Serious Neurological Adverse Events of Ceftriaxone
Abstract
1. Introduction
2. Results
2.1. General Characteristics of Serious Case Reports
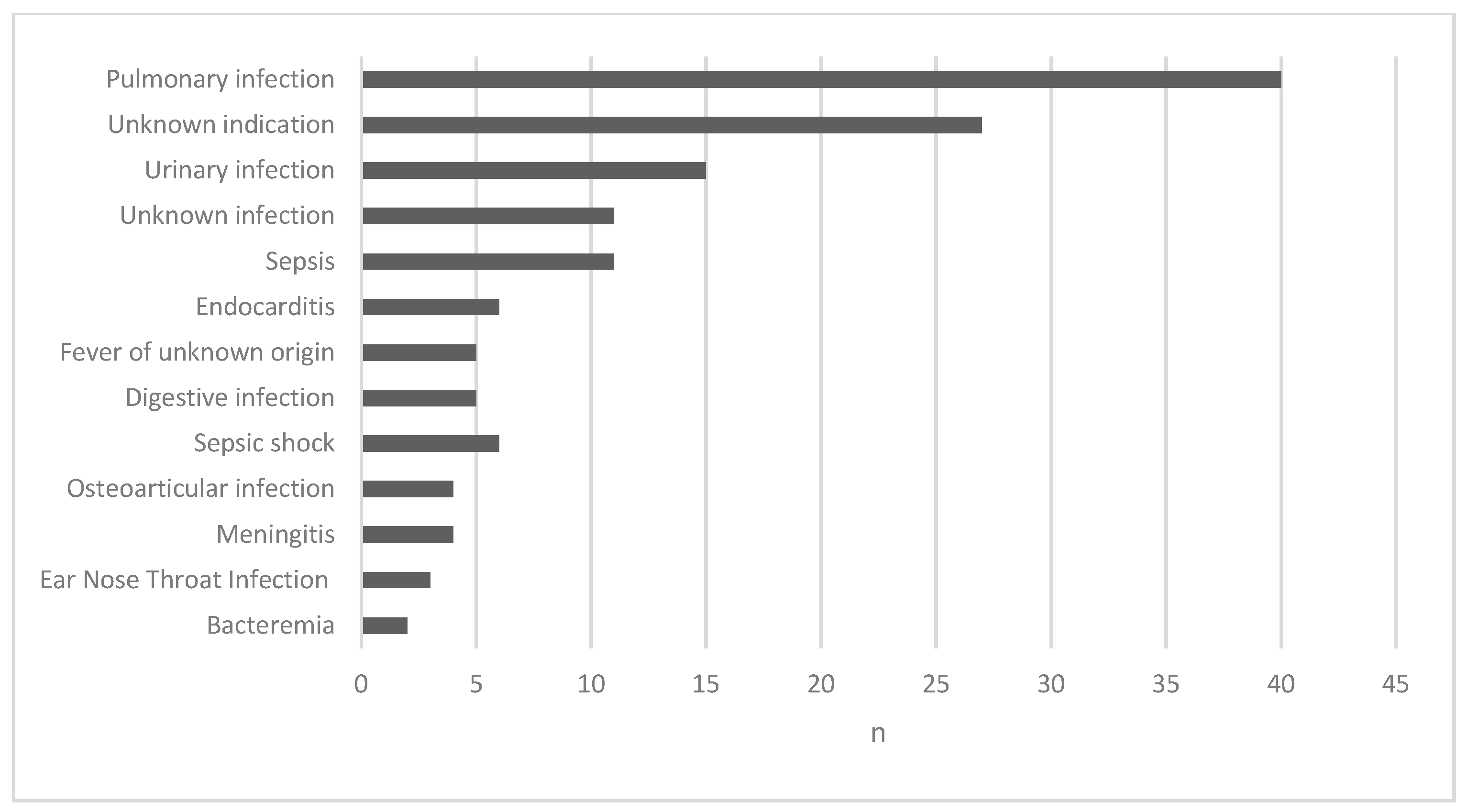
2.2. Administration Route, Daily Dose and Indications
2.3. Concomitant Administration of Antibiotics
2.4. Type of Serious ADRs Reported
2.5. Explorations
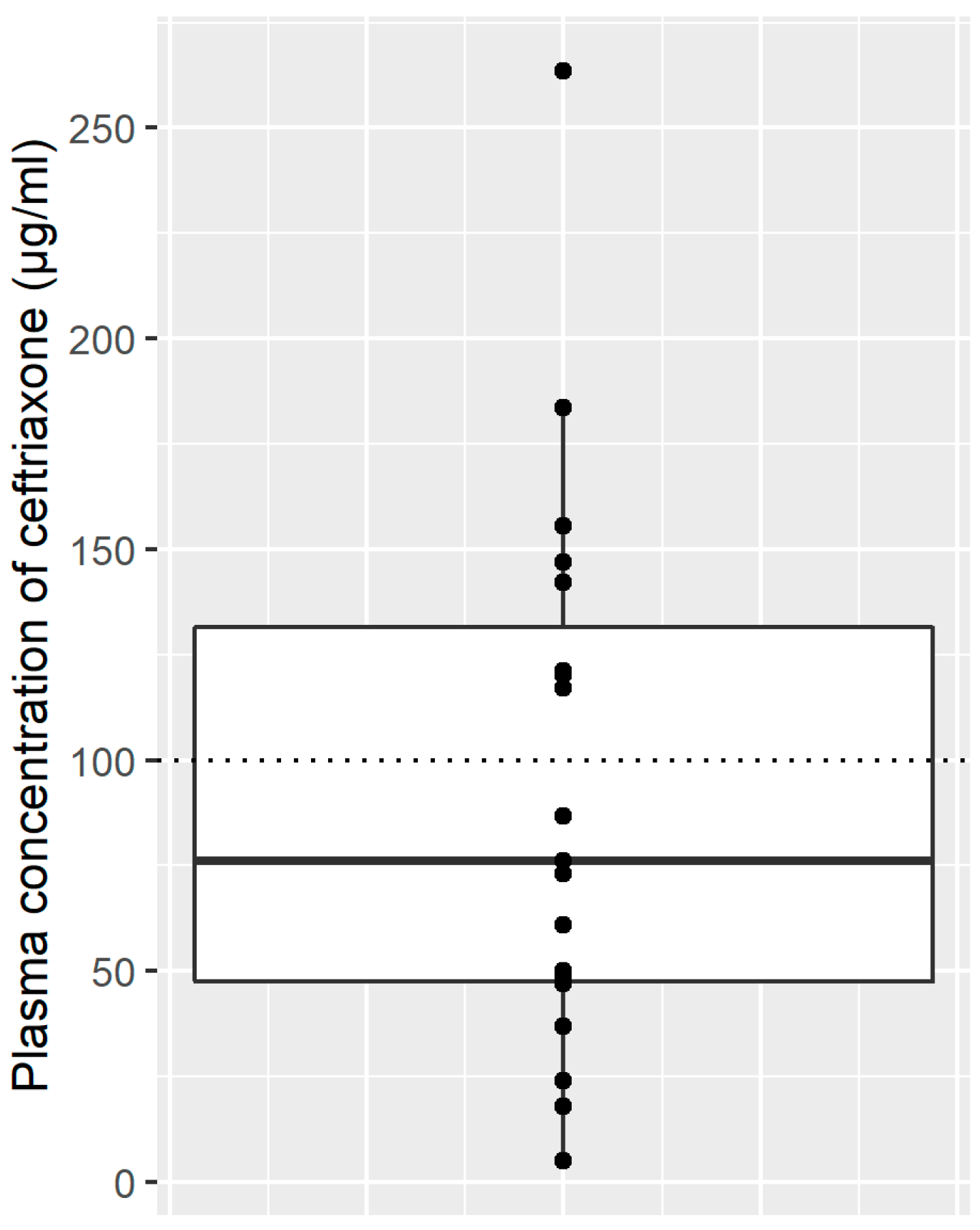
2.5.1. Plasma Concentrations
2.5.2. Electroencephalograms
3. Discussion
4. Materials and Methods
4.1. Data Set
4.2. Population and Cases
4.3. Statistical Analysis
4.4. Data Availability Statement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khodakaram, K.; Barmano, N. Uncommon reaction to a common prescription. Lancet 2011, 378, 288. [Google Scholar] [CrossRef]
- Tamma, P.D.; Avdic, E.; Li, D.X.; Dzintars, K.; Cosgrove, S.E. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern. Med. 2017, 177, 1308–1315. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Darby, R.R.; Raibagkar, P.; Castro, L.N.G.; Berkowitz, A.L. Antibiotic-associated encephalopathy. Neurology 2016, 86, 963–971. [Google Scholar] [CrossRef]
- Nishikubo, M.; Kanamori, M.; Nishioka, H. Levofloxacin-associated neurotoxicity in a patient with a high concentration of levofloxacin in the blood and cerebrospinal fluid. Antibiotics 2019, 8, 78. [Google Scholar] [CrossRef]
- Deshayes, S.; Coquerel, A.; Verdon, R. Neurological adverse effects attributable to β-lactam antibiotics: A literature review. Drug Saf. 2017, 40, 1171–1198. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Sun, S.-F.; Gaynor, P. A Case of ertapenem neurotoxicity resulting in vocal tremor and altered mentation in a dialysis dependent liver transplant patient. Antibiotics 2018, 8, 1. [Google Scholar] [CrossRef]
- Chow, K.M.; Hui, A.C.; Szeto, C.C. Neurotoxicity induced by beta-lactam antibiotics: From bench to bedside. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Grill, M.F.; Maganti, R.K. Neurotoxic effects associated with antibiotic use: Management considerations. Br. J. Clin. Pharm. 2011, 72, 381–393. [Google Scholar] [CrossRef]
- Sutter, R.; Rüegg, S.; Tschudin-Sutter, S. Seizures as adverse events of antibiotic drugs: A systematic review. Neurology 2015, 85, 1332–1341. [Google Scholar] [CrossRef]
- Patel, I.H.; Chen, S.; Parsonnet, M.; Hackman, M.R.; Brooks, M.A.; Konikoff, J.; Kaplan, S.A. Pharmacokinetics of ceftriaxone in humans. J. Antimicrob. Agents Chemother. 1981, 20, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Joynt, G.M. The pharmacokinetics of once-daily dosing of ceftriaxone in critically Ill patients. J. Antimicrob. Chemother. 2001, 47, 421–429. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, J.E.; Barriga, F.J.; Santamaria, J.; Iranzo, A.; Pareja, J.A.; Revilla, M.; dela Rosa, C.R. Nonconvulsive status epilepticus associated with cephalosporins in patients with renal failure. Am. J. Med. 2001, 111, 115–119. [Google Scholar] [CrossRef]
- Bora, I.; Demir, A.B.; Uzun, P. Nonconvulsive status epilepticus cases arising in connection with cephalosporins. Epilepsy Behav. Case Rep. 2016, 6, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Chedrawi, A.K.; Gharaybeh, S.I.; Al-Ghwery, S.A.; Al-Mohaimeed, S.A.; Alshahwan, S.A. Cephalosporin-induced nonconvulsive status epilepticus in a uremic child. Pediatr. Neurol. 2004, 30, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Roncon-Albuquerque, R.; Pires, I.; Martins, R.; Real, R.; Sousa, G.; von Hafe, P. Ceftriaxone-induced acute reversible encephalopathy in a patient treated for a urinary tract infection. Neth. J. Med. 2009, 67, 72–75. [Google Scholar]
- Kim, K.B.; Kim, S.M.; Park, W.; Kim, J.S.; Kwon, S.K.; Kim, H.-Y. Ceftiaxone-induced neurotoxicity: Case report, pharmacokinetic considerations, and literature review. J. Korean Med. Sci. 2012, 27, 1120–1123. [Google Scholar] [CrossRef]
- Sharma, N.; Gupta, A.; Batish, S. Ceftriaxone-induced acute reversible encephalopathy in a patient with enteric fever. Indian J. Pharmacol. 2012, 44, 124–125. [Google Scholar] [CrossRef]
- Safadi, S.; Mao, M.; Dillon, J.J. Ceftriaxone-induced acute encephalopathy in a peritoneal dialysis patient. Case Rep. Nephrol. 2014, 2014, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hagiya, H.; Miyawaki, K.; Yamamoto, N.; Yoshida, H.; Kitagawa, A.; Asaoka, T.; Eguchi, H.; Akeda, Y.; Tomono, K. Ceftriaxone-induced neurotoxicity in a patient after pancreas-kidney transplantation. Intern. Med. 2017, 56, 3103–3107. [Google Scholar] [CrossRef]
- Inoue, Y.; Doi, Y.; Arisato, T.; Sugioka, S.; Koga, K.; Nishioka, K.; Sugawara, A. Three cases of hemodialysis patients receiving high-dose ceftriaxone: Serum concentrations and its neurotoxicity. Kidney Int. Rep. 2017, 2, 984–987. [Google Scholar] [CrossRef]
- Dubin, I.; Schattner, A. Iatrogenic coma: Ceftriaxone-associated encephalopathy. Postgrad. Med. J. 2018, 94, 357–358. [Google Scholar] [CrossRef]
- Triplett, J.D.; Lawn, N.D.; Chan, J.; Dunne, J.W. Cephalosporin-related neurotoxicity: Metabolic encephalopathy or non-convulsive status epilepticus? J. Clin. Neurosci. 2019, 67, 163–166. [Google Scholar] [CrossRef]
- Sato, Y.; Kawashima, E.; Yoshimura, A.; Morita, H.; Wakasugi, H.; Iijima, S.; Wakayama, Y. Reversible choreoathetosis after the administration of ceftriaxone sodium in patients with end-stage renal disease. Am. J. Med. Sci. 2010, 340, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Naito, S.; Numasawa, Y.; Asada, M.; Shoji, N.; Zeniya, M.; Takahashi, D.; Sato, H.; Iimori, S.; Nomura, N.; et al. Encephalopathy induced by high plasma and cerebrospinal fluid ceftriaxone concentrations in a hemodialysis patient. Intern. Med. 2019, 58, 1775–1779. [Google Scholar] [CrossRef]
- Lacroix, C.; Kheloufi, F.; Montastruc, F.; Bennis, Y.; Pizzoglio, V.; Micallef, J. Serious central nervous system side effects of cephalosporins: A national analysis of serious reports registered in the french pharmacovigilance database. J. Neurol. Sci. 2019, 398, 196–201. [Google Scholar] [CrossRef]
- La Consommation D’antibiotiques en France en 2016; Rapport ANSM; ANSM: Paris, France, 2017.
- Report on Surveillance of Antibiotic Consumption 2016–2018; WHO: Geneva, Switzerland, 2018.
- Yuk, J.H.; Nightingale, C.H.; Quintiliani, R. Clinical pharmacokinetics of ceftriaxone. Clin. Pharmacokinet. 1989, 17, 223–235. [Google Scholar] [CrossRef]
- Résumé Des Caractéristiques Du Produit—Rocéphine 1g/10mL, Poudre et Solvant Pour Solution Injectable (IV). Available online: http://agence-prd.ansm.sante.fr/php/ecodex/rcp/R0263188.htm (accessed on 29 March 2021).
- Merino-Bohórquez, V.; Docobo-Pérez, F.; Valiente-Méndez, A.; Delgado-Valverde, M.; Cameán, M.; Hope, W.W.; Pascual, Á.; Rodríguez-Baño, J. Population pharmacokinetics of piperacillin in non-critically ill patients with bacteremia caused by enterobacteriaceae. Antibiotics 2021, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Mattappalil, A.; Mergenhagen, K.A. Neurotoxicity with antimicrobials in the elderly: A review. Clin. Ther. 2014, 36, 1489–1511. [Google Scholar] [CrossRef] [PubMed]
- Grandison, M.K.; Boudinot, F.D. Age-related changes in protein binding of drugs: Implications for therapy. Clin. Pharm. 2000, 38, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Hämmerlein, A.; Derendorf, H.; Lowenthal, D.T. Pharmacokinetic and pharmacodynamic changes in the elderly. Clinical implications. Clin. Pharm. 1998, 35, 49–64. [Google Scholar] [CrossRef]
- Schleibinger, M.; Steinbach, C.L.; Töpper, C.; Kratzer, A.; Liebchen, U.; Kees, F.; Salzberger, B.; Kees, M.G. Protein binding characteristics and pharmacokinetics of ceftriaxone in intensive care unit patients: Unbound ceftriaxone in ICU patients. Br. J. Clin. Pharmacol. 2015, 80, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, M.; Dailly, E.; Le Turnier, P.; Garot, D.; Guimard, T.; Bernard, L.; Tattevin, P.; Vandamme, Y.-M.; Hoff, J.; Lemaitre, F.; et al. High-dose ceftriaxone for bacterial meningitis and optimization of administration scheme based on nomogram. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Hartman, S.J.F.; Brüggemann, R.J.; Orriëns, L.; Dia, N.; Schreuder, M.F.; de Wildt, S.N. Pharmacokinetics and target attainment of antibiotics in critically ill children: A systematic review of current literature. Clin. Pharm. 2019, 36, 45–64. [Google Scholar] [CrossRef]
- Bos, J.C.; Prins, J.M.; Mistício, M.C.; Nunguiane, G.; Lang, C.N.; Beirão, J.C.; Mathôt, R.A.A.; van Hest, R.M. Pharmacokinetics and pharmacodynamic target attainment of ceftriaxone in adult severely ill Sub-Saharan African patients: A population pharmacokinetic modelling study. J. Antimicrob. Chemother. 2018, 73, 1620–1629. [Google Scholar] [CrossRef]
- Garot, D.; Respaud, R.; Lanotte, P.; Simon, N.; Mercier, E.; Ehrmann, S.; Perrotin, D.; Dequin, P.-F.; Le Guellec, C. Population pharmacokinetics of ceftriaxone in critically ill septic patients: A reappraisal. Br. J. Clin. Pharmacol. 2011, 72, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, J.; Carrié, C.; d’Houdain, N.; Djabarouti, S.; Petit, L.; Xuereb, F.; Legeron, R.; Biais, M.; Breilh, D. Are standard dosing regimens of ceftriaxone adapted for critically ill patients with augmented creatinine clearance? Antimicrob. Agents Chemother. 2019, 63, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.M.; Szeto, C.C.; Hui, A.C.-F.; Li, P.K.-T. Mechanisms of antibiotic neurotoxicity in renal failure. Int. J. Antimicrob. Agents 2004, 23, 213–217. [Google Scholar] [CrossRef]
- Le Turnier, P.; Navas, D.; Garot, D.; Guimard, T.; Bernard, L.; Tattevin, P.; Vandamme, Y.M.; Hoff, J.; Chiffoleau, A.; Dary, M.; et al. Tolerability of high-dose ceftriaxone in cns infections: A prospective multicentre cohort study. J. Antimicrob. Chemother. 2019, 74, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Grill, M.F.; Maganti, R. Cephalosporin-induced neurotoxicity: Clinical manifestations, potential pathogenic mechanisms, and the role of electroencephalographic monitoring. Ann. Pharm. 2008, 42, 1843–1850. [Google Scholar] [CrossRef]
- Chen, Z.; He, Y.; Wang, Z.J. The beta-lactam antibiotic, ceftriaxone, inhibits the development of opioid-induced hyperalgesia in mice. Neurosci. Lett. 2012, 509, 69–71. [Google Scholar] [CrossRef]
- Sari, Y.; Sakai, M.; Weedman, J.M.; Rebec, G.V.; Bell, R.L. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011, 46, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Sari, Y.; Toalston, J.E.; Rao, P.S.S.; Bell, R.L. Effects of ceftriaxone on ethanol, nicotine or sucrose intake by alcohol-preferring (P) rats and its association with GLT-1 expression. Neuroscience 2016, 326, 117–125. [Google Scholar] [CrossRef]
- Knackstedt, L.A.; Melendez, R.I.; Kalivas, P.W. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol. Psychiatry 2010, 67, 81–84. [Google Scholar] [CrossRef]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically Ill patients—Guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique—SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation—SFAR). Crit. Care 2019, 23, 1–20. [Google Scholar] [CrossRef]
- Lechtig-Wasserman, S.; Liebisch-Rey, H.; Diaz-Pinilla, N.; Blanco, J.; Fuentes-Barreiro, Y.-V.; Bustos, R.-H. Carbapenem Therapeutic drug monitoring in critically ill adult patients and clinical outcomes: A systematic review with meta-analysis. Antibiotics 2021, 10, 177. [Google Scholar] [CrossRef]
- Fernández-Torre, J.L.; Martínez-Martínez, M.; González-Rato, J.; Maestro, I.; Alonso, I.; Rodrigo, E.; Horcajada, J.P. Cephalosporin-induced nonconvulsive status epilepticus: Clinical and electroencephalographic features. Epilepsia 2005, 46, 1550–1552. [Google Scholar] [CrossRef]
- Passarelli, V.; da Conceição, M.P.O.M.; Trés, E.S.; Alves Junior, J.F.; Baldocchi, M.A. Stimulus-induced rhythmic, periodic, or ictal discharges (SIRPDs) associated with seizures in cefepime neurotoxicity. Arq. Neuro-Psiquiatr. 2014, 72, 643–644. [Google Scholar] [CrossRef]
- Research, C. for D.E. and Drug Safety and Availability—FDA Drug Safety Communication: Cefepime and Risk of Seizure in Patients Not Receiving Dosage Adjustments for Kidney Impairment. Available online: https://www.fda.gov/Drugs/DrugSafety/ucm309661.htm (accessed on 29 March 2021).
- Céfépime: Rappel Des Risques de Réactions Graves Lors Du Non Respect Des Posologies Recommandées Notamment en Cas de d’insuffisance Rénale—Point d’Information—ANSM: Agence Nationale de Sécurité Du Médicament et Des Produits de Santé. Available online: http://ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Cefepime-rappel-des-risques-de-reactions-graves-lors-du-non-respect-des-posologies-recommandees-notamment-en-cas-de-d-insuffisance-renale-Point-d-Information (accessed on 29 March 2021).
- Céfépime (Axepim® et Ses Génériques): Rappel Des Risques D’effets Indésirables Neurologiques Graves Lors Du Non-Respect Des Posologies Recommandées Notamment En Cas D’insuffisance Rénale—Lettre Aux Professionnels de Santé. Available online: http://ansm.sante.fr/S-informer/Informations-de-securite-Lettres-aux-professionnels-de-sante/Cefepime-Axepim-R-et-ses-generiques-rappel-des-risques-d-effets-indesirables-neurologiques-graves-lors-du-non-respect-des-posologies-recommandees-notamment-en-cas-d-insuffisance-renale-Lettre-aux-professionnels-de-sante (accessed on 29 March 2021).
- Montastruc, J.-L.; Sommet, A.; Lacroix, I.; Olivier, P.; Durrieu, G.; Damase-Michel, C.; Lapeyre-Mestre, M.; Bagheri, H. Pharmacovigilance for evaluating adverse drug reactions: Value, organization, and methods. Joint Bone Spine 2016, 73, 629–732. [Google Scholar] [CrossRef]
- Arimone, Y.; Bidault, I.; Dutertre, J.-P.; Gérardin, M.; Guy, C.; Haramburu, F.; Hillaire-Buys, D.; Meglio, C.; Penfornis, C.; Théophile, H.; et al. Updating the french method for the causality assessment of adverse drug reactions. Therapies 2013, 68, 69–76. [Google Scholar] [CrossRef] [PubMed]
| Patients’ Characteristics | |
|---|---|
| Female; n (%) | 84 (55.3) |
| Age (years); median (Q1; Q3) | 74.5 (63; 84.3) |
| Age > 65 years old; n (%) | 106 (69.7) |
| Creatinine clearance (mL/min); median (Q1; Q3) | 35 (20; 59.5) |
| Patient | Age | Sex | Renal Function | Dose (g/Day) | Through Concentration (µg/mL) | Indication | Neurological Manifestations | Electroencephalogram Findings | Days to Onset | Days to Remission | Treatment | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 83 | F | CKD | 2 | - | Pneumonia | Drowsiness, myoclonus | - | 4 | 5 | Discontinue, AED | [12] |
| 2 | 78 | F | CKD | 2 | - | Meningitis | Drowsiness, myoclonus | - | 6 | 5 | Discontinue, AED | [12] |
| 3 | 12 | F | CKD | 100 mg/kg | - | Sepsis | Confusion, visual hallucinations, facial myoclonus (rechallenge +) | Bursts and runs of generalized spike and spike wave discharges | 3 | 2 | Discontinue, AED | [14] |
| 4 | 60 | F | ARF | 2 | - | Hypogastric pain, fever | Altered mental status, apathy, somnolence | Periodic generalized triphasic waves | 4 | 2 | Discontinue | [15] |
| 5 | 65 | F | CKD | 2 | - | Chill, fever | Altered mental status, generalized myoclonic jerks | Generalized slowing with superimposed almost continuous or periodic bursts of sharp waves or sharp and slow wave activity | 5 | 2 | Discontinue | [16] |
| 6 | 8 | M | Normal | 1 | - | Diarrhea, fever | Altered mental status, apathy, somnolence | - | 3 | 3 | Discontinue | [17] |
| 7 | 37 | F | CKD, PD | 2 | - | Peritonitis | Agitation, paranoia, visual hallucinations | Moderate diffuse nonspecific slowing without epileptogenic activity | 3 | 1.5 | Discontinue | [18] |
| 8 | 24 | F | CKD | 2 | - | Recurrent urinary tract infection | Confusion after general tonico-clonic seizure | Continuous rhythmic generalized 2 to 3 Hz sharp- wave activity, extensive epileptiform activity | 3 | 3 | Discontinue, AED | [13] |
| 9 | 71 | M | CKD | 2 | - | Wound infection | Meaningless speech, inability to walk, sleepiness | Diffuse slow- wave activity | 5 | 5 | Discontinue, AED | [13] |
| 10 | 56 | M | CKD, HD | 4 (days 1–7) 2 (days 8–15) | - | Sepsis | Altered mental status, facial myoclonus, sporadic phonation | Bursts of generalized, high-voltage slow-wave activity | 7 | 5 | Discontinue | [19] |
| 11 | 72 | M | CKD | 4 (days 1–7) 2 (days 8–10) | 472 (day 8) 173 (day 10) | Pneumonia | Altered mental status, spasms of legs | Diffuse slow-wave activity | 8 | 6 | Discontinue | [20] |
| 12 | 75 | F | CKD | 2 | 304 (day 4) 331 (day 6) 422 (day 9) | Diverticulitis | Agitation, hyperkinesia, confusion | Slow-wave activity | 9 | 4 | Discontinue | [20] |
| 13 | 68 | F | CKD | 4 (days 1–7) 2 (days 8–23) | 172 (day 2) 178 (day 4) 188 (day 7) | Pyogenic arthritis | - | - | - | - | - | [20] |
| 14 | 76 | M | RI | 4 | - | Endocarditis | Agitation, confusion, coma | Triphasic waves | 14 | 2 | Discontinue | [21] |
| 15 | 70 | F | ARF | 4 | - | Meningitis | Encephalopathy, myoclonus | Severe slowing triphasic waves | 3 | - | - | [22] |
| 16 | 80 | F | Normal | 2.5 | - | Pneumonia | Encephalopathy | Moderate slowing triphasic waves | 2 | - | - | [22] |
| 17 | 80 | F | HD | 4 | - | Cellulitis | Choreoathetosis | - | 5 | 12 | Discontinue | [23] |
| 18 | 72 | F | HD | 1 | - | Catheter-related infection | Choreoathetosis | - | 2 | 1 | Discontinue | [23] |
| 19 | 76 | M | HD | 2 | - | Pneumonia | Choreoathetosis | - | 6 | - | Discontinue | [23] |
| 20 | 76 | M | HD | 2 | - | Catheter-related infection | Choreoathetosis | - | 5 | 2 | Discontinue | [23] |
| 21 | 86 | F | HD | 1 (days 1–3) 2 (days 3–13) | 130 (day 9) (LCR 10.2) | H. cinaedi bacteremia | Altered mental status, decreased level of consciousness, myoclonic jerks (right shoulder and arm) | Generalized triphasic waves | 13 | 4 | Discontinue | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacroix, C.; Bera-Jonville, A.-P.; Montastruc, F.; Velly, L.; Micallef, J.; Guilhaumou, R. Serious Neurological Adverse Events of Ceftriaxone. Antibiotics 2021, 10, 540. https://doi.org/10.3390/antibiotics10050540
Lacroix C, Bera-Jonville A-P, Montastruc F, Velly L, Micallef J, Guilhaumou R. Serious Neurological Adverse Events of Ceftriaxone. Antibiotics. 2021; 10(5):540. https://doi.org/10.3390/antibiotics10050540
Chicago/Turabian StyleLacroix, Clémence, Annie-Pierre Bera-Jonville, François Montastruc, Lionel Velly, Joëlle Micallef, and Romain Guilhaumou. 2021. "Serious Neurological Adverse Events of Ceftriaxone" Antibiotics 10, no. 5: 540. https://doi.org/10.3390/antibiotics10050540
APA StyleLacroix, C., Bera-Jonville, A.-P., Montastruc, F., Velly, L., Micallef, J., & Guilhaumou, R. (2021). Serious Neurological Adverse Events of Ceftriaxone. Antibiotics, 10(5), 540. https://doi.org/10.3390/antibiotics10050540





