Colistin Dosing Regimens against Pseudomonas aeruginosa in Critically Ill Patients: An Application of Monte Carlo Simulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbiology
2.2. Pharmacokinetic Parameters
2.3. PK/PD Index and PDT
2.4. Monte Carlo Simulation
3. Results
3.1. Microbiology
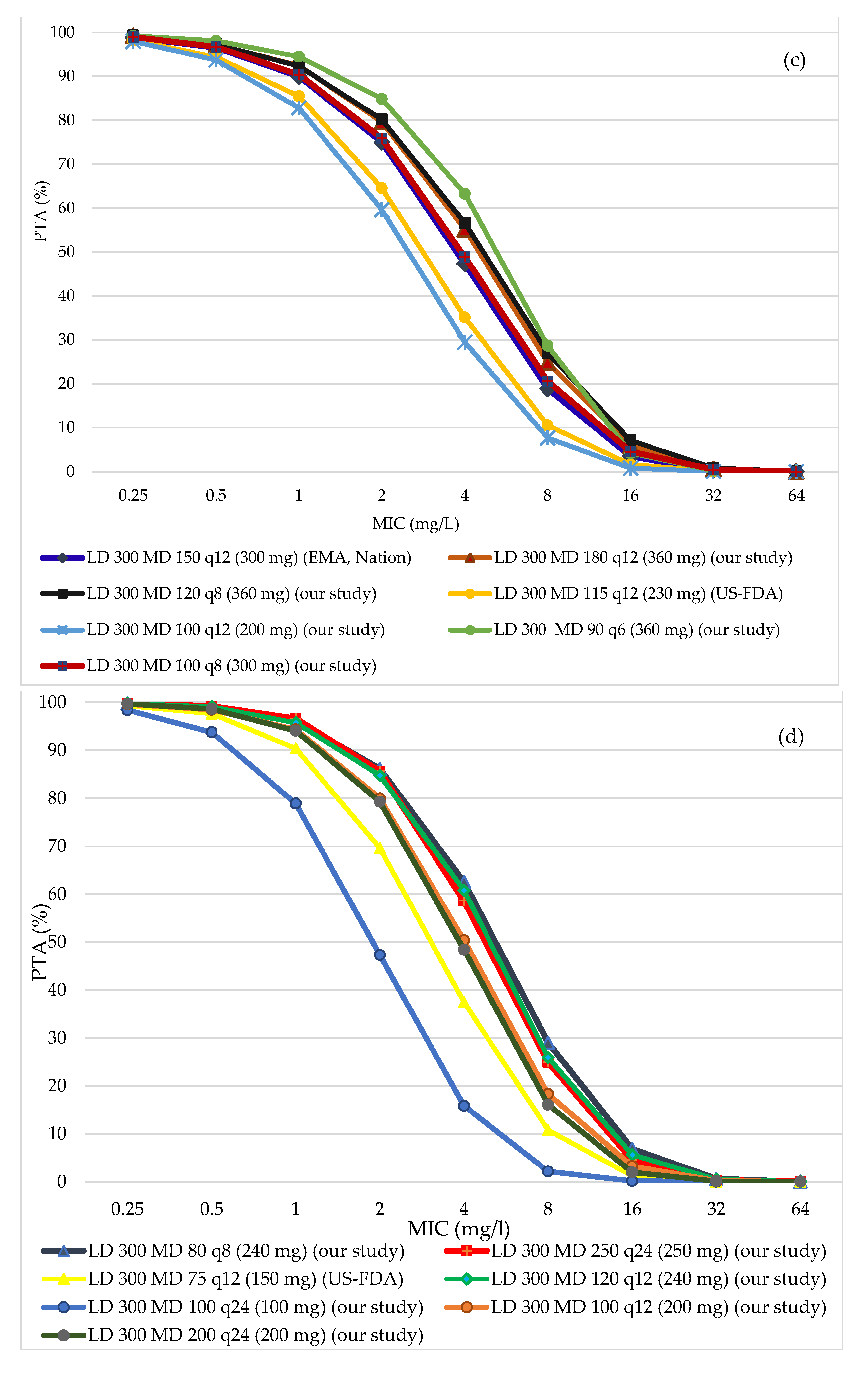
3.2. PTA of All Regimens in Different Ranges of Creatinine Clearance (CrCl)
3.3. CFR and Nephrotoxicity Risk
3.4. The Recommendation of Our Study
4. Discussion
Limitation of Our Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- National Nosocomial Infections Surveillance. System Report, Data Summary from January 1992 through June 2004, Issued October 2004. Am. J. Infect. Control 2004, 32, 470–485. [Google Scholar] [CrossRef]
- Park, S.-Y.; Park, H.J.; Moon, S.M.; Park, K.-H.; Chong, Y.P.; Kim, M.-N.; Kim, S.-H.; Lee, S.-O.; Kim, Y.S.; Woo, J.H. Impact of adequate empirical combination therapy on mortality from bacteremic pseudomonas aeruginosa pneumonia. BMC Infect. Dis. 2012, 12, 308. [Google Scholar] [CrossRef]
- Peña, C.; Cabot, G.; Gómez-Zorrilla, S.; Zamorano, L.; Ocampo-Sosa, A.; Murillas, J.; Almirante, B.; Pomar, V.; Aguilar, M.; Granados, A. Influence of virulence genotype and resistance profile in the mortality of pseudomonas aeruginosa bloodstream infections. Clin. Infect. Dis. 2014, 60, 539–548. [Google Scholar] [CrossRef]
- Lambert, M.-L.; Suetens, C.; Savey, A.; Palomar, M.; Hiesmayr, M.; Morales, I.; Agodi, A.; Frank, U.; Mertens, K.; Schumacher, M. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to european intensive-care units: A cohort study. Lancet Infect. Dis. 2011, 11, 30–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.-L.; Huang, A.-W.; Liu, S.-L.; Liu, W.-J.; Zhang, N.; Lu, X.-Z. Mortality attributable to carbapenem-resistant pseudomonas aeruginosa bacteremia: A meta-analysis of cohort studies. Emerg. Microbes Infect. 2016, 5, e27. [Google Scholar] [CrossRef]
- Matos, E.C.O.D.; Andriolo, R.B.; Rodrigues, Y.C.; Lima, P.D.L.D.; Carneiro, I.C.D.R.S.; Lima, K.V.B. Mortality in patients with multidrug-resistant pseudomonas aeruginosa infections: A meta-analysis. Rev. Soc. Bras. Med. Trop. 2018, 51, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Tabak, Y.P.; Merchant, S.; Ye, G.; Vankeepuram, L.; Gupta, V.; Kurtz, S.G.; Puzniak, L.A. Incremental clinical and economic burden of suspected respiratory infections due to multi-drug-resistant pseudomonas aeruginosa in the united states. J. Hosp. Infect. 2019, 103, 134–141. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2013. Available online: www.cdc.gov/drugresistance/threat-report-2013 (accessed on 22 May 2020).
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2019. Available online: www.cdc.gov/DrugResistance/Biggest-Threats.html (accessed on 22 May 2020).
- Li, J.; Nation, R.L.; Turnidge, J.D.; Milne, R.W.; Coulthard, K.; Rayner, C.R.; Paterson, D.L. Colistin: The re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect. Dis. 2006, 6, 589–601. [Google Scholar] [CrossRef]
- Biedenbach, D.J.; Giao, P.T.; Hung Van, P.; Su Minh Tuyet, N.; Thi Thanh Nga, T.; Phuong, D.M.; Vu Trung, N.; Badal, R.E. Antimicrobial-resistant pseudomonas aeruginosa and acinetobacter baumannii from patients with hospital-acquired or ventilator-associated pneumonia in vietnam. CLIN 2016, 38, 2098–2105. [Google Scholar] [CrossRef]
- Phu, V.D.; Wertheim, H.F.; Larsson, M.; Nadjm, B.; Dinh, Q.D.; Nilsson, L.E.; Rydell, U.; Le, T.T.; Trinh, S.H.; Pham, H.M.; et al. Burden of hospital acquired infections and antimicrobial use in vietnamese adult intensive care units. PLoS ONE 2016, 11, e0147544. [Google Scholar] [CrossRef]
- Tran, G.M.; Ho-Le, T.P.; Ha, D.T.; Tran-Nguyen, C.H.; Nguyen, T.S.M.; Pham, T.T.N.; Nguyen, T.A.; Nguyen, D.A.; Hoang, H.Q.; Tran, N.V.; et al. Patterns of antimicrobial resistance in intensive care unit patients: A study in vietnam. BMC Infect. Dis. 2017, 17, 429. [Google Scholar] [CrossRef]
- Ngoc Van, T.T.; Quang-Thinh, T.; Cù, T.; Nhac Vu, H.T. Investigation of the antibiotic resistance: The case of buu dien general hospital in ho chi minh city. Int J Pharm Pharm Sci 2019, 9, 116–119. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of Mics and Zone Diameters. Version 10.0. 2020. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 22 January 2021).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement m100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Nation, R.L.; Garonzik, S.M.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Forrest, A.; Paterson, D.L.; Li, J.; Silveira, F.P. Dosing guidance for intravenous colistin in critically-ill patients. Clin. Infect. Dis. 2017, 64, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Plachouras, D.; Karvanen, M.; Friberg, L.E.; Papadomichelakis, E.; Antoniadou, A.; Tsangaris, I.; Karaiskos, I.; Poulakou, G.; Kontopidou, F.; Armaganidis, A.; et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob. Agents Chemother. 2009, 53, 3430–3436. [Google Scholar] [CrossRef] [PubMed]
- Garonzik, S.; Li, J.; Thamlikitkul, V.; Paterson, D.; Shoham, S.; Jacob, J.; Silveira, F.; Forrest, A.; Nation, R. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 2011, 55, 3284–3294. [Google Scholar] [CrossRef]
- Cheah, S.E.; Wang, J.; Nguyen, V.T.; Turnidge, J.D.; Li, J.; Nation, R.L. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against pseudomonas aeruginosa and acinetobacter baumannii in mouse thigh and lung infection models: Smaller response in lung infection. J. Antimicrob. Chemother. 2015, 70, 3291–3297. [Google Scholar]
- Jaruratanasirikul, S.; Thengyai, S.; Wongpoowarak, W.; Wattanavijitkul, T.; Tangkitwanitjaroen, K.; Sukarnjanaset, W.; Jullangkoon, M.; Samaeng, M. Population pharmacokinetics and monte carlo dosing simulations of meropenem during the early phase of severe sepsis and septic shock in critically ill patients in intensive care units. Antimicrob. Agents Chemother. 2015, 59, 2995–3001. [Google Scholar] [CrossRef]
- Wicha, S.G.; Haak, T.; Zink, K.; Kees, F.; Kloft, C.; Kees, M.G. Population pharmacokinetics and target attainment analysis of moxifloxacin in patients with diabetic foot infections. J. Clin. Pharmacol. 2015, 55, 639–646. [Google Scholar] [CrossRef]
- Canut, A.; Isla, A.; Rodríguez-Gascón, A. Pharmacokinetic/pharmacodynamic analysis to evaluate ceftaroline fosamil dosing regimens for the treatment of community-acquired bacterial pneumonia and complicated skin and skin-structure infections in patients with normal and impaired renal function. Int. J. Antimicrob. Agents 2015, 45, 399–405. [Google Scholar] [CrossRef]
- Sorli, L.; Luque, S.; Grau, S.; Berenguer, N.; Segura, C.; Montero, M.M.; Alvarez-Lerma, F.; Knobel, H.; Benito, N.; Horcajada, J.P. Trough colistin plasma level is an independent risk factor for nephrotoxicity: A prospective observational cohort study. BMC Infect. Dis. 2013, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Fda Approved Drug Products Label and Approval History for Coly-Mycin m, nda 050108. Available online: https://www.Accessdata.Fda.Gov/scripts/cder/daf/index.Cfm?Event=overview.Process&applno=050108 (accessed on 1 May 2021).
- European Medicines Agency. Polymyxin-containing Medicines. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/polymyxin-containing-medicines (accessed on 1 May 2021).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Palavutitotai, N.; Jitmuang, A.; Tongsai, S.; Kiratisin, P.; Angkasekwinai, N. Epidemiology and risk factors of extensively drug-resistant pseudomonas aeruginosa infections. PLoS ONE 2018, 13, e0193431. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.V.; Thi Do, N.T.; Chandna, A.; Nguyen, T.V.; Pham, C.V.; Doan, P.M.; Nguyen, A.Q.; Thi Nguyen, C.K.; Larsson, M.; Escalante, S.; et al. Antibiotic use and resistance in emerging economies: A situation analysis for viet nam. BMC Public Health 2013, 13, 1158. [Google Scholar] [CrossRef] [PubMed]
- Jitaree, K.; Sathirakul, K.; Houngsaitong, J.; Asuphon, O.; Saelim, W.; Thamlikitkul, V.; Montakantikul, P. Pharmacokinetic/pharmacodynamic (pk/pd) simulation for dosage optimization of colistin against carbapenem-resistant klebsiella pneumoniae and carbapenem-resistant escherichia coli. Antibiotics 2019, 8, 125. [Google Scholar] [CrossRef]
- Gregoire, N.; Mimoz, O.; Megarbane, B.; Comets, E.; Chatelier, D.; Lasocki, S.; Gauzit, R.; Balayn, D.; Gobin, P.; Marchand, S.; et al. New colistin population pharmacokinetic data in critically ill patients suggesting an alternative loading dose rationale. Antimicrob. Agents Chemother. 2014, 58, 7324–7330. [Google Scholar] [CrossRef] [PubMed]
- Nation, R.L.; Garonzik, S.M.; Li, J.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Paterson, D.L.; Turnidge, J.D.; Forrest, A.; Silveira, F.P. Updated us and european dose recommendations for intravenous colistin: How do they perform? Clin. Infect. Dis. 2016, 62, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Wi, Y.M.; Kwon, Y.J.; Kim, S.R.; Chang, S.H.; Cho, S. Association between colistin dose and development of nephrotoxicity. Crit. Care Med. 2015, 43, 1187–1193. [Google Scholar] [CrossRef]
- Forrest, A.; Garonzik, S.M.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Paterson, D.L.; Li, J.; Silveira, F.P.; Nation, R.L. Pharmacokinetic/toxicodynamic analysis of colistin-associated acute kidney injury in critically ill patients. Antimicrob. Agents Chemother. 2017, 61, e01367-17. [Google Scholar] [CrossRef] [PubMed]
- Miano, T.A.; Lautenbach, E.; Wilson, F.P.; Guo, W.; Borovskiy, Y.; Hennessy, S. Attributable risk and time course of colistin-associated acute kidney injury. Clin. J. Am. Soc. Nephrol. 2018, 13, 542–550. [Google Scholar] [CrossRef]
- Smith, D.A.; Beaumont, K.; Maurer, T.S.; Di, L. Relevance of half-life in drug design. J. Med. Chem. 2018, 61, 4273–4282. [Google Scholar] [CrossRef]
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; et al. International consensus guidelines for the optimal use of the polymyxins: Endorsed by the american college of clinical pharmacy (accp), european society of clinical microbiology and infectious diseases (escmid), infectious diseases society of america (idsa), international society for anti-infective pharmacology (isap), society of critical care medicine (sccm), and society of infectious diseases pharmacists (sidp). Pharmacotherapy 2019, 39, 10–39. [Google Scholar] [PubMed]
- Kelesidis, T.; Falagas, M.E. The safety of polymyxin antibiotics. Expert Opin. Drug Saf. 2015, 14, 1687–1701. [Google Scholar] [CrossRef] [PubMed]
- Rigatto, M.H.; Behle, T.F.; Falci, D.R.; Freitas, T.; Lopes, N.T.; Nunes, M.; Costa, L.W.; Zavascki, A.P. Risk factors for acute kidney injury (aki) in patients treated with polymyxin b and influence of aki on mortality: A multicentre prospective cohort study. J. Antimicrob. Chemother. 2015, 70, 1552–1557. [Google Scholar] [CrossRef] [PubMed]



| Parameters | CMS (SD) | Parameters | Colistin (SD) |
|---|---|---|---|
| V1 (L) | 12.9 (5.2116) | V3/fm (L) | 57.2 (24.882) |
| V2 (L) | 16.1 (11.4149) | CLRCsl-pop/fm (L/h/CrCl) | 0.00834 |
| CLD1 (L/h) | 9.57 (7.66557) | CLNRc/fm (L/h) | 3.11 |
| CLRslope (L/h/CrCl) | 0.034 (0.02557) | fu | 0.49 (0.11) |
| CLNRcms (L/h) | 2.52 (1.00296) |
| Renal Function a | LD b | MD b | Regimen |
|---|---|---|---|
| 101–130 mL/min | 300 mg | 150 mg Q12h | US-FDA, EMA |
| 180 mg Q12h | Nation | ||
| 100 mg Q6h | Proposed | ||
| 120 mg Q6h | Proposed | ||
| 200 mg Q12h | Proposed | ||
| 100 mg Q4h | Proposed | ||
| 150 mg Q6h | Proposed | ||
| 80–100 mL/min | 300 mg | 150 mg Q12h | US-FDA, EMA |
| 180 mg Q12h | Nation | ||
| 150 mg Q8h | Proposed | ||
| 100 mg Q6h | Proposed | ||
| 120 mg Q6h | Proposed | ||
| 100 mg Q8h | Proposed | ||
| 51–79 mL/min | 300 mg | 150 mg Q12h | EMA, Nation |
| 115 mg Q12h | US-FDA | ||
| 180 mg Q12h | Proposed | ||
| 120 mg Q8h | Proposed | ||
| 100 mg Q8h 100 mg Q12h | Proposed Proposed | ||
| 30–50 mL/min | 300 mg | 75 mg Q12h | US-FDA |
| 125 mg Q12h | EMA | ||
| 110 mg Q12h | Nation | ||
| 250 mg Q24h 200 mg Q24h 100 mg Q12h | Proposed Proposed Proposed | ||
| 80 mg Q8h | Proposed | ||
| 120 mg Q12h | Proposed | ||
| 100 mg Q24h | Proposed | ||
| 11–29 mL/min | 300 mg | 90 mg Q36h | US-FDA |
| 90 mg Q12h | EMA | ||
| 80 mg Q12h | Nation | ||
| 150 mg Q24h | Proposed | ||
| 200 mg Q36h | Proposed | ||
| 120 mg Q24h 100 mg Q24h | Proposed Proposed | ||
| 60 mg Q12h | Proposed | ||
| 66 mg Q24h | Proposed | ||
| 1–10 mL/min | 300 mg | 60 mg Q12h | EMA |
| 70 mg Q12h | Nation | ||
| 80 mg Q24h 66 mg Q24h | Proposed Proposed | ||
| 33 mg Q24h | Proposed | ||
| 40 mg Q12h | Proposed |
| Total Included Isolates (n = 121) | Number (n) | Percentage (%) |
|---|---|---|
| Source of samples | ||
| Blood | 5 | 4.1 |
| Urine | 22 | 18.2 |
| Respiratory tract | 46 | 38 |
| Others (skin, body fluid, etc.) | 48 | 39.7 |
| Resistance pattern (antibiogram) | ||
| No resistance | 38 | 31.4 |
| Resistant to Ceftazidime | 25 | 20.7 |
| Resistant to Cefepime | 27 | 22.3 |
| Resistant to Ticarcilline/Clavulanic | 68 | 56.2 |
| Resistant to Piperacillin/Tazobactam | 35 | 28.9 |
| Resistant to Carbapenem | 35 | 28.9 |
| Resistant to Aminoglycoside | 30 | 24.8 |
| Resistant to Flouroquinolone | 39 | 32.2 |
| Resistant to Colistin | 1 | 0.8 |
| Multi-drug resistant | 30 | 24.8 |
| CrCl (mL/min) | Regimens (TDDs*) | PTA (%) | CFR (%) | AKI Risk (%) | |||
|---|---|---|---|---|---|---|---|
| MIC 0.5 mg/L (MIC90) | MIC 2 mg/L | EOT | D7 | ||||
| 101–130 | 150 mg Q12h (300 mg) | US-FDA, EMA | 83.65 | 43.75 | 82.82 | 24.63 | 16.75 |
| 180 mg Q12h (360 mg) | Nation | 87.1 | 49.99 | 86.26 | 29.23 | 20.17 | |
| 200 mg Q12h (400 mg) | Our study | 88.91 | 55.05 | 88.07 | 33.01 | 23.14 | |
| 100 mg Q6h (400 mg) | Our study | 90.93 | 60.55 | 90.09 | 43.38 | 30.33 | |
| 120 mg Q6h (480 mg) | Our study | 93.68 | 63.27 | 92.82 | 51.31 | 38.08 | |
| 100 mg Q4h (600 mg) | Our study | 94.6 | 74.72 | 93.77 | 62.77 | 50.51 | |
| 150 mg Q6h (600 mg) | Our study | 96.62 | 74.6 | 93.78 | 60.65 | 48.24 | |
| 80–100 | 150 mg Q12h (300 mg) | US-FDA, EMA | 91.32 | 57.85 | 90.47 | 36.59 | 26.24 |
| 180 mg Q12h (360 mg) | Nation | 93.37 | 64.36 | 92.52 | 42.58 | 31.83 | |
| 150 mg Q8h (450 mg) | Our study | 94.96 | 72.07 | 94.12 | 56.45 | 45.01 | |
| 100 mg Q6h (400 mg) | Our study | 95.12 | 73.69 | 94.28 | 59.45 | 46.34 | |
| 120 mg Q6h (480 mg) | Our study | 96.42 | 79.23 | 95.58 | 66.74 | 54.62 | |
| 100 mg Q8h (300 mg) | Our study | 91.79 | 60.15 | 90.94 | 42.57 | 30.66 | |
| 51–79 | 115 mg Q12h (230 mg) | US-FDA | 94.38 | 64.52 | 93.53 | 42.93 | 30.9 |
| 150 mg Q12h (300 mg) | EMA, Nation | 96.52 | 75.02 | 95.67 | 54 | 42 | |
| 180 mg Q12h (360 mg) | Our study | 97.21 | 79.6 | 96.37 | 59.64 | 48.22 | |
| 120 mg Q8h (360 mg) | Our study | 97.3 | 80.15 | 96.46 | 65.75 | 54.22 | |
| 100 mg Q8h (300 mg) | Our study | 96.72 | 75.88 | 95.87 | 59.81 | 46.89 | |
| 100 mg Q12h (200 mg) | Our study | 93.7 | 59.61 | 92.84 | 38.44 | 26 | |
| 30–50 | 75 mg Q12h (150 mg) | US-FDA | 97.66 | 69.6 | 96.79 | 48.2 | 34.7 |
| 125 mg Q12h (250 mg) | EMA | 99.18 | 86.35 | 98.34 | 68.73 | 56.18 | |
| 110 mg Q12h (220 mg)) | Nation | 98.71 | 83.11 | 97.86 | 63.45 | 50.74 | |
| 120 mg Q12h (240 mg) | Our study | 99.07 | 84.77 | 98.23 | 67.98 | 54.81 | |
| 80 mg Q8h (240 mg) | Our study | 98.95 | 86.15 | 98.11 | 73.17 | 61.38 | |
| 100 mg Q12h (200 mg) | Our study | 98.48 | 79.97 | 97.63 | 60.15 | 46.86 | |
| 200 mg Q24h (200 mg) | Our study | 98.62 | 79.26 | 97.77 | 41.24 | 30.2 | |
| 100 mg Q24h (100 mg) | Our study | 93.77 | 47.3 | 92.87 | 19.07 | 11.46 | |
| 250 mg Q24h (250 mg) | Our study | 99.18 | 85.6 | 98.34 | 47.49 | 37.15 | |
| 11–29 | 90 mg Q36h ((60 mg) | US-FDA | 97.01 | 39.17 | 96.06 | 11.51 | 5.79 |
| 90 mg Q12h (180 mg) | EMA | 99.77 | 92.21 | 98.94 | 78.29 | 66.68 | |
| 80 mg Q12h (160 mg) | Nation | 99.71 | 90.59 | 98.88 | 74.87 | 62.55 | |
| 120 mg Q24h (120 mg) | Our study | 99.39 | 81.28 | 98.54 | 45.21 | 33.16 | |
| 60 mg Q12h (120 mg) | Our study | 99.54 | 82.8 | 98.69 | 63.74 | 48.63 | |
| 66 mg Q24h (66 mg) | Our study | 97.33 | 52.72 | 96.42 | 23.17 | 13.08 | |
| 100 mg Q24h (100 mg) | Our study | 98.98 | 74.37 | 98.11 | 39.51 | 26.54 | |
| 150 mg Q24h (150 mg) | Our study | 99.76 | 88.35 | 98.92 | 53.79 | 41.79 | |
| 200 mg Q36h (133 mg) | Our study | 99.78 | 83.31 | 98.94 | 32.3 | 23.16 | |
| 1–10 | 60 mg Q12h (120 mg) | EMA | 99.96 | 95.24 | 99.14 | 83.68 | 72.27 |
| 70 mg Q12h (140 mg) | Nation | 99.97 | 95 | 99.15 | 87.61 | 78.67 | |
| 80 mg Q24h (80 mg) | Our study | 99.85 | 84.16 | 99 | 52.76 | 39.14 | |
| 40 mg Q12h (80 mg) | Our study | 99.77 | 85.99 | 98.93 | 68.95 | 53.11 | |
| 66 mg Q24h (66 mg) | Our study | 99.6 | 76.33 | 98.74 | 45.19 | 32.46 | |
| 33 mg Q24h (33 mg) | Our study | 95.6 | 40.12 | 94.65 | 17.79 | 9.52 | |
| CrCl (mL/min) | Regimens (TDDs*) | PTA (%) | CFR (%) | AKI Risk (%) | Alternative Regimens (TDDs*) | PTA (%) | CFR (%) | AKI Risk (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| EOT | D7 | EOT | D7 | |||||||
| 101–130 | 100 mg Q6h (400 mg) | 90.93 | 90.09 | 43.38 | 30.33 | |||||
| 80–100 | 100 mg Q8h (300 mg) | 91.79 | 90.94 | 42.57 | 30.66 | 150 mg Q12h (300 mg) (US-FDA) | 91.32 | 90.47 | 36.59 | 26.24 |
| 51–79 | 100 mg Q12h (200 mg) | 93.7 | 92.84 | 38.44 | 26 | |||||
| 30–50 | 100 mg Q24h (100 mg) | 93.77 | 92.87 | 19.07 | 11.46 | |||||
| 11–29 | 66 mg Q24h (66 mg) | 97.33 | 96.42 | 23.17 | 13.08 | |||||
| 1–10 | 33 mg Q24h (33 mg) | 95.6 | 94.65 | 17.79 | 9.52 | |||||
| CrCl (mL/min) | Regimens (TDDs) | PTA (%) | AKI Risk (%) | Alternative Regimens (TDDs) | PTA (%) | AKI Risk (%) | ||
|---|---|---|---|---|---|---|---|---|
| EOT | D7 | EOT | D7 | |||||
| 101–130 | 120 mg Q6h (480 mg) | 84.19 | 43.09 | 29.18 | ||||
| 80–100 | 100 mg Q6 (400 mg) | 88.16 | 59.45 | 46.34 | ||||
| 51–79 | 100 mg Q8h (300 mg) | 90.4 | 59.81 | 46.89 | 150 mg Q12h (300 mg) | 89.99 | 54 | 42 |
| 30–50 | 100 mg Q12 (200 mg) | 94.35 | 60.15 | 46.86 | 200 mg Q24h (200 mg) | 94.08 | 41.24 | 30.2 |
| 11–29 | 100 mg Q24 (100 mg) | 93.85 | 39.51 | 26.54 | ||||
| 1–10 | 66 mg Q24h (66 mg) | 95.6 | 45.19 | 32.46 | ||||
| CrCl (mL/min) | Regimens (TDDs) | PTA (%) | AKI Risk (%) | Alternative Regimens (TDDs) | PTA (%) | AKI Risk (%) | ||
|---|---|---|---|---|---|---|---|---|
| EOT | D7 | EOT | D7 | |||||
| 101–130 | Not recommend | |||||||
| 80–100 | 120 mg Q6h (480 mg) | 79.23 | 66.74 | 54.62 | ||||
| 51–79 | 120 mg Q8h (360 mg) | 80.15 | 65.75 | 54.22 | 180 mg Q12h (360 mg) | 79.6 | 59.64 | 48.22 |
| 30–50 | 120 mg Q12h (240 mg) | 84.77 | 67.98 | 54.81 | 250 mg Q24h (250 mg) | 85.6 | 47.49 | 37.15 |
| 11–29 | 120 mg Q24h (120 mg) | 81.28 | 45.21 | 33.16 | ||||
| 1–10 | 80 mg Q24h (80 mg) | 84.16 | 52.76 | 39.14 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.T.K.; Montakantikul, P.; Tragulpiankit, P.; Houngsaitong, J.; Shuib, M.F. Colistin Dosing Regimens against Pseudomonas aeruginosa in Critically Ill Patients: An Application of Monte Carlo Simulation. Antibiotics 2021, 10, 595. https://doi.org/10.3390/antibiotics10050595
Nguyen VTK, Montakantikul P, Tragulpiankit P, Houngsaitong J, Shuib MF. Colistin Dosing Regimens against Pseudomonas aeruginosa in Critically Ill Patients: An Application of Monte Carlo Simulation. Antibiotics. 2021; 10(5):595. https://doi.org/10.3390/antibiotics10050595
Chicago/Turabian StyleNguyen, Van Thi Khanh, Preecha Montakantikul, Pramote Tragulpiankit, Jantana Houngsaitong, and Mohd Fazli Shuib. 2021. "Colistin Dosing Regimens against Pseudomonas aeruginosa in Critically Ill Patients: An Application of Monte Carlo Simulation" Antibiotics 10, no. 5: 595. https://doi.org/10.3390/antibiotics10050595
APA StyleNguyen, V. T. K., Montakantikul, P., Tragulpiankit, P., Houngsaitong, J., & Shuib, M. F. (2021). Colistin Dosing Regimens against Pseudomonas aeruginosa in Critically Ill Patients: An Application of Monte Carlo Simulation. Antibiotics, 10(5), 595. https://doi.org/10.3390/antibiotics10050595





