Antibiotic Resistance in the Wastewater Treatment Plants. Front. Microbiol. 2018, 8, 2603. [Google Scholar] [CrossRef] [Green Version]
- Zhai, W.; Yang, F.; Mao, D.; Luo, Y. Fate and removal of various antibiotic resistance genes in typical pharmaceutical wastewater treatment systems. Environ. Sci. Pollut. Res. 2016, 23, 12030–12038. [Google Scholar] [CrossRef]
- Rousham, E.K.; Unicomb, L.; Islam, M.A. Human, animal and environmental contributors to antibiotic resistance in low-resource settings: Integrating behavioural, epidemiological and One Health approaches. Proc. R. Soc. B Boil. Sci. 2018, 285, 20180332. [Google Scholar] [CrossRef]
- Ibekwe, A.M.; Murinda, S.E.; Debroy, C.; Reddy, G.B. Potential pathogens, antimicrobial patterns and genotypic diversity ofEscherichia coliisolates in constructed wetlands treating swine wastewater. FEMS Microbiol. Ecol. 2016, 92, fiw006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, H.K.; Rehman, M.Y.A.; Malik, R.N. Fate and toxicity of pharmaceuticals in water environment: An insight on their occurrence in South Asia. J. Environ. Manag. 2020, 271, 111030. [Google Scholar] [CrossRef]
- Hussain, S.A.; Prasher, S.O. Understanding the Sorption of Ionophoric Pharmaceuticals in a Treatment Wetland. Wetlands 2011, 31, 563–571. [Google Scholar] [CrossRef]
- Dires, S.; Birhanu, T.; Ambelu, A.; Sahilu, G. Antibiotic resistant bacteria removal of subsurface flow constructed wetlands from hospital wastewater. J. Environ. Chem. Eng. 2018, 6, 4265–4272. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, P.; Yang, Q. Occurrence and diversity of antibiotic resistance in untreated hospital wastewater. Sci. Total Environ. 2018, 621, 990–999. [Google Scholar] [CrossRef]
- Lu, H.; Wang, T.; Lu, S.; Liu, H.; Wang, H.; Li, C.; Liu, X.; Guo, X.; Zhao, X.; Liu, F. Performance and bacterial community dynamics of hydroponically grown Iris pseudacorus L. during the treatment of antibiotic-enriched wastewater at low/normal temperature. Ecotoxicol. Environ. Saf. 2021, 213, 111997. [Google Scholar] [CrossRef]
- de Oliveira, M.; Frihling, B.E.F.; Velasques, J.; Filho, F.J.C.M.; Cavalheri, P.S.; Migliolo, L. Pharmaceuticals residues and xenobiotics contaminants: Occurrence, analytical techniques and sustainable alternatives for wastewater treatment. Sci. Total Environ. 2019, 705, 135568. [Google Scholar] [CrossRef] [PubMed]
- Riaz, L.; Anjum, M.; Yang, Q.; Safeer, R.; Sikandar, A.; Ullah, H.; Shahab, A.; Yuan, W.; Wang, Q. Chapter 23. Treatment technologies and management options of antibiotics and AMR/ARGs. In Advances in Environmental Pollution Research Series, Antibiotics and Antimicrobial Resistance Genes in the Environment; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 369–393. ISBN 9780128188828. [Google Scholar] [CrossRef]
- Huang, X.-F.; Ye, G.-Y.; Yi, N.-K.; Lu, L.-J.; Zhang, L.; Yang, L.-Y.; Xiao, L.; Liu, J. Effect of plant physiological characteristics on the removal of conventional and emerging pollutants from aquaculture wastewater by constructed wetlands. Ecol. Eng. 2019, 135, 45–53. [Google Scholar] [CrossRef]
- Yi, X.; Tran, N.H.; Yin, T.; He, Y.; Gin, K.Y.-H. Removal of selected PPCPs, EDCs, and antibiotic resistance genes in landfill leachate by a full-scale constructed wetlands system. Water Res. 2017, 121, 46–60. [Google Scholar] [CrossRef]
- Abou-Kandil, A.; Shibli, A.; Azaizeh, H.; Wolff, D.; Wick, A.; Jadoun, J. Fate and removal of bacteria and antibiotic resistance genes in horizontal subsurface constructed wetlands: Effect of mixed vegetation and substrate type. Sci. Total Environ. 2020, 759, 144193. [Google Scholar] [CrossRef]
- Karimi, B.; Ehrampoush, M.H.; Jabary, H. Indicator pathogens, organic matter and LAS detergent removal from wastewater by constructed subsurface wetlands. J. Environ. Health Sci. Eng. 2014, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Sleytr, K.; Tietz, A.; Langergraber, G.; Haberl, R. Investigation of bacterial removal during the filtration process in constructed wetlands. Sci. Total Environ. 2007, 380, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.-S.; Su, H.-C.; Ying, G.-G.; Liu, F.; Liu, S.-S.; He, L.-Y.; Chen, Z.-F.; Yang, Y.-Q.; Chen, F.-R. Removal of antibiotics and antibiotic resistance genes in rural wastewater by an integrated constructed wetland. Environ. Sci. Pollut. Res. 2014, 22, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Constructed Wetlands for Wastewater Treatment. Water 2010, 2, 530–549. [Google Scholar] [CrossRef] [Green Version]
- Bôto, M.; Almeida, C.M.R.; Mucha, A.P. Potential of Constructed Wetlands for Removal of Antibiotics from Saline Aquaculture Effluents. Water 2016, 8, 465. [Google Scholar] [CrossRef]
- Bayati, M.; Ho, T.L.; Vu, D.C.; Wang, F.; Rogers, E.; Cuvellier, C.; Huebotter, S.; Inniss, E.C.; Udawatta, R.; Jose, S.; et al. Assessing the efficiency of constructed wetlands in removing PPCPs from treated wastewater and mitigating the ecotoxicological impacts. Int. J. Hyg. Environ. Health 2020, 231, 113664. [Google Scholar] [CrossRef]
- Santos, F.; Almeida, C.M.R.; Ribeiro, I.; Mucha, A.P. Potential of constructed wetland for the removal of antibiotics and antibiotic resistant bacteria from livestock wastewater. Ecol. Eng. 2019, 129, 45–53. [Google Scholar] [CrossRef]
- Ávila, C.; García-Galán, M.J.; Borrego, C.M.; Rodríguez-Mozaz, S.; García, J.; Barceló, D. New insights on the combined removal of antibiotics and ARGs in urban wastewater through the use of two configurations of vertical subsurface flow constructed wetlands. Sci. Total Environ. 2020, 755, 142554. [Google Scholar] [CrossRef]
- Ilyas, H.; Van Hullebusch, E.D. Performance Comparison of Different Constructed Wetlands Designs for the Removal of Personal Care Products. Int. J. Environ. Res. Public Health 2020, 17, 3091. [Google Scholar] [CrossRef]
- Engida, T.; Wu, J.; Xu, D.; Wu, Z. Review paper on treatment of industrial and domestic wastewaters using uasb reactors integrated into constructed wetlands for sustainable reuse. Appl. Ecol. Environ. Res. 2020, 18, 3101–3129. [Google Scholar] [CrossRef]
- Nguyen, X.C.; Tran, T.P.; Hoang, V.H.; Nguyen, T.P.; Chang, S.W.; Nguyen, D.D.; Guo, W.; Kumar, A.; La, D.D.; Bach, Q.-V. Combined biochar vertical flow and free-water surface constructed wetland system for dormitory sewage treatment and reuse. Sci. Total Environ. 2020, 713, 136404. [Google Scholar] [CrossRef]
- Zurita, F.; Carreon, A. Performance of three pilot-scale hybrid constructed wetlands for total coliforms and Escherichia coli removal from primary effluent—A 2-year study in a subtropical climate. J. Water Health 2014, 13, 446–458. [Google Scholar] [CrossRef] [Green Version]
- Rahman, E.; Bin Halmi, M.I.E.; Samad, M.Y.B.A.; Uddin, K.; Mahmud, K.; Shukor, M.Y.A.; Abdullah, S.R.S.; Shamsuzzaman, S.M. Design, Operation and Optimization of Constructed Wetland for Removal of Pollutant. Int. J. Environ. Res. Public Health 2020, 17, 8339. [Google Scholar] [CrossRef] [PubMed]
- Tilak, A.S.; Wani, S.P.; Patil, M.; Datta, A. Evaluating Wastewater Treatment Efficiency of Two Field Scale Subsurface Flow Constructed Wetlands. Curr. Sci. 2016, 110, 1764–1772. [Google Scholar] [CrossRef] [Green Version]
- Capodaglio, A.G. Integrated, Decentralized Wastewater Management for Resource Recovery in Rural and Peri-Urban Areas. Resources 2017, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- United Nations. The Sustainable Development Goals Report 2019; United Nations publication issued by the Department of Economic and Social Affairs; United Nations: New York, NY, USA, 2019; p. 64. Available online: https://unstats.un.org/sdgs/report/2019/The-Sustainable-Development-Goals-Report-2019.pdf (accessed on 27 November 2021).
- Badza, T.; Tesfamariam, E.H.; Cogger, C. Sludge Stabilization Process, Drying Depth and Polymeric Material Addition: Implication on Nitrogen Content, Selected Chemical Properties and Land Requirement in Sand Drying Beds. Energies 2020, 13, 6753. [Google Scholar] [CrossRef]
- Vigueros, L.C.; Camperos, E.R. Vermicomposting of sewage sludge: A new technology for Mexico. Water Sci. Technol. 2002, 46, 153–158. [Google Scholar] [CrossRef]
- Ludibeth, S.-M.; Marina, I.-E.; Vicenta, E.M. Vermicomposting of Sewage Sludge: Earthworm Population and Agronomic Advantages. Compos. Sci. Util. 2012, 20, 11–17. [Google Scholar] [CrossRef]
- Boruszko, D. Vermicomposting as an Alternative Method of Sludge Treatment. J. Ecol. Eng. 2020, 21, 22–28. [Google Scholar] [CrossRef]
- Bina, B.; Movahedian, H.; Kord, I. The Effect of Lime Stabilization on the Microbiological Quality of Sewage Sludge. Iran. J. Environ. Health Sci. Eng. 2004, 1, 38–42. [Google Scholar]
- Shingare, R.P.; Thawale, P.R.; Raghunathan, K.; Mishra, A.; Kumar, S. Constructed wetland for wastewater reuse: Role and efficiency in removing enteric pathogens. J. Environ. Manag. 2019, 246, 444–461. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands, 2nd ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2009; p. 1016. ISBN 978-1-56670-526-4. Available online: https://sswm.info/sites/default/files/reference_attachments/KADLEC%20WALLACE%202009%20Treatment%20Wetlands%202nd%20Edition_0.pdf (accessed on 27 November 2021).
- Álvarez, J.A.; Ávila, C.; Otter, P.; Kilian, R.; Istenič, D.; Rolletschek, M.; Molle, P.; Khalil, N.; Ameršek, I.; Mishra, V.K.; et al. Constructed wetlands and solar-driven disinfection technologies for sustainable wastewater treatment and reclamation in rural India: SWINGS project. Water Sci. Technol. 2017, 76, 1474–1489. [Google Scholar] [CrossRef]
- Vivant, A.-L.; Boutin, C.; Prost-Boucle, S.; Papias, S.; Hartmann, A.; Depret, G.; Ziebal, C.; Le Roux, S.; Pourcher, A.-M. Free water surface constructed wetlands limit the dissemination of extended-spectrum beta-lactamase producing Escherichia coli in the natural environment. Water Res. 2016, 104, 178–188. [Google Scholar] [CrossRef]
- Berglund, B.; Khan, G.A.; Weisner, S.E.; Ehde, P.M.; Fick, J.; Lindgren, P.-E. Efficient removal of antibiotics in surface-flow constructed wetlands, with no observed impact on antibiotic resistance genes. Sci. Total Environ. 2014, 476–477, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.-N.; Wang, X.-Z.; He, X.-J.; Wang, Z.; Li, W.-X. Effects of antibiotics on microbial community structure and microbial functions in constructed wetlands treated with artificial root exudates. Environ. Sci. Process. Impacts 2019, 22, 217–226. [Google Scholar] [CrossRef]
- Almuktar, S.A.A.A.N.; Abed, S.N.; Scholz, M. Wetlands for wastewater treatment and subsequent recycling of treated effluent: A review. Environ. Sci. Pollut. Res. 2018, 25, 23595–23623. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Asolekar, S.R.; Sharma, S.K. Post-treatment and reuse of secondary effluents using natural ltreatment systems: The Indian practices. Environ. Monit. Assess. 2015, 187, 612. [Google Scholar] [CrossRef] [PubMed]
- Hiller, C.; Hübner, U.; Fajnorova, S.; Schwartz, T.; Drewes, J. Antibiotic microbial resistance (AMR) removal efficiencies by conventional and advanced wastewater treatment processes: A review. Sci. Total Environ. 2019, 685, 596–608. [Google Scholar] [CrossRef]
- Triggiano, F.; Calia, C.; Diella, G.; Montagna, M.T.; De Giglio, O.; Caggiano, G. The Role of Urban Wastewater in the Environmental Transmission of Antimicrobial Resistance: The Current Situation in Italy (2010–2019). Microorganisms 2020, 8, 1567. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Gernjak, W.; Krzeminski, P.; Malato, S.; McArdell, C.S.; Perez, J.A.S.; Schaar, H.; Fatta-Kassinos, D. Best available technologies and treatment trains to address current challenges in urban wastewater reuse for irrigation of crops in EU countries. Sci. Total Environ. 2019, 710, 136312. [Google Scholar] [CrossRef]
- King, T.; Schmidt, S.; Essack, S. Antibiotic resistant Klebsiella spp. from a hospital, hospital effluents and wastewater treatment plants in the uMgungundlovu District, KwaZulu-Natal, South Africa. Sci. Total Environ. 2019, 712, 135550. [Google Scholar] [CrossRef] [PubMed]
- Sydnor, E.R.M.; Perl, T.M. Hospital Epidemiology and Infection Control in Acute-Care Settings. Clin. Microbiol. Rev. 2011, 24, 141–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaheer, R.; Cook, S.R.; Barbieri, R.; Goji, N.; Cameron, A.; Petkau, A.; Polo, R.O.; Tymensen, L.; Stamm, C.; Song, J.; et al. Surveillance of Enterococcus spp. reveals distinct species and antimicrobial resistance diversity across a One-Health continuum. Sci. Rep. 2020, 10, 3937, Corrected in Sci Rep. 2020, 10, 13401. [Google Scholar] [CrossRef]
- Hayakawa, K.; Marchaim, D.; Martin, E.T.; Tiwari, N.; Yousuf, A.; Sunkara, B.; Pulluru, H.; Kotra, H.; Hasan, A.; Bheemreddy, S.; et al. Comparison of the Clinical Characteristics and Outcomes Associated with Vancomycin-Resistant Enterococcus faecalis and Vancomycin-Resistant E. faecium Bacteremia. Antimicrob. Agents Chemother. 2012, 56, 2452–2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huijbers, P.M.; Larsson, D.J.; Flach, C.-F. Surveillance of antibiotic resistant Escherichia coli in human populations through urban wastewater in ten European countries. Environ. Pollut. 2020, 261, 114200. [Google Scholar] [CrossRef]
- Duong, H.A.; Phung, T.V.; Nguyen, T.N.; Thi, L.-A.P.; Pham, H.V. Occurrence, Distribution, and Ecological Risk Assessment of Antibiotics in Selected Urban Lakes of Hanoi, Vietnam. J. Anal. Methods Chem. 2021, 2021, 1–13. [Google Scholar] [CrossRef]
- Felis, E.; Kalka, J.; Sochacki, A.; Kowalska, K.; Bajkacz, S.; Harnisz, M.; Korzeniewska, E. Antimicrobial pharmaceuticals in the aquatic environment-occurrence and environmental implications. Eur. J. Pharmacol. 2019, 866, 172813. [Google Scholar] [CrossRef]
- Miller, S.I. Antibiotic Resistance and Regulation of the Gram-Negative Bacterial Outer Membrane Barrier by Host Innate Immune Molecules. mBio 2016, 7, e01541-16. [Google Scholar] [CrossRef] [Green Version]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta. 2009, 1794, 808–816. [Google Scholar] [CrossRef] [Green Version]
- Hendricks, R.; Pool, E.J. The effectiveness of sewage treatment processes to remove faecal pathogens and antibiotic residues. J. Environ. Sci. Health Part A 2012, 47, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Shi, Y.; Gao, L.; Liu, J.; Cai, Y. Occurrence and removal of antibiotics in a municipal wastewater reclamation plant in Beijing, China. Chemosphere 2013, 92, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Batchu, S.R.; Panditi, V.R.; O’Shea, K.E.; Gardinali, P.R. Photodegradation of antibiotics under simulated solar radiation: Implications for their environmental fate. Sci. Total Environ. 2014, 470–471, 299–310. [Google Scholar] [CrossRef]
- Chopra, I.; Shales, S. Susceptibility of Protein Synthesis in Escherichia coli to Tetracycline and Minocycline. Microbiology 1981, 124, 187–189. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Yang, L.; Wang, J. Denitrification performance and microbial diversity in a packed-bed bioreactor using PCL as carbon source and biofilm carrier. Appl. Microbiol. Biotechnol. 2012, 97, 2725–2733. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Li, C.; Yu, J.; Xu, Q.; Wei, S.; Tian, Z.; Yang, Z.; Yang, W.; Shen, J. Insight into adsorption of combined antibiotic-heavy metal contaminants on graphene oxide in water. Sep. Purif. Technol. 2019, 236, 116278. [Google Scholar] [CrossRef]
- Moles, S.; Valero, P.; Escuadra, S.; Mosteo, R.; Gómez, J.; Ormad, M.P. Performance comparison of commercial TiO2: Separation and reuse for bacterial photo-inactivation and emerging pollutants photo-degradation. Environ. Sci. Pollut. Res. 2020, 27, 9099–9113. [Google Scholar] [CrossRef]
- van Grieken, R.; Marugán, J.; Pablos, C.; Furones, L.; López, A. Comparison between the photocatalytic inactivation of Gram-positive E. faecalis and Gram-negative E. coli faecal contamination indicator microorganisms. Appl. Catal. B Environ. 2010, 100, 212–220. [Google Scholar] [CrossRef]
- Jia, A.; Wan, Y.; Xiao, Y.; Hu, J. Occurrence and fate of quinolone and fluoroquinolone antibiotics in a municipal sewage treatment plant. Water Res. 2012, 46, 387–394. [Google Scholar] [CrossRef]
- Behera, S.K.; Kim, H.-W.; Oh, J.-E.; Park, H.-S. Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci. Total Environ. 2011, 409, 4351–4360. [Google Scholar] [CrossRef]
- Marx, C.; Günther, N.; Schubert, S.; Oertel, R.; Ahnert, M.; Krebs, P.; Kuehn, V. Mass flow of antibiotics in a wastewater treatment plant focusing on removal variations due to operational parameters. Sci. Total Environ. 2015, 538, 779–788. [Google Scholar] [CrossRef]
- Vieno, N.; Tuhkanen, T.; Kronberg, L. Elimination of pharmaceuticals in sewage treatment plants in Finland. Water Res. 2007, 41, 1001–1012. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T. Biodegradation and Adsorption of Antibiotics in the Activated Sludge Process. Environ. Sci. Technol. 2010, 44, 3468–3473. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, T. Mass flows and removal of antibiotics in two municipal wastewater treatment plants. Chemosphere 2011, 83, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.-C.; Tsai, Y.-T. Occurrence of pharmaceuticals in Taiwan’s surface waters: Impact of waste streams from hospitals and pharmaceutical production facilities. Sci. Total Environ. 2009, 407, 3793–3802. [Google Scholar] [CrossRef]
- De la Cruz, N.; Gimenez, J.; Esplugas, S.; Grandjean, D.; de Alencastro, L.; Pulgarín, C. Degradation of 32 emergent contaminants by UV and neutral photo-fenton in domestic wastewater effluent previously treated by activated sludge. Water Res. 2012, 46, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- Ben, W.; Zhu, B.; Yuan, X.; Zhang, Y.; Yang, M.; Qiang, Z. Occurrence, removal and risk of organic micropollutants in wastewater treatment plants across China: Comparison of wastewater treatment processes. Water Res. 2018, 130, 38–46. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, G.; Li, X.; Zou, S.; Li, P.; Hu, Z.; Li, J. Occurrence and elimination of antibiotics at four sewage treatment plants in the Pearl River Delta (PRD), South China. Water Res. 2007, 41, 4526–4534. [Google Scholar] [CrossRef]
- Hollender, J.; Zimmermann, S.G.; Koepke, S.; Krauss, M.; McArdell, C.S.; Ort, C.; Singer, H.; von Gunten, U.; Siegrist, H. Elimination of Organic Micropollutants in a Municipal Wastewater Treatment Plant Upgraded with a Full-Scale Post-Ozonation Followed by Sand Filtration. Environ. Sci. Technol. 2009, 43, 7862–7869. [Google Scholar] [CrossRef]
- Alajmi, H.M. Effect of Physical, Chemical and Biological Treatment on the Removal of Five Pharmaceuticals from Domestic Wastewater in Laboratory-Scale Reactors and a Full-Scale Plant. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 2014. Available online: https://theses.ncl.ac.uk/jspui/bitstream/10443/2469/1/Alajmi%2C%20H.%2014.pdf (accessed on 27 November 2021).
- Altmann, J.; Ruhl, A.S.; Zietzschmann, F.; Jekel, M. Direct comparison of ozonation and adsorption onto powdered activated carbon for micropollutant removal in advanced wastewater treatment. Water Res. 2014, 55, 185–193. [Google Scholar] [CrossRef]
- Nam, S.-W.; Choi, D.-J.; Kim, S.-K.; Her, N.; Zoh, K.-D. Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J. Hazard. Mater. 2014, 270, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Phoon, B.L.; Ong, C.C.; Saheed, M.S.M.; Show, P.-L.; Chang, J.-S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J. Fenton-like degradation of sulfamethoxazole using Fe-based magnetic nanoparticles embedded into mesoporous carbon hybrid as an efficient catalyst. Chem. Eng. J. 2018, 351, 1085–1094. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Z.; Shen, C.; Xu, Y. Inactivation of antibiotic-resistant bacteria and antibiotic resistance genes by electrochemical oxidation/electro-Fenton process. Water Sci. Technol. 2020, 81, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Rodríguez, S.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Assessment of Sulfate Radical-Based Advanced Oxidation Processes for Water and Wastewater Treatment: A Review. Water 2018, 10, 1828. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, J. Oxidative removal of carbamazepine by peroxymonosulfate (PMS) combined to ionizing radiation: Degradation, mineralization and biological toxicity. Sci. Total Environ. 2019, 658, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Zhuan, R.; Wang, J. Degradation of sulfamethoxazole by ionizing radiation: Kinetics and implications of additives. Sci. Total Environ. 2019, 668, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Al-Jassim, N.; Ansari, M.I.; Harb, M.; Hong, P.-Y. Removal of bacterial contaminants and antibiotic resistance genes by conventional wastewater treatment processes in Saudi Arabia: Is the treated wastewater safe to reuse for agricultural irrigation? Water Res. 2015, 73, 277–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinthaler, F.; Posch, J.; Feierl, G.; Wüst, G.; Haas, D.; Ruckenbauer, G.; Mascher, F.; Marth, E. Antibiotic resistance of E. coli in sewage and sludge. Water Res. 2003, 37, 1685–1690. [Google Scholar] [CrossRef]
- Varela, A.R.; Ferro, G.; Vredenburg, J.; Yanık, M.; Vieira, L.; Rizzo, L.; Lameiras, C.; Manaia, C.M. Vancomycin resistant enterococci: From the hospital effluent to the urban wastewater treatment plant. Sci. Total Environ. 2013, 450–451, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Munir, M.; Wong, K.; Xagoraraki, I. Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan. Water Res. 2011, 45, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Hiwrale, I.; Das, S.; Shukla, V.; Tyagi, L.; Pal, S.; Dafale, N.; Dhodapkar, R. Profiling of emerging contaminants and antibiotic resistance in sewage treatment plants: An Indian perspective. J. Hazard. Mater. 2020, 408, 124877. [Google Scholar] [CrossRef] [PubMed]
- Marín, I.; Goñi, P.; Lasheras, A.; Ormad, M. Efficiency of a Spanish wastewater treatment plant for removal potentially pathogens: Characterization of bacteria and protozoa along water and sludge treatment lines. Ecol. Eng. 2015, 74, 28–32. [Google Scholar] [CrossRef]
- Szostkova, M.; Vitez, T.; Marecek, J.; Losak, T. Microbial Contamination of Screenings from Wastewater Treatment Plants. Pol. J. Environ. Stud. 2012, 21, 1943–1947. [Google Scholar]
- WHO. Guidelines for Safe Use of Wastewater, Excreta and Greywater. (Volume IV: Excreta and Greywater Use in Agriculture); World Health Organization (WHO): Geneva, Switzerland, 2006; ISBN 9241546859. Available online: https://www.who.int/publications/i/item/9241546859 (accessed on 27 November 2021).
- Teshome, A.; Alemayehu, T.; Deriba, W.; Ayele, A.T. Antibiotic Resistance Profile of Bacteria Isolated from Wastewater Systems in Eastern Ethiopia. J. Environ. Public Health 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Hernández, M.L.; Pronk, M.; Garcia, H.; Boersma, A.; Brdjanovic, D.; van Loosdrecht, M.C.; Hooijmans, C.M. Removal of bacterial and viral indicator organisms in full-scale aerobic granular sludge and conventional activated sludge systems. Water Res. X 2019, 6, 100040. [Google Scholar] [CrossRef]
- Mao, G.; Song, Y.; Bartlam, M.; Wang, Y. Long-Term Effects of Residual Chlorine on Pseudomonas aeruginosa in Simulated Drinking Water Fed With Low AOC Medium. Front. Microbiol. 2018, 9, 879. [Google Scholar] [CrossRef]
- Owoseni, M.C.; Olaniran, A.O.; Okoh, A.I. Chlorine Tolerance and Inactivation of Escherichia coli recovered from Wastewater Treatment Plants in the Eastern Cape, South Africa. Appl. Sci. 2017, 7, 810. [Google Scholar] [CrossRef]
- Lv, L.; Jiang, T.; Zhang, S.; Yu, X. Exposure to Mutagenic Disinfection Byproducts Leads to Increase of Antibiotic Resistance in Pseudomonas aeruginosa. Environ. Sci. Technol. 2014, 48, 8188–8195. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, Y.; Geng, J.; Ren, H.; Zhang, Y.; Ding, L.; Xu, K. Inactivation of antibiotic resistance genes in municipal wastewater effluent by chlorination and sequential UV/chlorination disinfection. Sci. Total Environ. 2015, 512–513, 125–132. [Google Scholar] [CrossRef]
- Huang, J.-J.; Hu, H.-Y.; Tang, F.; Li, Y.; Lu, S.-Q.; Lu, Y. Inactivation and reactivation of antibiotic-resistant bacteria by chlorination in secondary effluents of a municipal wastewater treatment plant. Water Res. 2011, 45, 2775–2781. [Google Scholar] [CrossRef]
- Du, J.; Geng, J.; Ren, H.; Ding, L.; Xu, K.; Zhang, Y. Variation of antibiotic resistance genes in municipal wastewater treatment plant with A2O-MBR system. Environ. Sci. Pollut. Res. 2014, 22, 3715–3726. [Google Scholar] [CrossRef] [PubMed]
- Bouki, C.; Venieri, D.; Diamadopoulos, E. Detection and fate of antibiotic resistant bacteria in wastewater treatment plants: A review. Ecotoxicol. Environ. Saf. 2013, 91, 1–9. [Google Scholar] [CrossRef]
- Teklehaimanot, G.Z.; Genthe, B.; Kamika, I.; Momba, M. Prevalence of enteropathogenic bacteria in treated effluents and receiving water bodies and their potential health risks. Sci. Total Environ. 2015, 518–519, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.-B.; Guo, M.; Yang, J. Fate of Antibiotic Resistant Bacteria and Genes during Wastewater Chlorination: Implication for Antibiotic Resistance Control. PLoS ONE 2015, 10, e0119403. [Google Scholar] [CrossRef]
- Thayanukul, P.; Kurisu, F.; Kasuga, I.; Furumai, H. Evaluation of microbial regrowth potential by assimilable organic carbon in various reclaimed water and distribution systems. Water Res. 2013, 47, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-W.; Li, D.; Gu, A.Z.; Zeng, S.-Y.; He, M. Bacterial regrowth in water reclamation and distribution systems revealed by viable bacterial detection assays. Chemosphere 2016, 144, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, H.; Chandran, K. Propensity of activated sludge to amplify or attenuate tetracycline resistance genes and tetracycline resistant bacteria: A mathematical modeling approach. Chemosphere 2010, 78, 1071–1077. [Google Scholar] [CrossRef]
- Guo, M.-T.; Yuan, Q.-B.; Yang, J. Ultraviolet reduction of erythromycin and tetracycline resistant heterotrophic bacteria and their resistance genes in municipal wastewater. Chemosphere 2013, 93, 2864–2868. [Google Scholar] [CrossRef]
- Lüddeke, F.; Heß, S.; Gallert, C.; Winter, J.; Güde, H.; Löffler, H. Removal of total and antibiotic resistant bacteria in advanced wastewater treatment by ozonation in combination with different filtering techniques. Water Res. 2015, 69, 243–251. [Google Scholar] [CrossRef]
- Alexander, J.; Knopp, G.; Dötsch, A.; Wieland, A.; Schwartz, T. Ozone treatment of conditioned wastewater selects antibiotic resistance genes, opportunistic bacteria, and induce strong population shifts. Sci. Total Environ. 2016, 559, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A.; Stüber, J.; Herrmann, N.; McDowell, D.; Ried, A.; Kampmann, M.; Teiser, B. Ozonation: A tool for removal of pharmaceuticals, contrast media and musk fragrances from wastewater? Water Res. 2003, 37, 1976–1982. [Google Scholar] [CrossRef]
- Xu, P.; Janex, M.-L.; Savoye, P.; Cockx, A.; Lazarova, V. Wastewater disinfection by ozone: Main parameters for process design. Water Res. 2001, 36, 1043–1055. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of Enteric Bacteria in Constructed Treatment Wetlands with Emergent Macrophytes: A Review. J. Environ. Sci. Health Part A 2005, 40, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.C.; Nguyen, D.D.; Tran, Q.B.; Nguyen, T.T.H.; Tran, T.K.A.; Tran, T.C.P.; Nguyen, T.H.G.; Tran, T.N.T.; La, D.D.; Chang, S.W.; et al. Two-step system consisting of novel vertical flow and free water surface constructed wetland for effective sewage treatment and reuse. Bioresour. Technol. 2020, 306, 123095. [Google Scholar] [CrossRef]
- Quansah, A.; Ntaryamira, T.; Rwemera, J. Sludge Wastewater Management by Conventional Treatment Process: Case Study—Bujumbura Municipal Sewage. Int. J. Sci. 2018, 4, 52–65. [Google Scholar] [CrossRef] [Green Version]
- Bunce, J.T.; Ndam, E.; Ofiteru, I.D.; Moore, A.; Graham, D.W. A Review of Phosphorus Removal Technologies and Their Applicability to Small-Scale Domestic Wastewater Treatment Systems. Front. Environ. Sci. 2018, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Sheng, G.-P.; Lu, Y.-Z.; Zeng, R.J.; Yu, H.-Q. Removal of antibiotic resistance genes from wastewater treatment plant effluent by coagulation. Water Res. 2017, 111, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wang, B.; Gao, Y.; Liu, W.; Zhao, X.; Huang, X.; Yu, J. Occurrence and Fate of Antibiotics in the Aqueous Environment and Their Removal by Constructed Wetlands in China: A review. Pedosphere 2017, 27, 42–51. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, B.; Li, N.; Sardar, M.F.; Song, T.; Zhu, C.; Lv, X.; Li, H. Effects of UV disinfection on phenotypes and genotypes of antibiotic-resistant bacteria in secondary effluent from a municipal wastewater treatment plant. Water Res. 2019, 157, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Qiao, M.; Zhong, J.; Zhu, Y.; Guo, C.; Zhang, Q.; Yang, P.; Han, L.; Zhang, W.; Wu, Y.; et al. Characterization of antibiotic resistance genes and bacterial community in selected municipal and industrial sewage treatment plants beside Poyang Lake. Water Res. 2020, 174, 115603. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M. Occurrence and removal of antibiotic resistance genes in municipal wastewater and rural domestic sewage treatment systems in eastern China. Environ. Int. 2013, 55, 9–14. [Google Scholar] [CrossRef]
- Börjesson, S.; Dienues, O.; Jarnheimer, P.; Olsen, B.; Matussek, A.; Lindgren, P.-E. Quantification of genes encoding resistance to aminoglycosides, β-lactams and tetracyclines in wastewater environments by real-time PCR. Int. J. Environ. Health Res. 2009, 19, 219–230. [Google Scholar] [CrossRef]
- Pei, M.; Zhang, B.; He, Y.; Su, J.; Gin, K.; Lev, O.; Shen, G.; Hu, S. State of the art of tertiary treatment technologies for controlling antibiotic resistance in wastewater treatment plants. Environ. Int. 2019, 131, 105026. [Google Scholar] [CrossRef] [PubMed]
- Korzeniewska, E.; Harnisz, M. Relationship between modification of activated sludge wastewater treatment and changes in antibiotic resistance of bacteria. Sci. Total Environ. 2018, 639, 304–315. [Google Scholar] [CrossRef]
- Gao, P.; Munir, M.; Xagoraraki, I. Correlation of tetracycline and sulfonamide antibiotics with corresponding resistance genes and resistant bacteria in a conventional municipal wastewater treatment plant. Sci. Total Environ. 2012, 421–422, 173–183. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Stiborova, H.; Kracmarova, M.; Vesela, T.; Biesiekierska, M.; Cerny, J.; Balik, J.; Demnerova, K. Impact of Long-Term Manure and Sewage Sludge Application to Soil as Organic Fertilizer on the Incidence of Pathogenic Microorganisms and Antibiotic Resistance Genes. Agronomy 2021, 11, 1423. [Google Scholar] [CrossRef]
- Thakali, O.; Brooks, J.P.; Shahin, S.; Sherchan, S.P.; Haramoto, E. Removal of Antibiotic Resistance Genes at Two Conventional Wastewater Treatment Plants of Louisiana, USA. Water 2020, 12, 1729. [Google Scholar] [CrossRef]
- Huang, J.J.; Xi, J.Y.; Hu, H.Y.; Tang, F.; Pang, Y.C. Inactivation and regrowth of antibiotic-resistant bacteria by PAA disinfection in the secondary effluent of a municipal wastewater treatment plant. Biomed. Environ. Sci. 2013, 26, 865–868. [Google Scholar] [CrossRef]
- Jin, M.; Liu, L.; Wang, D.-N.; Yang, D.; Liu, W.-L.; Yin, J.; Yang, Z.-W.; Wang, H.-R.; Qiu, Z.-G.; Shen, Z.-Q.; et al. Chlorine disinfection promotes the exchange of antibiotic resistance genes across bacterial genera by natural transformation. ISME J. 2020, 14, 1847–1856. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yu, X.; McDonald, T.J.; Jinadatha, C.; Dionysiou, D.; Feng, M. Elimination of antibiotic resistance genes and control of horizontal transfer risk by UV-based treatment of drinking water: A mini review. Front. Environ. Sci. Eng. 2019, 13, 1–9. [Google Scholar] [CrossRef]
- Zheng, J.; Su, C.; Zhou, J.; Xu, L.; Qian, Y.; Chen, H. Effects and mechanisms of ultraviolet, chlorination, and ozone disinfection on antibiotic resistance genes in secondary effluents of municipal wastewater treatment plants. Chem. Eng. J. 2017, 317, 309–316. [Google Scholar] [CrossRef]
- Shekhawat, S.S.; Kulshreshtha, N.M.; Gupta, A.B. Investigation of chlorine tolerance profile of dominant gram negative bacteria recovered from secondary treated wastewater in Jaipur, India. J. Environ. Manag. 2019, 255, 109827. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, R.; Yang, Y.; Chen, B.; Cheng, Z.; Zhang, M.; Li, J.; Zhang, G.; Zou, S. Characterizing the antibiotic resistance genes in a river catchment: Influence of anthropogenic activities. J. Environ. Sci. 2018, 69, 125–132. [Google Scholar] [CrossRef]
- Sinha, R.P.; Häder, D.-P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Ávila, C.; Pelissari, C.; Sezerino, P.H.; Sgroi, M.; Roccaro, P.; García, J. Enhancement of total nitrogen removal through effluent recirculation and fate of PPCPs in a hybrid constructed wetland system treating urban wastewater. Sci. Total Environ. 2017, 584–585, 414–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanakis, A.I.; Akratos, C.S. Phytoremediation: Management of Environmental Contaminants, Volume 4. In Phytoremediation: Management of Environmental Contaminants; Springer: Cham, Switzerland, 2016; Volume 4, pp. 1–409. [Google Scholar] [CrossRef]
- Rosal, R.; Rodriguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E. Oxidation of dissolved organic matter in the effluent of a sewage treatment plant using ozone combined with hydrogen peroxide (O3/H2O2). Chem. Eng. J. 2009, 149, 311–318. [Google Scholar] [CrossRef]
- van Gijn, K.; Sohier, J.; Maasdam, R.; de Wilt, H.; Rijnaarts, H.; Langenhoff, A. Optimizing Micropollutant Removal by Ozonation; Interference of Effluent Organic Matter Fractions. Ozone Sci. Eng. 2021, 43, 579–591. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, Z.; Fang, W.; Gao, G. Composition of bacterial communities in municipal wastewater treatment plant. Sci. Total Environ. 2019, 689, 1181–1191. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Wang, Q. Free Ammonia Pretreatment for Anaerobic Sludge Digestion Reduces the Spread of Antibiotic Resistance. bioRxiv 2020, 2–12. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Moustafa, M.T. Treating wastewater under zero waste principle using wetland mesocosms. Front. Environ. Sci. Eng. 2020, 15, 1–14. [Google Scholar] [CrossRef]
- Jamwal, P.; Raj, A.V.; Raveendran, L.; Shirin, S.; Connelly, S.; Yeluripati, J.; Richards, S.; Rao, L.; Helliwell, R.; Tamburini, M. Evaluating the performance of horizontal sub-surface flow constructed wetlands: A case study from southern India. Ecol. Eng. 2021, 162, 106170. [Google Scholar] [CrossRef]
- Lopardo, C.R.; Zhang, L.; Mitsch, W.J.; Urakawa, H. Comparison of nutrient retention efficiency between vertical-flow and floating treatment wetland mesocosms with and without biodegradable plastic. Ecol. Eng. 2019, 131, 120–130. [Google Scholar] [CrossRef]
- Si, Z.; Wang, Y.; Song, X.; Cao, X.; Zhang, X.; Sand, W. Mechanism and performance of trace metal removal by continuous-flow constructed wetlands coupled with a micro-electric field. Water Res. 2019, 164, 114937. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shen, Q. Phytoremediation of cadmium-contaminated wetland soil with Typha latifolia L. and the underlying mechanisms involved in the heavy-metal uptake and removal. Environ. Sci. Pollut. Res. 2019, 27, 4905–4916. [Google Scholar] [CrossRef] [PubMed]
- Vivant, A.-L.; Boutin, C.; Prost-Boucle, S.; Papias, S.; Ziebal, C.; Pourcher, A.-M. Fate of two strains of extended-spectrum beta-lactamase producing Escherichia coli in constructed wetland microcosm sediments: Survival and change in antibiotic resistance profiles. Water Sci. Technol. 2019, 79, 1550–1560. [Google Scholar] [CrossRef]
- Stokes, J.M.; Lopatkin, A.J.; Lobritz, M.A.; Collins, J.J. Bacterial Metabolism and Antibiotic Efficacy. Cell Metab. 2019, 30, 251–259. [Google Scholar] [CrossRef]
- Chen, L.; Huang, F.; Zhang, C.; Zhang, J.; Liu, F.; Guan, X. Effects of norfloxacin on nitrate reduction and dynamic denitrifying enzymes activities in groundwater. Environ. Pollut. 2021, 273, 116492. [Google Scholar] [CrossRef]
- Semedo, M.; Song, B.; Sparrer, T.; Phillips, R. Antibiotic Effects on Microbial Communities Responsible for Denitrification and N2O Production in Grassland Soils. Front. Microbiol. 2018, 9, 2121. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Yin, G.; Liu, M.; Zhou, J.; Zheng, Y.; Gao, J.; Zong, H.; Yang, Y.; Gao, L.; Tong, C. Effects of Sulfamethazine on Denitrification and the Associated N2O Release in Estuarine and Coastal Sediments. Environ. Sci. Technol. 2014, 49, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.-S.; Bai, Y.-H.; Chen, Q.-Q.; Shen, Y.-Y.; Huang, B.-C.; Jin, R.-C. Deciphering the toxic effects of antibiotics on denitrification: Process performance, microbial community and antibiotic resistance genes. J. Environ. Manag. 2020, 262, 110375. [Google Scholar] [CrossRef] [PubMed]
- Faulwetter, J.L.; Gagnon, V.; Sundberg, C.; Chazarenc, F.; Burr, M.D.; Brisson, J.; Camper, A.K.; Stein, O.R. Microbial processes influencing performance of treatment wetlands: A review. Ecol. Eng. 2009, 35, 987–1004. [Google Scholar] [CrossRef]
- Helt, C.D.; Weber, K.P.; Legge, R.L.; Slawson, R.M. Antibiotic resistance profiles of representative wetland bacteria and faecal indicators following ciprofloxacin exposure in lab-scale constructed mesocosms. Ecol. Eng. 2012, 39, 113–122. [Google Scholar] [CrossRef]
- Yang, L.; Wen, Q.; Zhao, Y.; Chen, Z.; Wang, Q.; Bürgmann, H. New insight into effect of antibiotics concentration and process configuration on the removal of antibiotics and relevant antibiotic resistance genes. J. Hazard. Mater. 2019, 373, 60–66. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Meng, G.; Zhang, C.; Guo, R. Efficiency and kinetics of conventional pollutants and tetracyclines removal in integrated vertical-flow constructed wetlands enhanced by aeration. J. Environ. Manag. 2020, 273, 111120. [Google Scholar] [CrossRef]
- Chen, J.; Tong, T.; Jiang, X.; Xie, S. Biodegradation of sulfonamides in both oxic and anoxic zones of vertical flow constructed wetland and the potential degraders. Environ. Pollut. 2020, 265, 115040. [Google Scholar] [CrossRef] [PubMed]
- Nivala, J.; Kahl, S.; Boog, J.; van Afferden, M.; Reemtsma, T.; Müller, R.A. Dynamics of emerging organic contaminant removal in conventional and intensified subsurface flow treatment wetlands. Sci. Total Environ. 2018, 649, 1144–1156. [Google Scholar] [CrossRef] [PubMed]
- Kahl, S.; Nivala, J.; van Afferden, M.; Müller, R.A.; Reemtsma, T. Effect of design and operational conditions on the performance of subsurface flow treatment wetlands: Emerging organic contaminants as indicators. Water Res. 2017, 125, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Almeida, C.M.R.; Pereira, A.C.; Ribeiro, I.L.; Reis, I.; Carvalho, P.; Basto, M.C.; Mucha, A.P. Microbial community dynamics associated with veterinary antibiotics removal in constructed wetlands microcosms. Bioresour. Technol. 2015, 182, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Pelissari, C.; Ávila, C.; Trein, C.M.; García, J.; de Armas, R.D.; Sezerino, P.H. Nitrogen transforming bacteria within a full-scale partially saturated vertical subsurface flow constructed wetland treating urban wastewater. Sci. Total Environ. 2017, 574, 390–399. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Nurul, S.; Schmitt, H.; Sutton, N.B.; Murk, T.A.; Blokland, M.; Rijnaarts, H.H.; Langenhoff, A.A. Evaluation of attenuation of pharmaceuticals, toxic potency, and antibiotic resistance genes in constructed wetlands treating wastewater effluents. Sci. Total Environ. 2018, 631–632, 1572–1581. [Google Scholar] [CrossRef]
- Liu, X.-H.; Guo, X.; Liu, Y.; Lu, S.; Xi, B.; Zhang, J.; Wang, Z.; Bi, B. A review on removing antibiotics and antibiotic resistance genes from wastewater by constructed wetlands: Performance and microbial response. Environ. Pollut. 2019, 254, 112996. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, B.; Wang, H.; Lai, X.; Li, F.; Salam, M.M.A.; Pan, F.; Zhao, Y. The simultaneous antibiotics and nitrogen removal in vertical flow constructed wetlands: Effects of substrates and responses of microbial functions. Bioresour. Technol. 2020, 310, 123419. [Google Scholar] [CrossRef] [PubMed]
- Sochacki, A.; Felis, E.; Bajkacz, S.; Nowrotek, M.; Miksch, K. Removal and transformations of diclofenac and sulfamethoxazole in a two-stage constructed wetland system. Ecol. Eng. 2018, 122, 159–168. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed wetlands for wastewater treatment. Encycl. Ecol. 2011, 45, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.-J.; Kim, L.-H.; Zoh, K.-D. Removal characteristics and mechanism of antibiotics using constructed wetlands. Ecol. Eng. 2016, 91, 85–92. [Google Scholar] [CrossRef]
- Panja, S.; Sarkar, D. Removal of Tetracycline and Ciprofloxacin from Wastewater by Vetiver Grass (Chrysopogon zizanioides (L.) Roberty) as a Function of Nutrient Concentrations. Environ. Sci. Pollut. Res. 2020, 27, 34951–34965. [Google Scholar] [CrossRef]
- Kurade, M.B.; Xiong, J.-Q.; Govindwar, S.P.; Roh, H.-S.; Saratale, G.D.; Jeon, B.-H.; Lim, H. Uptake and biodegradation of emerging contaminant sulfamethoxazole from aqueous phase using Ipomoea aquatica. Chemosphere 2019, 225, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Christofilopoulos, S.; Syranidou, E.; Gkavrou, G.; Manousaki, E.; Kalogerakis, N. The role of halophyteJuncus acutusL. in the remediation of mixed contamination in a hydroponic greenhouse experiment. J. Chem. Technol. Biotechnol. 2016, 91, 1665–1674. [Google Scholar] [CrossRef]
- Alhaddad, F.A.; Abu-Dieyeh, M.H.; ElAzazi, E.-S.M.; Ahmed, T.A. Salt tolerance of selected halophytes at the two initial growth stages for future management options. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Freedman, A.; Gross, A.; Shelef, O.; Rachmilevitch, S.; Arnon, S. Salt uptake and evapotranspiration under arid conditions in horizontal subsurface flow constructed wetland planted with halophytes. Ecol. Eng. 2014, 70, 282–286. [Google Scholar] [CrossRef]
- Glenn, E.P.; Brown, J.J.; Blumwald, E. Salt Tolerance and Crop Potential of Halophytes. CRC Crit. Rev. Plant Sci. 1999, 18, 227–255. [Google Scholar] [CrossRef]
- Martin, L.; Theophile, F.; Etienne, P.T.; Akoa, A. Removal of faecal bacteria and nutrients from domestic wastewater in a horizontal surface flow wetland vegetated with Echinochloa pyramidalis. Afr. J. Environ. Sci. Technol. 2012, 6, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.; Hench, K.; Garbutt, K.; Sexstone, A.; Bissonnette, G.; Skousen, J. Treatment of Domestic Wastewater by Three Plant Species in Constructed Wetlands. Water Air Soil Pollut. 2001, 128, 283–295. [Google Scholar] [CrossRef]
- Russo, N.; Pino, A.; Toscano, A.; Cirelli, G.L.; Caggia, C.; Arioli, S.; Randazzo, C.L. Occurrence, diversity, and persistence of antibiotic resistant enterococci in full-scale constructed wetlands treating urban wastewater in Sicily. Bioresour. Technol. 2018, 274, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Lamori, J.G.; Xue, J.; Rachmadi, A.T.; Lopez, G.U.; Kitajima, M.; Gerba, C.P.; Pepper, I.L.; Brooks, J.P.; Sherchan, S. Removal of fecal indicator bacteria and antibiotic resistant genes in constructed wetlands. Environ. Sci. Pollut. Res. 2019, 26, 10188–10197. [Google Scholar] [CrossRef]
- Maiga, Y.; von Sperling, M.; Mihelcic, J. Part 4 Management of Risk from Excreta and Wastewater. In Constructed Wetlands; Rose, J.B., Jiménez-Cisneros, B., Eds.; Michigan State University: E. Lansing, MI, USA, 2017; Available online: http://www.waterpathogens.org/book/constructed-wetlands (accessed on 27 November 2021).
- Fang, H.; Zhang, Q.; Nie, X.; Chen, B.; Xiao, Y.; Zhou, Q.; Liao, W.; Liang, X. Occurrence and elimination of antibiotic resistance genes in a long-term operation integrated surface flow constructed wetland. Chemosphere 2017, 173, 99–106. [Google Scholar] [CrossRef]
- Hien, P.T.; Tadashi, T.; Kazuhiro, M. Removal of Tetracycline and Tetracycline Resistance Genes from Municipal Wastewater in Microcosm Fill-and-Drain Constructed Wetlands. Jpn. J. Water Treat. Biol. 2017, 53, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Pazda, M.; Rybicka, M.; Stolte, S.; Bielawski, K.P.; Stepnowski, P.; Kumirska, J.; Wolecki, D.; Mulkiewicz, E.; Pazda, M. Identification of Selected Antibiotic Resistance Genes in Two Different Wastewater Treatment Plant Systems in Poland: A Preliminary Study. Molecules 2020, 25, 2851. [Google Scholar] [CrossRef]
- Song, H.-L.; Zhang, S.; Guo, J.; Yang, Y.-L.; Zhang, L.-M.; Li, H.; Yang, X.-L.; Liu, X. Vertical up-flow constructed wetlands exhibited efficient antibiotic removal but induced antibiotic resistance genes in effluent. Chemosphere 2018, 203, 434–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decamp, O.; Warren, A. Investigation of Escherichia coli removal in various designs of subsurface flow wetlands used for wastewater treatment. Ecol. Eng. 2000, 14, 293–299. [Google Scholar] [CrossRef]
- Miller, J.H.; Novak, J.T.; Knocke, W.R.; Epruden, A. Survival of Antibiotic Resistant Bacteria and Horizontal Gene Transfer Control Antibiotic Resistance Gene Content in Anaerobic Digesters. Front. Microbiol. 2016, 7, 263. [Google Scholar] [CrossRef]
- Chen, J.; Ying, G.-G.; Wei, X.-D.; Liu, Y.-S.; Liu, S.-S.; Hu, L.-X.; He, L.-Y.; Chen, Z.-F.; Chen, F.-R.; Yang, Y.-Q. Removal of antibiotics and antibiotic resistance genes from domestic sewage by constructed wetlands: Effect of flow configuration and plant species. Sci. Total Environ. 2016, 571, 974–982. [Google Scholar] [CrossRef]
- Šereš, M.; Innemanová, P.; Hnátková, T.; Rozkošný, M.; Stefanakis, A.; Semerád, J.; Cajthaml, T. Evaluation of Hybrid Constructed Wetland Performance and Reuse of Treated Wastewater in Agricultural Irrigation. Water 2021, 13, 1165. [Google Scholar] [CrossRef]
- Abdel-Mohsein, H.S.; Feng, M.; Fukuda, Y.; Tada, C. Remarkable Removal of Antibiotic-Resistant Bacteria During Dairy Wastewater Treatment Using Hybrid Full-scale Constructed Wetland. Water Air Soil Pollut. 2020, 231, 1–12. [Google Scholar] [CrossRef]
- Chen, J.; Deng, W.-J.; Liu, Y.-S.; Hu, L.-X.; He, L.-Y.; Zhao, J.-L.; Wang, T.-T.; Ying, G.-G. Fate and removal of antibiotics and antibiotic resistance genes in hybrid constructed wetlands. Environ. Pollut. 2019, 249, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhao, Y.; Wang, C.; Zhang, H.; Chen, Q.; Zhang, X.; Zhang, L.; Wu, J.; Wu, Z.; Zhou, Q. Removal performance of antibiotics and antibiotic resistance genes in swine wastewater by integrated vertical-flow constructed wetlands with zeolite substrate. Sci. Total Environ. 2020, 721, 137765. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zheng, J.; Liu, C.; Liu, L.; Liu, Y.; Fan, H. Removal of antibiotics and resistance genes from swine wastewater using vertical flow constructed wetlands: Effect of hydraulic flow direction and substrate type. Chem. Eng. J. 2017, 308, 692–699. [Google Scholar] [CrossRef]
- Liu, L.; Liu, C.; Zheng, J.; Huang, X.; Wang, Z.; Liu, Y.; Zhu, G. Elimination of veterinary antibiotics and antibiotic resistance genes from swine wastewater in the vertical flow constructed wetlands. Chemosphere 2013, 91, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Valipour, A.; Ahn, Y.-H. Constructed wetlands as sustainable ecotechnologies in decentralization practices: A review. Environ. Sci. Pollut. Res. 2015, 23, 180–197. [Google Scholar] [CrossRef] [PubMed]
- Quiñónez-Dìaz, M.D.J.; Karpiscak, M.M.; Ellman, E.D.; Gerba, C.P. Removal of pathogenic and indicator microorganisms by a constructed wetland receiving untreated domestic wastewater. J. Environ. Sci. Health Part A 2001, 36, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Nnadozie, C.F.; Odume, O.N. Freshwater environments as reservoirs of antibiotic resistant bacteria and their role in the dissemination of antibiotic resistance genes. Environ. Pollut. 2019, 254, 113067. [Google Scholar] [CrossRef]
- Wu, S.; Carvalho, P.N.; Müller, J.A.; Manoj, V.R.; Dong, R. Sanitation in constructed wetlands: A review on the removal of human pathogens and fecal indicators. Sci. Total Environ. 2016, 541, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Li, Y.; Liu, X.; Xu, K. The tolerance mechanism and accumulation characteristics of Phragmites australis to sulfamethoxazole and ofloxacin. Chemosphere 2020, 253, 126695. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.-G.; Toze, S.; Hanna, J.; Yu, X.-Y.; Dillon, P.J.; Kookana, R.S. Decay of endocrine-disrupting chemicals in aerobic and anoxic groundwater. Water Res. 2008, 42, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhang, S.; Wang, P.; Wang, C. Effects of water flow on submerged macrophyte-biofilm systems in constructed wetlands. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Q1. Protocols for AR | Q2. Treatment of WW, Sludge and Discharge of Effluents | Q3. Use of Natural Treatment System |
|---|---|---|
| What are the protocols/interventions implemented in India for the control of AR in the environment? | What are the technologies that assist in reducing the load of pollutants from wastewater? Are they able to reduce antibiotics, ARB and ARG load? | Are CWs efficient enough for the control of antibiotic resistance from the effluent of treatment plants? How effective is it, and can they be implemented at a larger scale? |
| Source: | ||
| National action plan on antimicrobial resistance (2017) | WW and sludge generated through urban sectors in both liquid and solid state or a mixture of both Main pollutants: antibiotic drugs, ARG/ARB | Any water matrix contaminated through domestic and industrial wastewater, e.g., ponds, lakes, rivers, canals Main pollutant: antibiotic drugs, ARG/ARB |
| Primary objective: | ||
| Effective understanding of AMR through trainings, awareness and practice of health care in the community Optimize the use of antibiotics/antimicrobials in veterinary and human use through awareness, stewardship, and effective regulatory mechanisms for markets Promote innovations and research on AMR and collaborate with international organizations | Treatment of WW: primary, secondary and tertiary treatment through conventional methods in WWTP, as well as aerobic or anaerobic digestion Post-treatment of sludge: drying [50], vermicomposting [51,52,53], liming [54] | Use of NTS for efficient reduction of coliforms and AR from effluents of conventional treatment technologies [55] Various designs and configurations, use of substrates and plant species assist in better removal [56] |
| Pathways of exposure/disposal: | ||
| Veterinary and human uses, wastewater from domestic and industrial discharges, agricultural runoff | Treated effluents can be polished if CWs are coupled as tertiary units in WWTP [57] CW further assist in reducing the pollutant load of pathogens, bacteria, coliform, ARG/ARB [58] and other emergent pollutants like pesticides, antibiotics [59,60] | Disposal of treated effluent for multifarious uses such as reuse in toilets, aquaculture, gardening and agricultural purposes [61], direct discharge to water bodies, recharge to groundwater [62] |
| AR in the environment: Wastewater treatment plants, sludge, effluent discharges from pharmaceutical industries, domestic and other industrial wastewater, hospital discharges, agricultural runoff, poultry and veterinary wastes, swine and feedlot wastewater, soil and sediments | ||
| Types of recent studies in AR: Clinical studies: case studies on humans, animals, plants Environmental studies: laboratory and field-based studies on water, soil, agricultural farms, poultry, review of previous study and comparative study involving effects of anthropogenic activities | ||
| Type of CW | Source/Type of Wastewater | Antibiotic-Resistant Bacteria/Antibiotic-Resistant Genes | Removal Efficiency | References |
|---|---|---|---|---|
| Hybrid/integrated flow CW | Mixed wastewater from restaurant, hostel & brewery | E. coli and thermotolerant coliforms | 99.5% | [204] |
| Dairy wastewater | Total coliforms, fecal coliforms, fecal streptococci, and E. coli | 99.93–99.99% | [205] | |
| Raw domestic wastewater | sul2, ermB, cmlA and floR | 87.8% to 99.1% | [206] | |
| Domestic wastewater | Enterococci, HF183, intI1, and ermF | 84.0%, 66.6%, 67.2% & 13.1% | [195] | |
| Raw landfill leachate | Sulfonamide resistance (sul1, sul2, and dfrA), aminoglycoside resistance (aac6), tetracycline resistance (tetO), quinolone resistance (qnrA), and intl1, beta-lactam resistance (blaNDM1, blaKPC, and blaCTX) and macrolide-lincosamide resistance (ermB) | >90% | [32] | |
| Rural domestic wastewater | sul1, sul2, tetM, and tetO | >99% | [36] | |
| Vertical flow CW | Urban wastewater | sul1, sul2, qnrS, blaTEM, ermB | 46–97%, 33–97%, 9–99%, 18–97% & 11–98% | [41] |
| Swine wastewater | sul1, sul2, sul3, tetM and tetO | 95.1% | [207] | |
| Swine wastewater | Tetracycline-resistance genes (tet) and integrase gene of Class 1 integrons | 33.2 to 99.1%, | [208] | |
| Raw domestic wastewater | Sulfonamide resistance genes (sul1, sul2 and sul3), four tetracycline resistance genes (tetG, tetM, tetO and tetX), two macrolide resistance genes (ermB and ermC), two chloramphenicol resistance genes (cmlA and floR) and 16S rRNA (bacteria) | 63.9 and 84.0% | [203] | |
| Swine wastewater | Tetracycline resistance (tet) genes such as tetM, tetO, and tetW | Reduced by 50% | [209] | |
| Horizontal flow CW | Raw domestic wastewater | int1, tetM, sul1, ermB and total ARGs | 50.7–89.4%, 85.9–97%, 49.6–92.9%, 58.2–96.7% & 79.9–94.3% | [33] |
| Hospital wastewater | Tetracycline-, erythromycin- and ampicillin-resistant and higher multidrug-resistant bacteria | 80.8% to 93.2% | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazra, M.; Durso, L.M. Performance Efficiency of Conventional Treatment Plants and Constructed Wetlands towards Reduction of Antibiotic Resistance. Antibiotics 2022, 11, 114. https://doi.org/10.3390/antibiotics11010114
Hazra M, Durso LM. Performance Efficiency of Conventional Treatment Plants and Constructed Wetlands towards Reduction of Antibiotic Resistance. Antibiotics. 2022; 11(1):114. https://doi.org/10.3390/antibiotics11010114
Chicago/Turabian StyleHazra, Moushumi, and Lisa M. Durso. 2022. "Performance Efficiency of Conventional Treatment Plants and Constructed Wetlands towards Reduction of Antibiotic Resistance" Antibiotics 11, no. 1: 114. https://doi.org/10.3390/antibiotics11010114
APA StyleHazra, M., & Durso, L. M. (2022). Performance Efficiency of Conventional Treatment Plants and Constructed Wetlands towards Reduction of Antibiotic Resistance. Antibiotics, 11(1), 114. https://doi.org/10.3390/antibiotics11010114