Presepsin (Soluble CD14 Subtype) as an Early Marker of Neonatal Sepsis and Septic Shock: A Prospective Diagnostic Trial
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics and Kinetics of Biomarkers
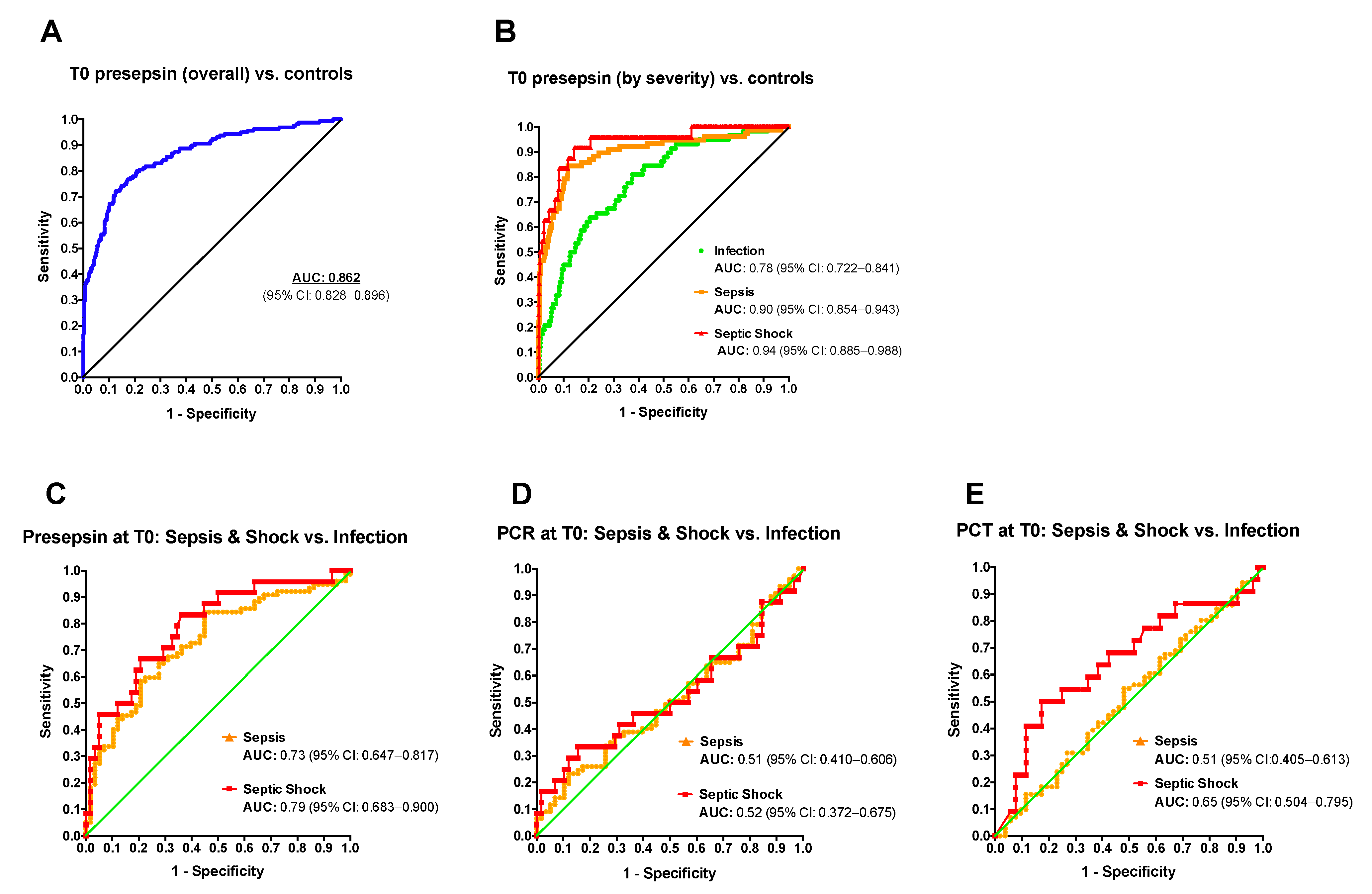
2.2. Diagnostic Accuracy of Presepsin for Neonatal Sepsis
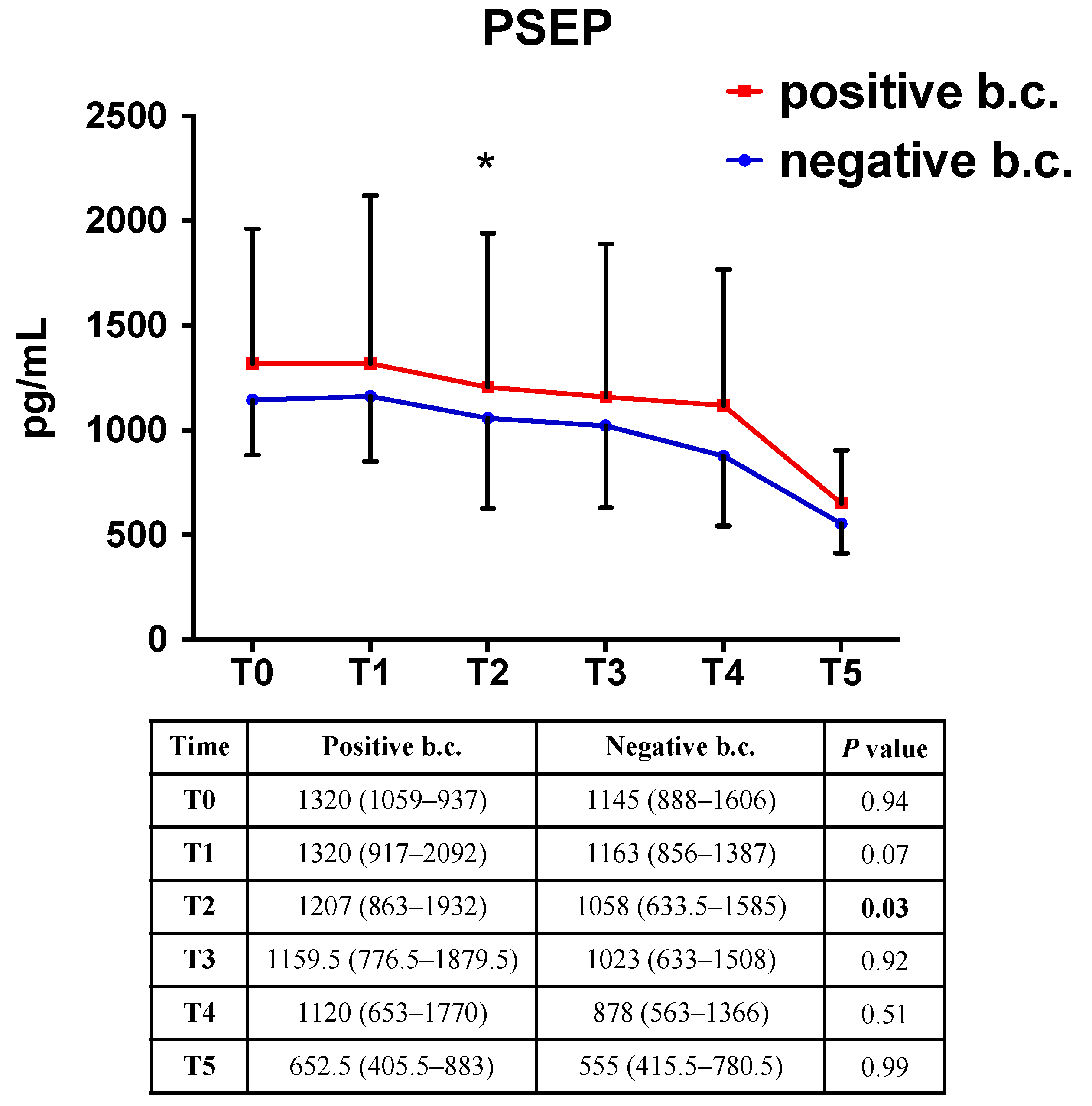
2.3. Presepsin Correlation with Positive Blood Culture
3. Discussion
4. Materials and Methods
4.1. Study Design and Inclusion Criteria
4.2. Definitions
4.3. Collection of Samples and Measurement of Biomarkers
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shane, A.L.; Sanchez, P.J.; Stoll, B.J. Neonatal sepsis. Lancet 2017, 390, 1770–1780. [Google Scholar] [CrossRef]
- Pugni, L.; Ronchi, A.; Bizzarri, B.; Consonni, D.; Pietrasanta, C.; Ghirardi, B.; Fumagalli, M.; Ghirardello, S.; Mosca, F. Exchange Transfusion in the Treatment of Neonatal Septic Shock: A Ten-Year Experience in a Neonatal Intensive Care Unit. Int. J. Mol. Sci. 2016, 17, 695. [Google Scholar] [CrossRef] [PubMed]
- Kermorvant-Duchemin, E.; Laborie, S.; Rabilloud, M.; Lapillonne, A.; Claris, O. Outcome and prognostic factors in neonates with septic shock. Pediatr. Crit. Care Med. 2008, 9, 186–191. [Google Scholar] [CrossRef]
- Mwaniki, M.K.; Atieno, M.; Lawn, J.E.; Newton, C.R. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: A systematic review. Lancet 2012, 379, 445–452. [Google Scholar] [CrossRef]
- Han, Y.Y.; Carcillo, J.A.; Dragotta, M.A.; Bills, D.M.; Watson, R.S.; Westerman, M.E.; Orr, R.A. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics 2003, 112, 793–799. [Google Scholar] [CrossRef]
- Wynn, J.L.; Wong, H.R. Pathophysiology and treatment of septic shock in neonates. Clin. Perinatol. 2010, 37, 439–479. [Google Scholar] [CrossRef]
- Wynn, J.L.; Wong, H.R.; Shanley, T.P.; Bizzarro, M.J.; Saiman, L.; Polin, R.A. Time for a neonatal-specific consensus definition for sepsis. Pediatr. Crit. Care Med. 2014, 15, 523–528. [Google Scholar] [CrossRef]
- Kellogg, J.A.; Manzella, J.P.; Bankert, D.A. Frequency of low-level bacteremia in children from birth to fifteen years of age. J. Clin. Microbiol. 2000, 38, 2181–2185. [Google Scholar] [CrossRef]
- Sharma, D.; Farahbakhsh, N.; Shastri, S.; Sharma, P. Biomarkers for diagnosis of neonatal sepsis: A literature review. J. Matern. Fetal Neonatal Med. 2018, 31, 1646–1659. [Google Scholar] [CrossRef]
- Zou, Q.; Wen, W.; Zhang, X.C. Presepsin as a novel sepsis biomarker. World J. Emerg. Med. 2014, 5, 16–19. [Google Scholar] [CrossRef]
- Ruan, L.; Chen, G.Y.; Liu, Z.; Zhao, Y.; Xu, G.Y.; Li, S.F.; Li, C.N.; Chen, L.S.; Tao, Z. The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: A meta-analysis and systematic review. Crit. Care 2018, 22, 316. [Google Scholar] [CrossRef] [PubMed]
- Bellos, I.; Fitrou, G.; Pergialiotis, V.; Thomakos, N.; Perrea, D.N.; Daskalakis, G. The diagnostic accuracy of presepsin in neonatal sepsis: A meta-analysis. Eur. J. Pediatr. 2018, 177, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Parri, N.; Trippella, G.; Lisi, C.; De Martino, M.; Galli, L.; Chiappini, E. Accuracy of presepsin in neonatal sepsis: Systematic review and meta-analysis. Expert Rev. Anti Infect. Ther. 2019, 17, 223–232. [Google Scholar] [CrossRef]
- van Maldeghem, I.; Nusman, C.M.; Visser, D.H. Soluble CD14 subtype (sCD14-ST) as biomarker in neonatal early-onset sepsis and late-onset sepsis: A systematic review and meta-analysis. BMC Immunol. 2019, 20, 17. [Google Scholar] [CrossRef]
- Pugni, L.; Pietrasanta, C.; Milani, S.; Vener, C.; Ronchi, A.; Falbo, M.; Arghittu, M.; Mosca, F. Presepsin (Soluble CD14 Subtype): Reference Ranges of a New Sepsis Marker in Term and Preterm Neonates. PLoS ONE 2015, 10, e0146020. [Google Scholar] [CrossRef]
- Poggi, C.; Vasarri, M.V.; Boni, L.; Pugni, L.; Mosca, F.; Dani, C. Reference ranges of Presepsin in preterm infants in the first 48 h of life: A multicenter observational study. Clin. Chim. Acta 2020, 508, 191–196. [Google Scholar] [CrossRef]
- Cotten, C.M.; Taylor, S.; Stoll, B.; Goldberg, R.N.; Hansen, N.I.; Sanchez, P.J.; Ambalavanan, N.; Benjamin, D.K.; NICHD Neonatal Research Network. Prolonged Duration of Initial Empirical Antibiotic Treatment Is Associated With Increased Rates of Necrotizing Enterocolitis and Death for Extremely Low Birth Weight Infants. Pediatrics 2009, 123, 58–66. [Google Scholar] [CrossRef]
- Alexander, V.N.; Northrup, V.; Bizzarro, M.J. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J. Pediatr. 2011, 159, 392–397. [Google Scholar] [CrossRef]
- Shozushima, T.; Takahashi, G.; Matsumoto, N.; Kojika, M.; Okamura, Y.; Endo, S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J. Infect. Chemother. 2011, 17, 764–769. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Y.X.; Yin, Q.; Zhao, Y.Z.; Li, C.S. Diagnostic value and prognostic evaluation of Presepsin for sepsis in an emergency department. Crit. Care 2013, 17, R244. [Google Scholar] [CrossRef]
- Kweon, O.J.; Choi, J.H.; Park, S.K.; Park, A.J. Usefulness of presepsin (sCD14 subtype) measurements as a new marker for the diagnosis and prediction of disease severity of sepsis in the Korean population. J. Crit. Care 2014, 29, 965–970. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nishimura, T.; Kaga, S.; Uchida, K.; Tachibana, Y.; Esaki, M.; Fukushima, W.; Kondo, K.; Mizobata, Y. Diagnostic accuracy of presepsin for sepsis by the new Sepsis-3 definitions. Am. J. Emerg. Med. 2019, 37, 1936–1941. [Google Scholar] [CrossRef]
- Poggi, C.; Bianconi, T.; Gozzini, E.; Generoso, M.; Dani, C. Presepsin for the detection of late-onset sepsis in preterm newborns. Pediatrics 2015, 135, 68–75. [Google Scholar] [CrossRef]
- Gad, G.I.; Shinkar, D.M.; El-Din, M.M.K.; Nagi, H.M. The Utility of Soluble CD14 Subtype in Early Diagnosis of Culture-Proven Early-Onset Neonatal Sepsis and Prediction of Outcome. Am. J. Perinatol. 2020, 37, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, A.; Arthamin, M.Z.; Indriana, K.; Anshory, M.; Hur, M.; Di Somma, S.; GREAT Network. Comparison between presepsin and procalcitonin in early diagnosis of neonatal sepsis. J. Matern. Fetal Neonatal Med. 2019, 32, 3903–3908. [Google Scholar] [CrossRef]
- Miyosawa, Y.; Akazawa, Y.; Kamiya, M.; Nakamura, C.; Takeuchi, Y.; Kusakari, M.; Nakamura, T. Presepsin as a predictor of positive blood culture in suspected neonatal sepsis. Pediatr. Int. 2018, 60, 157–161. [Google Scholar] [CrossRef]
- Montaldo, P.; Rosso, R.; Santantonio, A.; Chello, G.; Giliberti, P. Presepsin for the detection of early-onset sepsis in preterm newborns. Pediatr. Res. 2017, 81, 329–334. [Google Scholar] [CrossRef]
- Xiao, T.; Chen, L.P.; Zhang, L.H.; Lai, F.H.; Zhang, L.; Qiu, Q.F.; Que, R.L.; Xie, S.; Wu, D.C. The clinical significance of sCD14-ST for blood biomarker in neonatal hematosepsis: A diagnostic accuracy study. Medicine 2017, 96, e6823. [Google Scholar] [CrossRef]
- Ozdemir, A.A.; Elgormus, Y. Diagnostic Value of Presepsin in Detection of Early-Onset Neonatal Sepsis. Am. J. Perinatol. 2017, 34, 550–556. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Lukacs, S.L.; Schoendorf, K.C.; Schuchat, A. Trends in sepsis-related neonatal mortality in the United States, 1985–1998. Pediatr. Infect. Dis. J. 2004, 23, 599–603. [Google Scholar] [CrossRef] [PubMed]

| Group 1 (n = 58) | Group 2 (n = 77) | Group 3 (n = 24) | p-Value | |
|---|---|---|---|---|
| Infection | Sepsis | Septic Shock | ||
| GA, mean (SD), weeks | 33.8 (5.8) | 31 (5.2) | 30 (4.5) | 0.002 |
| BW, mean (SD), grams | 2205 (1224.8) | 1612.2 (1065.5) | 1425.8 (921.7) | 0.002 |
| Male, n (%) | 33 (56.9) | 52 (67.5) | 12 (51) | 0.22 |
| SGA, n (%) | 10 (17.2) | 16 (20.8) | 4 (16.7) | 0.84 |
| Clinical chorioamnionitis, n (%) | 6 (10.4) | 9 (11.7) | 4 (16.7) | 0.73 |
| Apgar score at 5 min, median, (IQR) | 9 (8–10) | 8 (8–9) | 8 (7–9) | 0.003 |
| Age at T0, median (IQR) | 2 (1–22.3) | 23 (12–37.5) | 10.5 (1–29.8) | <0.001 |
| ETT at T0, n (%) | 5 (8.9) | 28 (38.9) | 14 (58.3) | <0.001 |
| CVC at T0, n (%) | 19 (33.9) | 51 (70.8) | 19 (79.2) | <0.001 |
| No. of clinical signs at T0, median (IQR) | 2 (1–3) | 4 (3–5) | 6 (5–8) | <0.001 |
| Fever at T0, n (%) | 1 (1.7) | 35 (45.5) | 12 (50) | <0.001 |
| Oligoanuria at T0, n (%) | 0 | 2 (2.6) | 5 (20.8) | <0.001 |
| White blood cells at T0, median (IQR), cell * 109/L | 13,415 (9150–18,620) | 12,230 (7240–20,180) | 5120 (2800–14,080) | 0.072 |
| Platelets at T0, mean (SD), *109/L | 266 (134) | 248 (142) | 197 (152) | 0.125 |
| Lactate at T0, mean (SD), mmol/L | 2.8 (2) | 2.1 (1.8) | 3.5 (2.3) | 0.004 |
| Inotropic drugs at T0, n (%) | 2 (3.4) | 6 (7.8) | 20 (83.3) | <0.001 |
| Hydrocortisone at T0, n (%) | 0 | 2 (2.6) | 13 (54.2) | <0.001 |
| Positive blood culture at T0, n (%) | 15/58 (25.8) | 46/77 (59.7) | 16/24 (66.7) | <0.001 |
| Gram-negative bacteria, n (%) | 1 (1.72) | 16 (20.8) | 8 (33.3) | <0.001 |
| Days of antibiotic therapy, mean (SD) | 9 (4.4) | 12 (5.6) | 13 (6.9) | 0.001 |
| Sepsis-related mortality, n (%) | 0 | 4/77 (5.2) | 3/24 (12.5) | 0.042 |
| Presepsin at T0, median (IQR), pg/mL | 977.5 (709–1239) | 1361 (1082–2065) | 1557.5 (1149.5–2386) | <0.001 |
| CRP at T0, median (IQR), mg/dL | 3.8 (1.5–6.3) | 3.8 (1–6.7) | 3.4 (0.7–7.9) | 0.49 |
| PCT at T0, median (IQR), ng/mL | 3 (0.8–17) | 2.4 (0.8–14.3) | 19.8 (1.8–31.4) | 0.14 |
| Time | Infection | Sepsis | Septic Shock | Adjusted p-Value 1 | Adjusted p-Value 2 |
|---|---|---|---|---|---|
| PSEP (pg/mL) | |||||
| T0 | 977.7, (709–1239) | 1361, (1082–2065) | 1557.5, (1149.5–2386) | <0.001 | <0.001 |
| T1 | 957, (782–1233) | 1311, (961.5–1851) | 1645, (1182–2366) | 0.001 | <0.001 |
| T2 | 875, (709–1227) | 1279, (759–1801) | 1789, (1113–2618) | 0.004 | <0.001 |
| T3 | 844.5, (633.0–1083) | 1159.5, (703–1874.5) | 1713.0, (1065–3087) | 0.002 | <0.001 |
| T4 | 772.5, (458–1141.0) | 1072.5, (799.0–1741) | 1740.0, (782–2547) | <0.001 | <0.001 |
| T5 | 528.0, (413–677) | 638.5, (372–929) | 681, (603–1468) | 0.91 | 0.13 |
| CRP (mg/dL) | |||||
| T0 | 3.8, (1.5–6.3) | 3.8, (1–6.7) | 3.4, (0.7–7.9) | 0.139 | 0.224 |
| T1 | |||||
| T2 | 2.6, (1.4–5.5) | 5.9, (2.2–11.3) | 8.2, (4.2–14.9) | 0.001 | <0.001 |
| T3 | |||||
| T4 | 1.3, (0.7–3.5) | 3.7, (1.1–8.7) | 7.5, (1.2–11.1) | 0.001 | 0.007 |
| T5 | 0.5, (0.3–0.7) | 0.5, (0.2–0.7) | 0.5, (0.2–0.7) | 0.266 | 0.537 |
| PCT (ng/mL) | |||||
| T0 | 3, (0.8–17) | 2.4, (0.8–14.3) | 19.8, (1.8–31.4) | 0.755 | 0.031 |
| T1 | |||||
| T2 | 2.2, (0.6–19.4) | 4.3, (1.3–23) | 18.5, (4.5–46.5) | 0.442 | 0.026 |
| T3 | |||||
| T4 | 0.8, (0.5–4.5) | 3.1, (0.9–10.8) | 7.7, (4–48.3) | 0.31 | 0.001 |
| T5 | 0.2, (0–0.3) | 0.2, (0.1–0.5) | 0.3, (0.1–0.3) | 0.454 | 0.874 |
| Max. Youden Index (pg/mL) | Sensitivity | Specificity | PPV | NPV | POS LR | NEG LR | |
|---|---|---|---|---|---|---|---|
| Reference Group: Healthy Neonates | |||||||
| OVERALL | 987.5 | 0.72 | 0.87 | 0.57 | 0.93 | 5.65 (4.54–7.02) | 0.32 (0.25–0.41) |
| Infection | 687.5 | 0.81 | 0.62 | 0.15 | 0.98 | 2.16 (1.84–2.53) | 0.30 (0.18 to 0.52) |
| Sepsis | 1013 | 0.84 | 0.88 | 0.45 | 0.98 | 7.16 (5.71–8.97) | 0.17 (0.10–0.30) |
| Septic shock | 971.5 | 0.92 | 0.86 | 0.18 | 1.00 | 6.42 (5.16–8.00) | 0.09 (0.03–0.37) |
| Reference Group: “Infection” Group | |||||||
| Sepsis | 1006 | 0.84 | 0.55 | 0.71 | 0.73 | 1.88 (1.39–2.55) | 0.28 (0.16–0.50) |
| Septic shock | 1139 | 0.83 | 0.64 | 0.49 | 0.90 | 2.3 (1.57–3.38) | 0.26 (0.10–0.65) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrasanta, C.; Ronchi, A.; Vener, C.; Poggi, C.; Ballerini, C.; Testa, L.; Colombo, R.M.; Spada, E.; Dani, C.; Mosca, F.; et al. Presepsin (Soluble CD14 Subtype) as an Early Marker of Neonatal Sepsis and Septic Shock: A Prospective Diagnostic Trial. Antibiotics 2021, 10, 580. https://doi.org/10.3390/antibiotics10050580
Pietrasanta C, Ronchi A, Vener C, Poggi C, Ballerini C, Testa L, Colombo RM, Spada E, Dani C, Mosca F, et al. Presepsin (Soluble CD14 Subtype) as an Early Marker of Neonatal Sepsis and Septic Shock: A Prospective Diagnostic Trial. Antibiotics. 2021; 10(5):580. https://doi.org/10.3390/antibiotics10050580
Chicago/Turabian StylePietrasanta, Carlo, Andrea Ronchi, Claudia Vener, Chiara Poggi, Claudia Ballerini, Lea Testa, Rosaria Maria Colombo, Elena Spada, Carlo Dani, Fabio Mosca, and et al. 2021. "Presepsin (Soluble CD14 Subtype) as an Early Marker of Neonatal Sepsis and Septic Shock: A Prospective Diagnostic Trial" Antibiotics 10, no. 5: 580. https://doi.org/10.3390/antibiotics10050580
APA StylePietrasanta, C., Ronchi, A., Vener, C., Poggi, C., Ballerini, C., Testa, L., Colombo, R. M., Spada, E., Dani, C., Mosca, F., & Pugni, L. (2021). Presepsin (Soluble CD14 Subtype) as an Early Marker of Neonatal Sepsis and Septic Shock: A Prospective Diagnostic Trial. Antibiotics, 10(5), 580. https://doi.org/10.3390/antibiotics10050580







