Ventilator-Associated Pneumonia in Patients with COVID-19: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
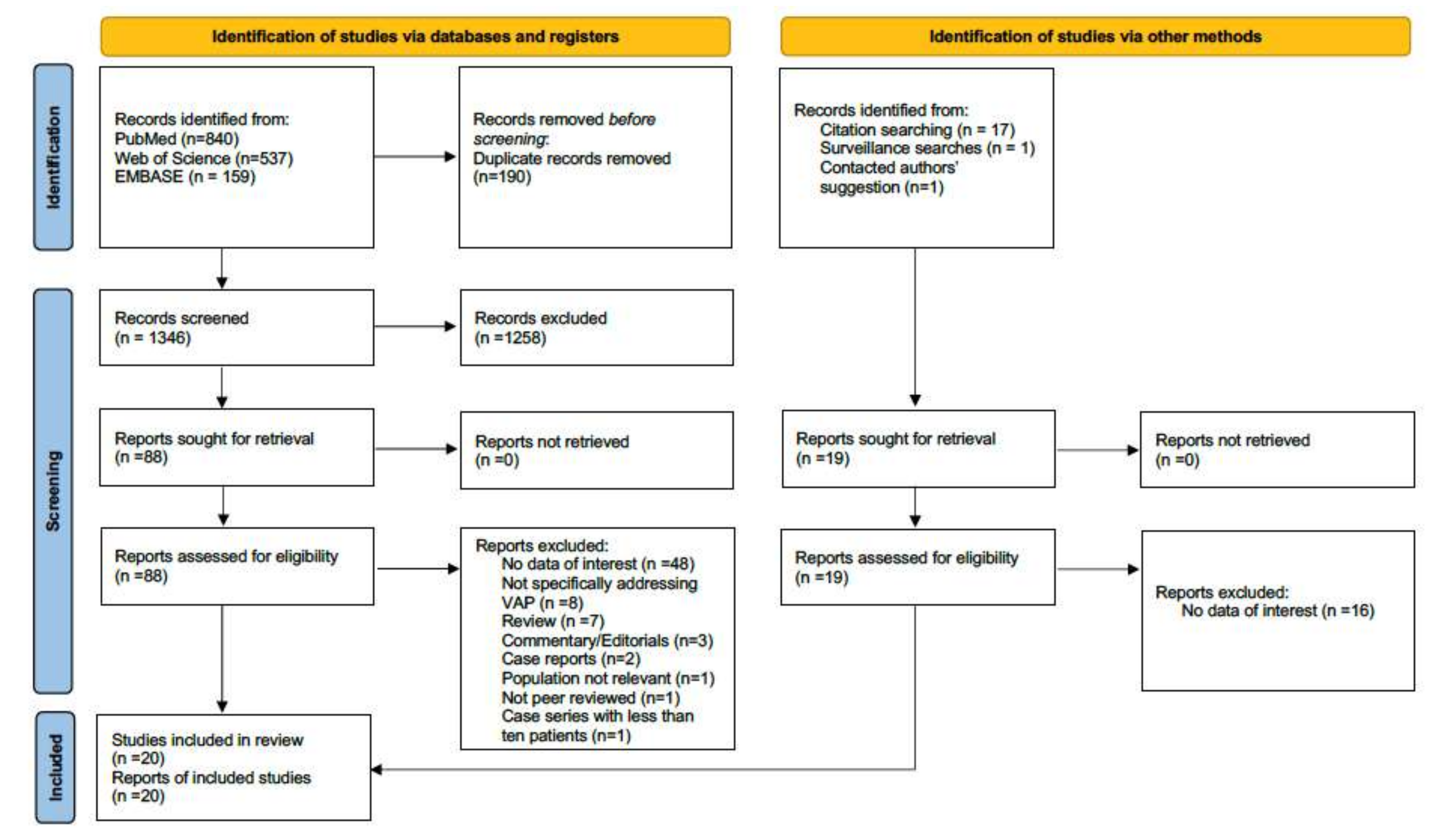
2. Materials and Methods
2.1. Qualitative Analysis
2.2. Quantitative Analysis
3. Results
3.1. Characteristics of Included Studies and Patients
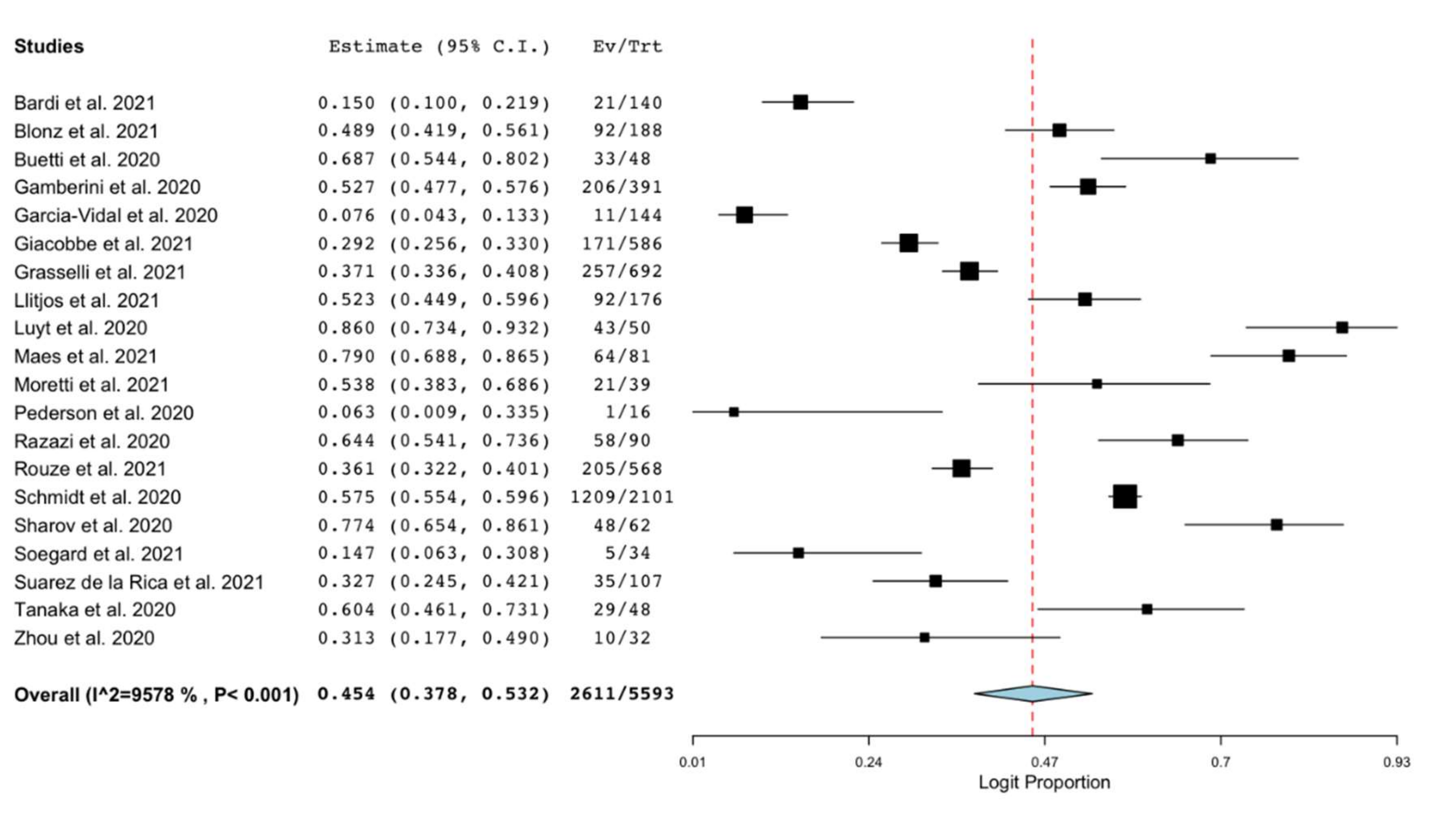
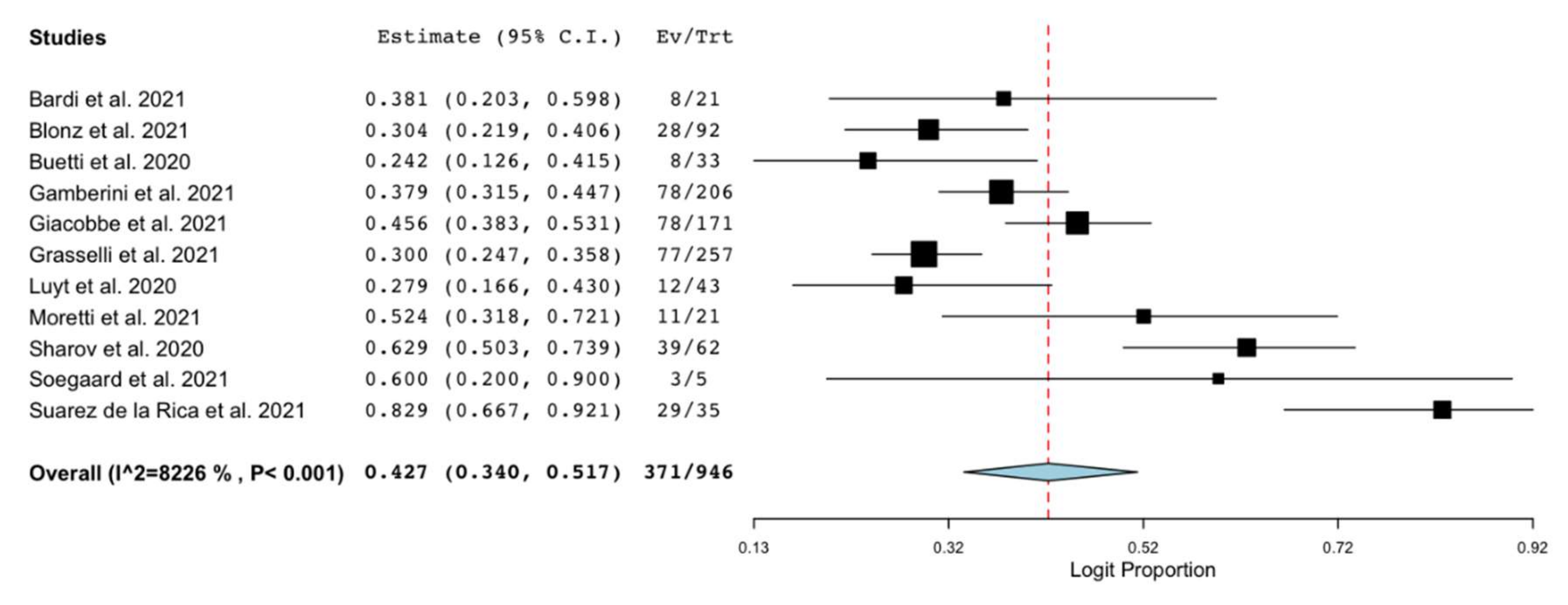
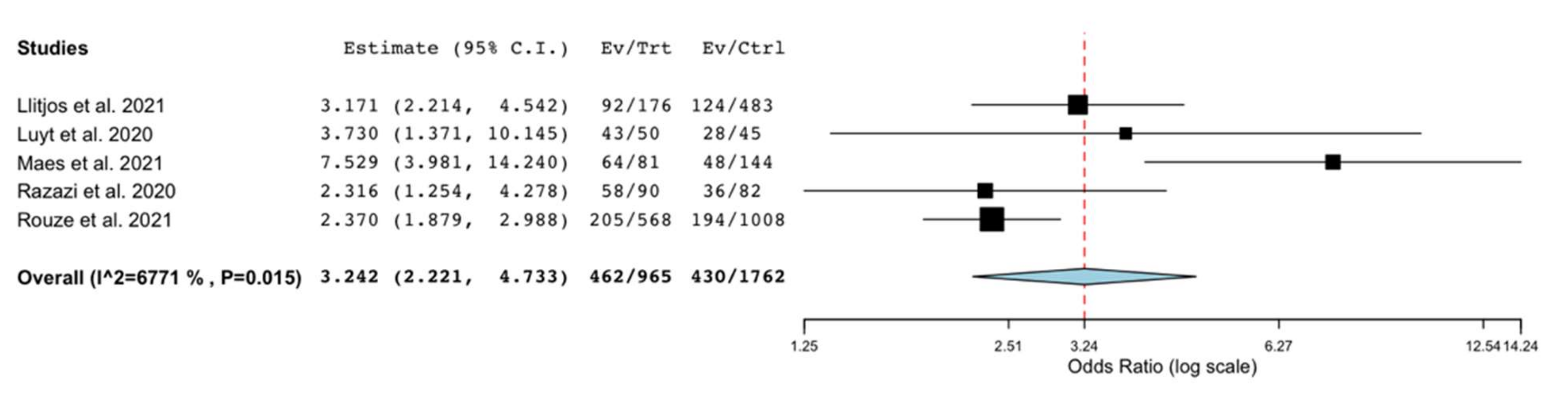
3.2. Outcomes
3.3. Sensitivity and Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, R.; Elhusseiny, K.M.; Yeh, Y.-C.; Sun, W.-Z. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes—A systematic review and meta-analysis. PLoS ONE 2021, 16, e0246318. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Klompas, M.; Luyt, C.E. Ventilator-associated pneumonia in adults: A narrative review. Intensive Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef]
- Xu, E.; Pérez-Torres, D.; Fragkou, P.C.; Zahar, J.R.; Koulenti, D. Nosocomial pneumonia in the era of multidrug-resistance: Updates in diagnosis and management. Microorganisms 2021, 9, 534. [Google Scholar] [CrossRef] [PubMed]
- NHSN—CDC. Pneumonia (Ventilator-Associated [VAP] and Non-Ventilator-Associated Pneumonia [PNEU]) Event; CDC: Atlanta, GA, USA, 2021. [Google Scholar]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Bassi, G.L.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M. A new definition of ventilator-Associated pneumonia: Far from perfect, better than before. Ann. Am. Thorac. Soc. 2013, 10, 644–645. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Hamilton, C.W.; Ernst, F.R. Economic Impact of Ventilator-Associated Pneumonia in a Large Matched Cohort. Infect. Control Hosp. Epidemiol. 2012, 33, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Cattaneo, E.; Florio, G.; Ippolito, M.; Zanella, A.; Cortegiani, A.; Huang, J.; Pesenti, A.; Einav, S. Mechanical ventilation parameters in critically ill COVID-19 patients: A scoping review. Crit. Care 2021, 25. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Battaglini, D.; Enrile, E.M.; Dentone, C.; Vena, A.; Robba, C.; Ball, L.; Bartoletti, M.; Coloretti, I.; Di Bella, S.; et al. Incidence and Prognosis of Ventilator-Associated Pneumonia in Critically Ill Patients with COVID-19: A Multicenter Study. J. Clin. Med. 2021, 10, 555. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.; Shojania, K.G.; McGowan, J.; Daniel, R.; Rader, T.; Iansavichene, A.E.; Ji, J.; Ansari, M.T.; Moher, D. Surveillance search techniques identified the need to update systematic reviews. J. Clin. Epidemiol. 2008, 61, 755–762. [Google Scholar] [CrossRef]
- Page, M.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (Minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Chapter 7.7.3.5—Medians and interquartile ranges. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019; Available online: https://handbook-5-1.cochrane.org/chapter_7/7_7_3_5_mediansand_interquartile_ranges.htm (accessed on 7 May 2021).
- Wallace; Byron, C.; Dahabreh, I.J.; Trikalinos; Lau, J.; Trow, P.; Schmid, C.H. Open Meta Analyst; Closing the gap between methodologists and end-users: R as a computational back-end. Stat. Softw. 2012, 49, 1–15. [Google Scholar]
- Blonz, G.; Kouatchet, A.; Chudeau, N.; Pontis, E.; Lorber, J.; Lemeur, A.; Planche, L.; Lascarrou, J.B.; Colin, G. Epidemiology and microbiology of ventilator-associated pneumonia in COVID-19 patients: A multicenter retrospective study in 188 patients in an un-inundated French region. Crit. Care 2021, 25. [Google Scholar] [CrossRef]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Azzam Lopez, A.; Diez-Remesal, Y.; Martinez Castro, N.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Rouzé, A.; Martin-Loeches, I.; Povoa, P.; Makris, D.; Artigas, A.; Bouchereau, M.; Lambiotte, F.; Metzelard, M.; Cuchet, P.; Boulle Geronimi, C.; et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: A European multicenter cohort study. Intensive Care Med. 2021, 47, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Hajage, D.; Demoule, A.; Pham, T.; Combes, A.; Dres, M.; Lebbah, S.; Kimmoun, A.; Mercat, A.; Beduneau, G.; et al. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021, 47, 60–73. [Google Scholar] [CrossRef]
- Razazi, K.; Arrestier, R.; Haudebourg, A.F.; Benelli, B.; Carteaux, G.; Decousser, J.-W.; Fourati, S.; Woerther, P.L.; Schlemmer, F.; Charles-Nelson, A.; et al. Risks of ventilator-associated pneumonia and invasive pulmonary aspergillosis in patients with viral acute respiratory distress syndrome related or not to Coronavirus 19 disease. Crit. Care 2020, 24, 1–11. [Google Scholar] [CrossRef]
- Buetti, N.; Mazzuchelli, T.; Lo Priore, E.; Balmelli, C.; Llamas, M.; Pallanza, M.; Elzi, L.; Consonni, V.; Trimboli, P.; Forni-Ogna, V.; et al. Early administered antibiotics do not impact mortality in critically ill patients with COVID-19. J. Infect. 2020, 81, e148–e149. [Google Scholar] [CrossRef]
- Luyt, C.E.; Sahnoun, T.; Gautier, M.; Vidal, P.; Burrel, S.; Pineton de Chambrun, M.; Chommeloux, J.; Desnos, C.; Arzoine, J.; Nieszkowska, A.; et al. Ventilator-associated pneumonia in patients with SARS-CoV-2-associated acute respiratory distress syndrome requiring ECMO: A retrospective cohort study. Ann. Intensive Care 2020, 10. [Google Scholar] [CrossRef]
- Llitjos, J.F.; Bredin, S.; Lascarrou, J.B.; Soumagne, T.; Cojocaru, M.; Leclerc, M.; Lepetit, A.; Gouhier, A.; Charpentier, J.; Piton, G.; et al. Increased susceptibility to intensive care unit-acquired pneumonia in severe COVID-19 patients: A multicentre retrospective cohort study. Ann. Intensive Care 2021, 11. [Google Scholar] [CrossRef]
- Gamberini, L.; Tonetti, T.; Spadaro, S.; Zani, G.; Mazzoli, C.A.; Capozzi, C.; Giampalma, E.; Bacchi Reggiani, M.L.; Bertellini, E.; Castelli, A.; et al. Factors influencing liberation from mechanical ventilation in coronavirus disease 2019: Multicenter observational study in fifteen Italian ICUs. J. Intensive Care 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Sharov, K.S. SARS-CoV-2-related pneumonia cases in pneumonia picture in Russia in March-May 2020: Secondary bacterial pneumonia and viral co-infections. J. Glob. Health 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, K.K.; Baettig, V.; Osthoff, M.; Marsch, S.; Leuzinger, K.; Schweitzer, M.; Meier, J.; Bassetti, S.; Bingisser, R.; Nickel, C.H.; et al. Community-acquired and hospital-acquired respiratory tract infection and bloodstream infection in patients hospitalized with COVID-19 pneumonia. J. Intensive Care 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Tanaka, S.; De Tymowski, C.; Assadi, M.; Zappella, N.; Jean-Baptiste, S.; Robert, T.; Peoch, K.; Lortat-Jacob, B.; Fontaine, L.; Bouzid, D.; et al. Lipoprotein concentrations over time in the intensive care unit COVID-19 patients: Results from the ApoCOVID study. PLoS ONE 2020, 15, 1–15. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395. [Google Scholar] [CrossRef]
- Maes, M.; Higginson, E.; Pereira-Dias, J.; Curran, M.D.; Parmar, S.; Khokhar, F.; Cuchet-Lourenço, D.; Lux, J.; Sharma-Hajela, S.; Ravenhill, B.; et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit. Care 2021, 25. [Google Scholar] [CrossRef]
- Pedersen, H.P.; Hildebrandt, T.; Poulsen, A.; Uslu, B.; Knudsen, H.H.; Roed, J.; Poulsen, T.D.; Nielsen, H.B. Initial experiences from patients with covid-19 on ventilatory support in denmark. Dan. Med. J. 2020, 67, 1–4. [Google Scholar]
- Suarez-de-la, A.; Falces-romero, I. Original Secondary infections in mechanically ventilated patients with COVID-19: An overlooked matter? Rev. Española Quimioter. 2021, 1–7. [Google Scholar] [CrossRef]
- Moretti, M.; Van Laethem, J.; Minini, A.; Pierard, D.; Malbrain, M.L.N.G. Ventilator-associated bacterial pneumonia in coronavirus 2019 disease, a retrospective monocentric cohort study. J. Infect. Chemother. 2021. [Google Scholar] [CrossRef]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-acquired infections in critically-ill COVID-19 patients. Chest 2021. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Kózka, M.; Sega, A.; Wojnar-Gruszka, K.; Tarnawska, A.; Gniadek, A. Risk factors of pneumonia associated with mechanical ventilation. Int. J. Environ. Res. Public Health 2020, 17, 656. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Xiao, W.; Song, T.; Wang, S. Incidence, Risk Factors, and Outcomes of Ventilator-Associated Pneumonia in Traumatic Brain Injury: A Meta-analysis. Neurocrit. Care 2020, 32, 272–285. [Google Scholar] [CrossRef]
- Grasselli, G.; Tonetti, T.; Protti, A.; Langer, T.; Girardis, M.; Bellani, G.; Laffey, J.; Carrafiello, G.; Carsana, L.; Rizzuto, C.; et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: A multicentre prospective observational study. Lancet Respir. Med. 2020, 8, 1201–1208. [Google Scholar] [CrossRef]
- Bekaert, M.; Timsit, J.F.; Vansteelandt, S.; Depuydt, P.; Vésin, A.; Garrouste-Orgeas, M.; Decruyenaere, J.; Clec’h, C.; Azoulay, E.; Benoit, D. Attributable mortality of ventilator-associated pneumonia: A reappraisal using causal analysis. Am. J. Respir. Crit. Care Med. 2011, 184, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, M.; Ramanan, M.; Bellina, D.; Catalisano, G.; Iozzo, P.; Di Guardo, A.; Moscarelli, A.; Grasselli, G.; Giarratano, A.; Bassetti, M.; et al. Personal protective equipment use by healthcare workers in intensive care unit during the early phase of COVID-19 pandemic in Italy: A secondary analysis of the PPE-SAFE survey. Ther. Adv. Infect. Dis. 2021, 8. [Google Scholar] [CrossRef]
- Muscedere, J.G.; Day, A.; Heyland, D.K. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and Hospital-acquired pneumonia. Clin. Infect. Dis. 2010, 51. [Google Scholar] [CrossRef] [PubMed]
| Authors (Year) [REF] | Design (Country) | COVID-19 ICU Patients * | Non-COVID-19 Comparison Population | Criteria Used for the Definition of VAP |
|---|---|---|---|---|
| Bardi et al. (2021) [16] | Single center retrospective study (Spain) | 140 patients with COVID-19 (RT-PCR) admitted to ICU Age 61 years [IQR 57–67] Female 23% Patients with VAP: 21 (15%) | NA | According to Centers for Disease Control and Prevention criteria and the Spanish Society of Infectious Diseases and Clinical Microbiology |
| Blonz et al. (2021) [15] | Single center retrospective study (France) | 188 patients with COVID-19 (RT-PCR) admitted to the ICU, who have been receiving IMV for more than 48 h Age: 64 years (±11) Female 22% Patients with VAP: 92 (49%) Duration of MV prior to VAP: 10 days | NA | Timing: at least 48 h of IMV Radiological: two successive chest radiographs or chest CT scans showing new or progressive lung infiltrates Clinical: at least one among (i) body temperature > 38.3 °C with no other cause, (ii) leukocytes < 4000/mm3 or >12,000/mm3, and at least one among (i) new onset of purulent sputum or change in sputum and (ii) worsening gas exchange Microbiological: at least one among (i) positive quantitative culture from minimally contaminated LRT specimen (PN 1), using plugged telescopic catheter with a threshold of 103 CFU/mL or a bronchoalveolar lavage with a threshold of 104 CFU/mL, (ii) positive quantitative culture from possibly contaminated LRT specimen (PN 2) using blind endotracheal aspirate with a threshold of 106 CFU/mL, and (iii) positive growth in culture of pleural fluid (PN 3) |
| Buetti et al. (2020) [20] | Single center retrospective study (Switzerland) | 48 patients with COVID-19 admitted to ICU Age 66 years [IQR 60–71] Female 23% Patients with VAP: 33 (69%) | NA | Radiological: new or progressive and persistent radiographic infiltrates Clinical suspicion Microbiological: positive microbiological cultures from lower respiratory tract specimens |
| Gamberini et al. (2020) [23] | Multicenter prospective observational study 15 ICUs (Italy) | 391 patients admitted to ICU with COVID-19 (RT-PCR) Age 66 years [IQR 59–72] Female 23% Patients with VAP: 206 (53%) | NA | Timing: on mechanical ventilation for >2 calendar days on the date of event, with day of ventilator placement being Day 1 and the ventilator was in place on the date of event or the day before Radiological: two or more serial chest imaging test results with at least one among new and persistent or progressive and persistent (i) Infiltrate; (ii) Consolidation; (iii) Cavitation Clinical: at least one among (i) fever (>38.0 °C or >100.4 °F), (ii) Leukopenia (≤4000 WBC/mm3) or leukocytosis (>12,000 WBC/mm3), (iii) for adults >70 years old, altered mental status with no other recognized cause, and at least two among (i) new onset of purulent sputum or change in character of sputum or increased respiratory secretions or increased suctioning requirements, (ii) new onset or worsening cough, or dyspnea, or tachypnea, (iii) rales or bronchial breath sounds or (iv) worsening gas exchange |
| Garcia-Vidal et al. (2020) [33] | Single center retrospective study (Spain) | 144 patients admitted to ICU with COVID-19 (RT-PCR) Patients with VAP: 11 (7.6%) | NA | NA |
| Giacobbe et al. (2021) [9] | Multicenter retrospective study 11 ICUs (Italy) | 586 patients admitted to the ICU with COVID-19 (RT-PCR for SARS-CoV-2) who have been receiving invasive mechanical ventilation Patients with VAP: 171 (29%) Age: 64 [IQR 57–71] Female 20% Patients with microbiologically confirmed VAP: 77 (45%, 92 no specimens analyzed) Duration of MV prior to VAP: 9 days [IQR 5–15] | NA | Timing: at least 48 h of IMV Radiological: new or changing chest X-ray infiltrate/s Clinical: both (i) new onset of body temperature ≥ 38 °C or ≤ 35 °C and/or leukocytosis or leukopenia or immature neutrophils and (ii) new onset of suctioned respiratory secretions and/or need for acute ventilator support system changes to enhance oxygenation Microbiological confirmation: (criteria not needed for the diagnosis) positive BALF culture for bacterial respiratory pathogens |
| Grasselli et al. (2021) [32] | Multicenter retrospective study 8 hospitals (Italy) | 692 patients admitted to the ICU with COVID-19 (RT-PCR for SARS-CoV-2) who had been receiving invasive mechanical ventilation Patients with VAP: 257 (37%) | NA | At least two among: (i) fever, leukocytosis/leucopenia, purulent secretions, (ii) new/progressive radiographic infiltrate, (iii) worsening oxygenation Microbiological: bronchoalveolar lavage ≥ 104 CFU/mL or endotracheal aspirate ≥ 105 CFU/mL |
| Llitjos et al. (2021) [22] | Multicenter retrospective study 7 ICUs (France) | 176 patients with COVID-19 (RT-PCR) admitted to the ICU who have been receiving invasive mechanical ventilation for at least 48 h Age 63 years [IQR 55–73] Female 24% Patients with VAP: 92 (52%) Duration of MV prior to VAP: 9 days [IQR 6–14] | - 435 patients with bacterial CAP who have been receiving invasive mechanical ventilation Age 66 years [IQR 56–79] Female 32% Patients with VAP: 113 (26%) Duration of MV prior to VAP: 9 days [IQR 6–12]; - 48 patients with viral CAP who have been receiving invasive mechanical ventilation Age 72 years [IQR 42–75] Female 48% Patients with VAP: 11 (23%) Duration of MV prior to VAP: 7 days [IQR 6.5–14] | ICU-acquired pneumonia Timing: at least 48 h of IMV Clinical: diagnosis was based on Clinical Pulmonary Infectious Score >6 Microbiological confirmation: (criteria not needed for the diagnosis) direct Gram staining and semi-quantitative culture. An independent physician retrospectively assessed the diagnostic accuracy of all episodes of ICU-acquired pneumonia |
| Luyt et al. (2020) [21] | Single center retrospective study (France) | 50 patients with COVID-19 associated ARDS admitted to the ICU and requiring ECMO Age 48 years [IQR 42–56] Female 28% Patients with VAP: 43 (86%) Duration of MV prior to VAP: 10 days [IQR 8–16] | 45 patients with severe influenza-associated ARDS requiring ECMO Age 58 years [IQR 48–64] Female 38% Patients with VAP: 28 (62%) Duration of MV before VAP: 14 (8–19) | Timing: at least 48 h of IMV Radiological: new and persistent pulmonary infiltrate on chest radiograph (not needed for patients with ARDS) Clinical: at least two criteria among (i) temperature ≥ 38 °C (ii) white blood cell count ≥ 10 Giga/L (iii) purulent tracheal secretions iv) increased minute ventilation (v) arterial oxygenation decline requiring modifications of the ventilator settings and/or (vi) need for increased vasopressor infusion Microbiological: significant quantitative growth (≥104 colony-forming units/mL) of distal BALF samples |
| Maes et al. (2021) [28] | Single center retrospective study (UK) | 81 patients with COVID-19 admitted to the ICU, who have been receiving invasive mechanical ventilation for more than 48 h Age 62 years [IQR 50–70] Female 31% Patients with VAP: 64 (79%) Duration of MV prior to VAP: 7 days [IQR 5–12] Patients with microbiologically confirmed VAP: 39 (48%) | 144 patients admitted to ICU without COVID-19 who have been receiving invasive mechanical ventilation for more than 48 h Age 62 years [IQR 49–72] Female 40% Patients with VAP: 48 (34%) Duration of MV prior to VAP: 6 days [IQR 4–9] Patients with microbiologically confirmed VAP: 19 (13%) | Timing: at least 48 h of IMV Radiological: new or worsening infiltrates on Chest X-ray or CT thorax Clinical: at least one among (i) fever > 38 °C with no other cause, (ii) leukopenia (<4000 WBC/mm3) or leukocytosis (≥12,000 WBC/mm3), (iii) new onset of suctioned respiratory secretions or suggestive auscultation or worsening gas exchanges Microbiological confirmation: (criteria not needed for the diagnosis) at least one among (i) positive quantitative culture from minimally contaminated LRT specimen (PN 1) or broncho-alveolar lavage with a threshold of ≥ 104 CFU/mL or detection by TaqMan array with Ct ≤ 32 and (ii) positive quantitative culture from possibly contaminated LRT specimen (PN 2) or quantitative culture of LRT specimen with a threshold of 105 CFU/mL |
| Moretti et al. (2021) [31] | Single center retrospective study (Belgium) | 39 patients with COVID-19 (RT-PCR) admitted to the ICU, who have been receiving invasive mechanical ventilation Age 62 [IQR 55–72] Female 28% Patients with VAP: 21 (69%) Duration of MV prior to VAP: 13 days [IQR 7–21] | NA | National Healthcare Safety Network (NHSN) 2017. Clinical: (required for iVAC, possible and probable VAP) Oxygenation problem occurred with hypothermia (temperature < 36 °C) or fever (temperature > 38 °C) or leukocytosis (>12,000 white blood cells/ mm3) or leukocytopenia (<4000 white blood cells/mm3) Microbiological: (i) (required for possible VAP) qualitative pulmonary infection (endotracheal aspiration or BAL showing on gram stain >25 neutrophils and <10 epithelial cells per low power field) or (ii) (required for probable VAP) a quantitative pulmonary infection (endotracheal aspiration or BAL growing, respectively, >105 CFU/mL and >104 CFU/mL) |
| Pedersen et al. (2020) [29] | Single center retrospective study (Denmark) | 16 patients with COVID-19 admitted to ICU 69.5 years (range: 56–84 years) Female 25% Patients with VAP: 1 (6%) | NA | NA |
| Razazi et al. (2020) [19] | Single center retrospective study (France) | 90 patients with COVID-19 (RT-PCR) associated ARDS admitted to ICU, who required mechanical ventilation for more than 48 h Age 59 [IQR 53–69] Female 18% Patients with VAP: 58 (64%) Duration of MV prior to VAP: 8 days [IQR 5–12] | 82 patients admitted to ICU with non-COVID-19 associated ARDS (50 with severe influenza) Age 63 [IQR 57–71] Female 34% Patients with VAP: 36 (44%) Duration of MV prior to VAP: 7 days [IQR 5–9] | Timing: at least 48 h of IMV Radiological: new or worsening infiltrates on chest X-ray Clinical: systemic signs of infection (new-onset fever, leukocytosis or leucopenia, increased need for vasopressors to maintain blood pressure), purulent secretions, and impaired oxygenation Microbiological: quantitative cultures of lower respiratory tract secretions sampled before administering new antibiotics using a blinded protected telescope catheter or bronchoscopy (103 and 104 colony forming units/ mL, respectively) |
| Rouzè et al. (2021) [17] | Multicenter retrospective study 36 ICUs (28 in France, 3 in Spain, 3 in Greece, 1 in Portugal, 1 in Ireland) | 568 patients with COVID-19, admitted to ICU, who have been receiving invasive mechanical ventilation for more than 48 h Age 64 years [IQR 55–71] Female 41% Patients with VAP: 205 (36%) | - 482 patients admitted to ICU with influenza pneumonia who required mechanical ventilation for more than 48 h Age 62 years [IQR 53–71] Female 49% Patients with VAP: 107 (22%) - 526 patients admitted to ICU with no viral infection who required mechanical ventilation for more than 48 h Age 65 years [IQR 55–74] Female 45% Patients with VAP: 87 (16%) | Timing: at least 48 h of IMV Clinical: at least two among (i) body temperature of more than 38.5 °C or less than 36.5 °C, (ii) leucocyte count greater than 12,000 cells per μL or less than 4000 cells per μL, and (iii) purulent tracheal secretions Radiological: new or progressive infiltrates on chest X-ray Microbiological: isolation in the endotracheal aspirate of at least 105 CFU per mL, or in bronchoalveolar lavage of at least 104 CFU per mL |
| Schmidt et al. (2020) [18] | Multicenter prospective cohort study 149 ICUs (France, Switzerland, Belgium) | 2101 patients with COVID-19 (RT-PCR) admitted to ICU and intubated on day 1 Patients with VAP: 1209/2101 (58%) | NA | Clinical suspicion Microbiological: quantitative distal bronchoalveolar lavage cultures growing ≥ 104 CFU/mL, blind protected specimen brush distal growing ≥ 103 CFU/mL, or endotracheal aspirates growing ≥ 106 CFU/mL |
| Sharov et al. (2020) [24] | Single center retrospective study (Russia) | 62 patients with COVID-19 (molecular biological techniques) and mechanically ventilated Age 54.2 years ± 15.3 Female 55% Patients with VAP: 48 (77%) Duration of MV prior to VAP: 9.1 days ± 5.6 | NA | Timing: at least after 3.5 days of IMV Radiological: AI-based algorithm of CT images Microbiological: biochemical methods |
| Søgaard et al. (2021) [25] | Single center retrospective study (Switzerland) | 34 patients with COVID-19 (RT-PCR) admitted to ICU who required mechanical ventilation Age 65 years [IQR 55–72] Female 24% Patients with VAP: 5 (15%) Patients with microbiologically confirmed VAP: 4 (12%) | NA | Timing: at least 48 h of IMV Radiological: consolidations consistent with bacterial pneumonia Clinical: indicators of worsening oxygenation, new fever and purulent respiratory secretions Microbiological confirmation: (criteria not needed for the diagnosis) positive culture for a respiratory pathogen |
| Suarez de la Rica et al. (2021) [30] | Single center retrospective study (Spain) | 107 patients with COVID-19 (RT-PCR) admitted to ICU and mechanically ventilated Age 62.2 years ± 10.6 Female 29% Patients with VAP: 35 (32.7%) | NA | According to Centers for Disease Control (CDC) criteria Clinical suspicion Radiological criteria Microbiological: at least 1 bacterial species by conventional culture, with a threshold of ≥105 colony forming units in endotracheal aspirates. |
| Tanaka et al. (2020) [26] | Single center retrospective study (France) | 48 patients with COVID-19 ARDS admitted to ICU Age 57 [IQR 46-64] Female 35% Patients with VAP: 29 (60.4%) | NA | According to the Infectious Diseases Society of America and the American Thoracic Society guidelines |
| Zhou et al. (2020) [27] | Multicenter retrospective study (China) | 32 patients with COVID-19 (according to WHO interim guidance) requiring invasive mechanical ventilation Patients with VAP: 10 (31%) | NA | According to ATS guidelines for treatment of hospital-acquired and ventilator-associated pneumonia |
| Authors (Year) [REF] | Microorganisms n Isolates (%) | Antimicrobial Resistance n Isolates (%) |
|---|---|---|
| Bardi et al. (2021) [16] | Enterobacteriaceae E. cloacae, 1 (5%) Gram-positive S. aureus, 5 (24%) Non-fermenting Gram-negative A. baumannii, 1 (5%) P. aeruginosa, 8 (38%) | MDR bacteria, 10 (48%) MRSA, 5 (24%) |
| Blonz et al. (2021) [15] | Enterobacteriaceae, 102 (49%) E. cloacae, 10 E. coli, 26 K. pneumoniae, 16 Gram-positive E. faecium, 1 (0.5%) S. aureus, 28 (14%) Non-fermenting Gram-negative A. baumannii, 4 (2%) P. aeruginosa, 31 (15%) | MRSA, 3 (1.4%) |
| Garcia-Vidal et al. (2020) [33] | Enterobacteriaceae K. pneumoniae 1 Non-fermenting Gram-negative P. aeruginosa 3 Gram-positive S. aureus 4 | NA |
| Giacobbe et al. (2021) [9] | Enterobacteriaceae E. coli, 2 (3%) E. aerogenes, 7 (9%) K. pneumoniae, 15 (19%) Gram-positive S. aureus, 18 (23%) Non-fermenting Gram-negative Acinetobacter spp., 9 (12%) P. aeruginosa, 27 (35%) | MRSA, 8 (10%) Carbapenem-resistant Gram-negative bacteria, 25 (32%) |
| Grasselli et al. (2021) [32] | Gram-negative 249 (64%) P. aeruginosa, 85 Enterobacterales (other) 53 Klebsiella spp., 43 E. coli, 31 A. baumanii, 6 Gram-positive 140 (36%) Staphylococcus aureus, 110 Enterococcus spp., 21 | NA |
| Llitjos et al. (2021) [22] | Enterobacteriaceae, 50 (NA) Non-fermenting Gram-negative, 20 (NA) Gram-positive cocci, 28 (NA) Polymicrobial, 24 (NA) | NA |
| Luyt et al. * (2020) [21] | Enterobacteriaceae, 30 (70%) E. cloacae, 3 Gram-positive S. aureus, 3 (7%) Non-fermenting Gram-negative, 18 (42%) P. aeruginosa, 16 | Inducible AmpC Enterobacteriaceae, 17 (40%) ESBL-PE, 2 (5%) MRSA, 2 (5%) |
| Maes et al. (2021) [28] | Enterobacteriaceae E. coli, 2 (NA) K. pneumoniae, 3 (NA) Gram-positive S. aureus, 2 (NA) Non-fermenting Gram-negative P. aeruginosa, 3 (NA) | NA |
| Moretti et al. (2021) [31] | Enterobacteriaceae Enterobacter spp., NA (11%) K. pneumoniae, NA (26%) Gram-positive S. aureus, NA (7%) Non-fermenting Gram-negative P. aeruginosa, NA (18%) | MDR, 67% XDR, 1 (5%) |
| Pedersen et al. [29] | Enterobacteriaceae E. cloacae 1 (100%) | |
| Razazi et al. (2020) * [19] | Enterobacteriaceae, 42 (72%) Enterobacter spp., 23 E. coli, 10 K. pneumoniae, 4 Non-fermenting Gram-negative, 24 (41%) Acinetobacter spp., 1 Pseudomonas spp., 16 Gram-positive, 4 (3%) S. aureus 2 | MDR VAP, 21 (23%) ESBL-PE, 18 (20%) CRE, 3 (3%) MRSA plus ESBL-PE, 1 (1%) |
| Rouzè et al. (2021) # [17] | Gram-negative bacilli, 240 (83.6%) Enterobacteriaceae Enterobacter spp., 54 E. coli, 24 Klebsiella spp., 33 Non-fermenting Gram-negative A. baumannii, 2 P. aeruginosa, 64 Gram-positive cocci, 56 (19.5%) Enterococcus spp., 9 S. aureus, 35 | Multidrug-resistant isolates, 67 (23%) MRSA, 8 (3%) |
| Sharov et al. (2020) [24] (data from the private scientific correspondence with Dr. K.S. Sharov and Dr. A.S. Gorenintseva from the Russian Academy of Sciences) | Enterobacteriaceae K. pneumoniae, 5 (8%) Gram-positive S. aureus, 8 (13%) | NA |
| Søgaard et al. (2021) [25] | Enterobacteriaceae E. coli, 1 (20%) Non-fermenting Gram-negative A. baumannii, 1 (20%) | MDR, 1 (20%) |
| Suarez de la Rica et al. (2021) [30] | Enterobacteriaceae Enterobacter spp., 1 (2.8%) E. coli, 4 (11.4%) Klebsiella spp., 9 (26%) Non-fermenting Gram-negative P. aeruginosa, 11 (31%) Gram-positive S. aureus, 8 (23%) | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ippolito, M.; Misseri, G.; Catalisano, G.; Marino, C.; Ingoglia, G.; Alessi, M.; Consiglio, E.; Gregoretti, C.; Giarratano, A.; Cortegiani, A. Ventilator-Associated Pneumonia in Patients with COVID-19: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 545. https://doi.org/10.3390/antibiotics10050545
Ippolito M, Misseri G, Catalisano G, Marino C, Ingoglia G, Alessi M, Consiglio E, Gregoretti C, Giarratano A, Cortegiani A. Ventilator-Associated Pneumonia in Patients with COVID-19: A Systematic Review and Meta-Analysis. Antibiotics. 2021; 10(5):545. https://doi.org/10.3390/antibiotics10050545
Chicago/Turabian StyleIppolito, Mariachiara, Giovanni Misseri, Giulia Catalisano, Claudia Marino, Giulia Ingoglia, Marta Alessi, Elisa Consiglio, Cesare Gregoretti, Antonino Giarratano, and Andrea Cortegiani. 2021. "Ventilator-Associated Pneumonia in Patients with COVID-19: A Systematic Review and Meta-Analysis" Antibiotics 10, no. 5: 545. https://doi.org/10.3390/antibiotics10050545
APA StyleIppolito, M., Misseri, G., Catalisano, G., Marino, C., Ingoglia, G., Alessi, M., Consiglio, E., Gregoretti, C., Giarratano, A., & Cortegiani, A. (2021). Ventilator-Associated Pneumonia in Patients with COVID-19: A Systematic Review and Meta-Analysis. Antibiotics, 10(5), 545. https://doi.org/10.3390/antibiotics10050545








