Validation and Application of an HPLC-UV Method for Routine Therapeutic Drug Monitoring of Cefiderocol
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Assay Validation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazmierczak, K.M.; Tsuji, M.; Wise, M.G.; Hackel, M.; Yamano, Y.; Echols, R.; Sahm, D.F. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014 Study). Int. J. Antimicrob. Agents 2019, 53, 177–184. [Google Scholar] [PubMed]
- Katsube, T.; Echols, R.; Wajima, T. Pharmacokinetic and Pharmacodynamic Profiles of Cefiderocol, a Novel Siderophore Cephalosporin. Clin. Infect. Dis. 2019, 69 (Suppl. S7), S552–S558. [Google Scholar] [CrossRef]
- Kawaguchi, N.; Katsube, T.; Echols, R.; Wajima, T. Population Pharmacokinetic Analysis of Cefiderocol, a Parenteral Siderophore Cephalosporin, in Healthy Subjects, Subjects with Various Degrees of Renal Function, and Patients with Complicated Urinary Tract Infection or Acute Uncomplicated Pyelonephritis. Antimicrob. Agents Chemother. 2018, 62, e01391-17. [Google Scholar] [CrossRef] [PubMed]
- Veiga, R.P.; Paiva, J.-A. Pharmacokinetics-pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit. Care 2018, 22, 233. [Google Scholar] [CrossRef]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Societe Francaise de Pharmacologie et Therapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Societe Francaise d’Anesthesie et Reanimation-SFAR). Crit. Care 2019, 23, 104. [Google Scholar]
- Abdul-Aziz, M.H.; Lipman, J.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Dulhunty, J.; Kaukonen, K.-M.; Koulenti, D.; Martin, C.; et al. Is prolonged infusion of piperacillin/tazobactam and meropenem in critically ill patients associated with improved pharmacokinetic/pharmacodynamic and patient outcomes? An observation from the Defining Antibiotic Levels in Intensive care unit patients (DALI) cohort. J. Antimicrob. Chemother. 2016, 71, 196–207. [Google Scholar]
- Scharf, C.; Liebchen, U.; Paal, M.; Taubert, M.; Vogeser, M.; Irlbeck, M.; Zoller, M.; Schroeder, I. The higher the better? Defining the optimal beta-lactam target for critically ill patients to reach infection resolution and improve outcome. J. Intensive Care 2020, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Boschung-Pasquier, L.; Atkinson, A.; Kastner, L.; Banholzer, S.; Haschke, M.; Buetti, N.; Furrer, D.; Hauser, C.; Jent, P.; Que, Y.; et al. Cefepime neurotoxicity: Thresholds and risk factors. A retrospective cohort study. Clin. Microbiol. Infect. 2020, 26, 333–339. [Google Scholar] [CrossRef]
- Beumier, M.; Casu, G.S.; Hites, M.; Wolff, F.; Cotton, F.; Vincent, J.L.; Jacobs, F.; Taccone, F.S. Elevated β-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol. 2015, 81, 497–506. [Google Scholar] [PubMed]
- Kadomura, S.; Takekuma, Y.; Sato, Y.; Sumi, M.; Kawamoto, K.; Itoh, T.; Sugawara, M. Higher incidence of acute kidney injury in patients treated with piperacillin/tazobactam than in patients treated with cefepime: A single-center retrospective cohort study. J. Pharm. Health Care Sci. 2019, 5, 13. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Xia, Y.; Chu, Y.; Zhong, H.; Li, J.; Liang, P.; Bu, Y.; Zhao, R.; Liao, Y.; et al. Recommendation of Antimicrobial Dosing Optimization during Continuous Renal Replacement Therapy. Front. Pharmacol. 2020, 11, 786. [Google Scholar] [CrossRef]
- König, C.; Braune, S.; Roberts, J.A.; Nierhaus, A.; Steinmetz, O.M.; Baehr, M.; Frey, O.R.; Langebrake, C.; Kluge, S. Population pharmacokinetics and dosing simulations of ceftazidime in critically ill patients receiving sustained low-efficiency dialysis. J. Antimicrob. Chemother. 2017, 72, 1433–1440. [Google Scholar] [CrossRef]
- Roberts, J.A.; Joynt, G.; Lee, A.; Choi, G.; Bellomo, R.; Kanji, S.; Mudaliar, M.Y.; Peake, S.L.; Stephens, D.; Taccone, F.S.; et al. The effect of renal replacement therapy and antibiotic dose on antibiotic concentrations in critically ill patients: Data from the multinational SMARRT Study. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Katsube, T.; Echols, R.; Ferreira, J.C.A.; Krenz, H.K.; Berg, J.K.; Galloway, C. Cefiderocol, a Siderophore Cephalosporin for Gram-Negative Bacterial Infections: Pharmacokinetics and Safety in Subjects with Renal Impairment. J. Clin. Pharmacol. 2017, 57, 584–591. [Google Scholar] [CrossRef]
- PubChem. Compound Summary for CID 77843966, Cefiderocol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cefiderocol (accessed on 26 February 2021).
- Schmitt, G.; Herbold, M.; Peters, F. Methodenvalidierung im Forensisch-Toxikologischen Labor: Auswertung von Validierungsdaten Nach den Richtlinien der GTFCh mit VALISTAT; Arvecon GmbH: Walldorf, Germany, 2003. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Saisho, Y.; Katsube, T.; White, S.; Fukase, H.; Shimada, J. Pharmacokinetics, Safety, and Tolerability of Cefiderocol, a Novel Siderophore Cephalosporin for Gram-Negative Bacteria, in Healthy Subjects. Antimicrob. Agents Chemother. 2018, 62, e02163-17. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. The European Society of Clinical Microbiology and Infectious Diseases. Breakpoint tables for interpretation of MICs and zone diameters. Version 11.0. Available online: http://www.eucast.org (accessed on 27 January 2021).
- Miyazaki, S.; Katsube, T.; Shen, H.; Tomek, C.; Narukawa, Y. Metabolism, Excretion, and Pharmacokinetics of [(14) C]-Cefiderocol (S-649266), a Siderophore Cephalosporin, in Healthy Subjects Following Intravenous Administration. J. Clin. Pharmacol. 2019, 59, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Kolar, M.; Sedlakova, M.H.; Urbanek, K.; Mlynarcik, P.; Roderova, M.; Hricova, K.; Mezerova, K.; Kucova, P.; Zapletalova, J.; Fiserova, K.; et al. Implementation of Antibiotic Stewardship in a University Hospital Setting. Antibiotics 2021, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Wendt, S.; Ranft, D.; Kern, W.V.; Salzberger, B.; Lübbert, C. Antibiotic stewardship (ABS). Part 1: Basics. Internist 2020, 61, 375–387. [Google Scholar] [CrossRef]
- Mortensen, J.S.; Jensen, B.P.; Zhang, M.; Doogue, M. Preanalytical Stability of Piperacillin, Tazobactam, Meropenem, and Ceftazidime in Plasma and Whole Blood Using Liquid Chromatography-Tandem Mass Spectrometry. Ther. Drug Monit. 2019, 41, 538–543. [Google Scholar] [CrossRef]
- Cusumano, J.A.; Klinker, K.P.; Huttner, A.; Luther, M.K.; Roberts, J.A.; LaPlante, K.L. Towards precision medicine: Therapeutic drug monitoring-guided dosing of vancomycin and beta-lactam antibiotics to maximize effectiveness and minimize toxicity. Am. J. Health-Syst. Pharm. 2020, 77, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.L.; Altshuler, J.; Guervil, D.J.; Hirsch, E.B.; Ostrosky-Zeichner, L.L.; Ericsson, C.D.; Tam, V.H. Cefepime free minimum concentration to minimum inhibitory concentration (fCmin/MIC) ratio predicts clinical failure in patients with Gram-negative bacterial pneumonia. Int. J. Antimicrob. Agents 2015, 45, 541–544. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Voulgaris, G.L.; Maliaros, A.; Samonis, G.; Falagas, M.E. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: A systematic review and meta-analysis of randomised trials. Lancet Infect. Dis. 2018, 18, 108–120. [Google Scholar] [CrossRef]
- Udy, A.A.; Morton, F.J.; Nguyen-Pham, S.; Jarrett, P.; Lassig-Smith, M.; Stuart, J.; Dunlop, R.; Starr, T.; Boots, R.J.; Lipman, J. A comparison of CKD-EPI estimated glomerular filtration rate and measured creatinine clearance in recently admitted critically ill patients with normal plasma creatinine concentrations. BMC Nephrol. 2013, 14, 250. [Google Scholar] [CrossRef] [PubMed]
- Baptista, J.P.; Neves, M.; Rodrigues, L.; Teixeira, L.; Pinho, J.; Pimentel, J. Accuracy of the estimation of glomerular filtration rate within a population of critically ill patients. J. Nephrol. 2014, 27, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Esposito, S.; Leone, S.; Lucini, V.; Pannacci, M.; Ma, L.; Drusano, G.L. Feedback dose alteration significantly affects probability of pathogen eradication in nosocomial pneumonia. Eur. Respir. J. 2009, 34, 394–400. [Google Scholar] [CrossRef]
- Van Lent-Evers, N.A.; Mathôt, R.A.; Geus, W.P.; van Hout, B.A.; Vinks, A.A. Impact of goal-oriented and model-based clinical pharmacokinetic dosing of aminoglycosides on clinical outcome: A cost-effectiveness analysis. Ther. Drug Monit. 1999, 21, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; The Infection Section of European Society of Intensive Care Medicine (ESICM); Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Wong, G.; Briscoe, S.; McWhinney, B.; Ally, M.; Ungerer, J.; Lipman, J.; Roberts, J.A. Therapeutic drug monitoring of β-lactam antibiotics in the critically ill: Direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J. Antimicrob. Chemother. 2018, 73, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
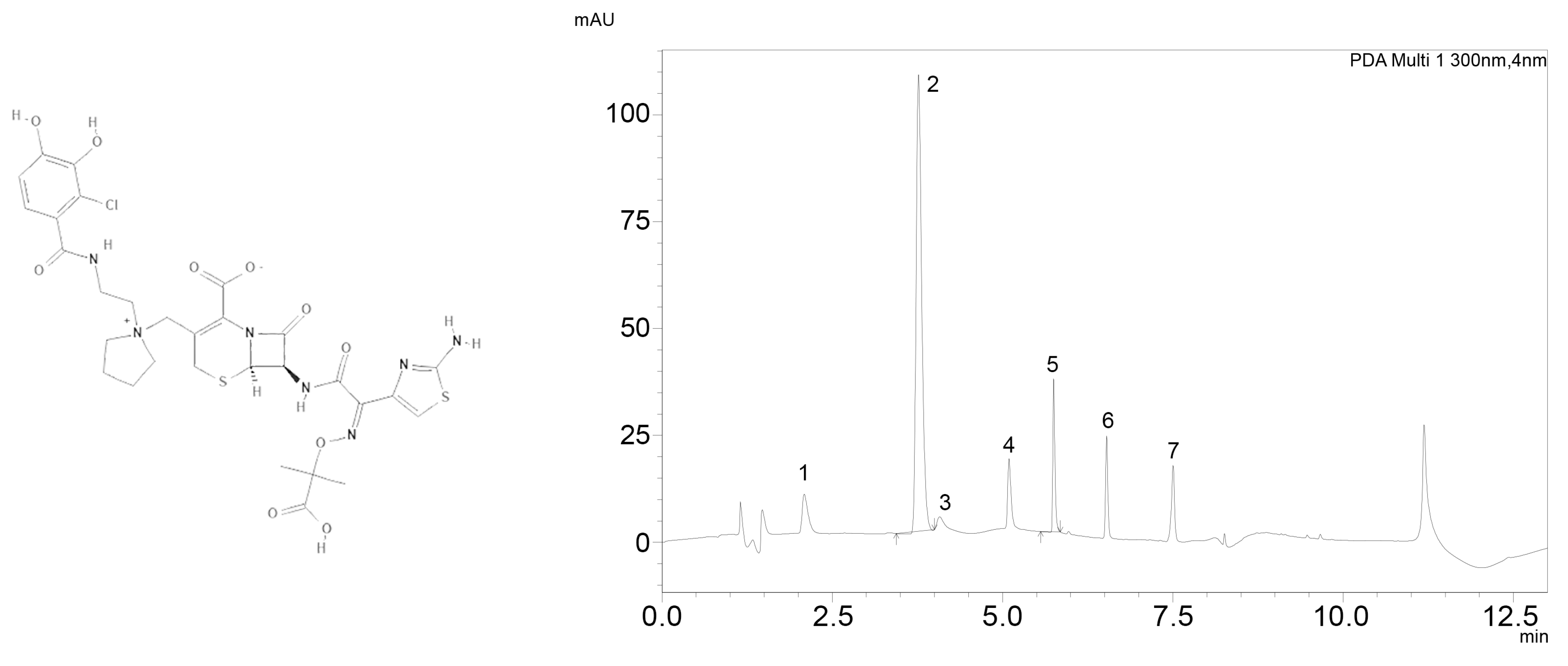
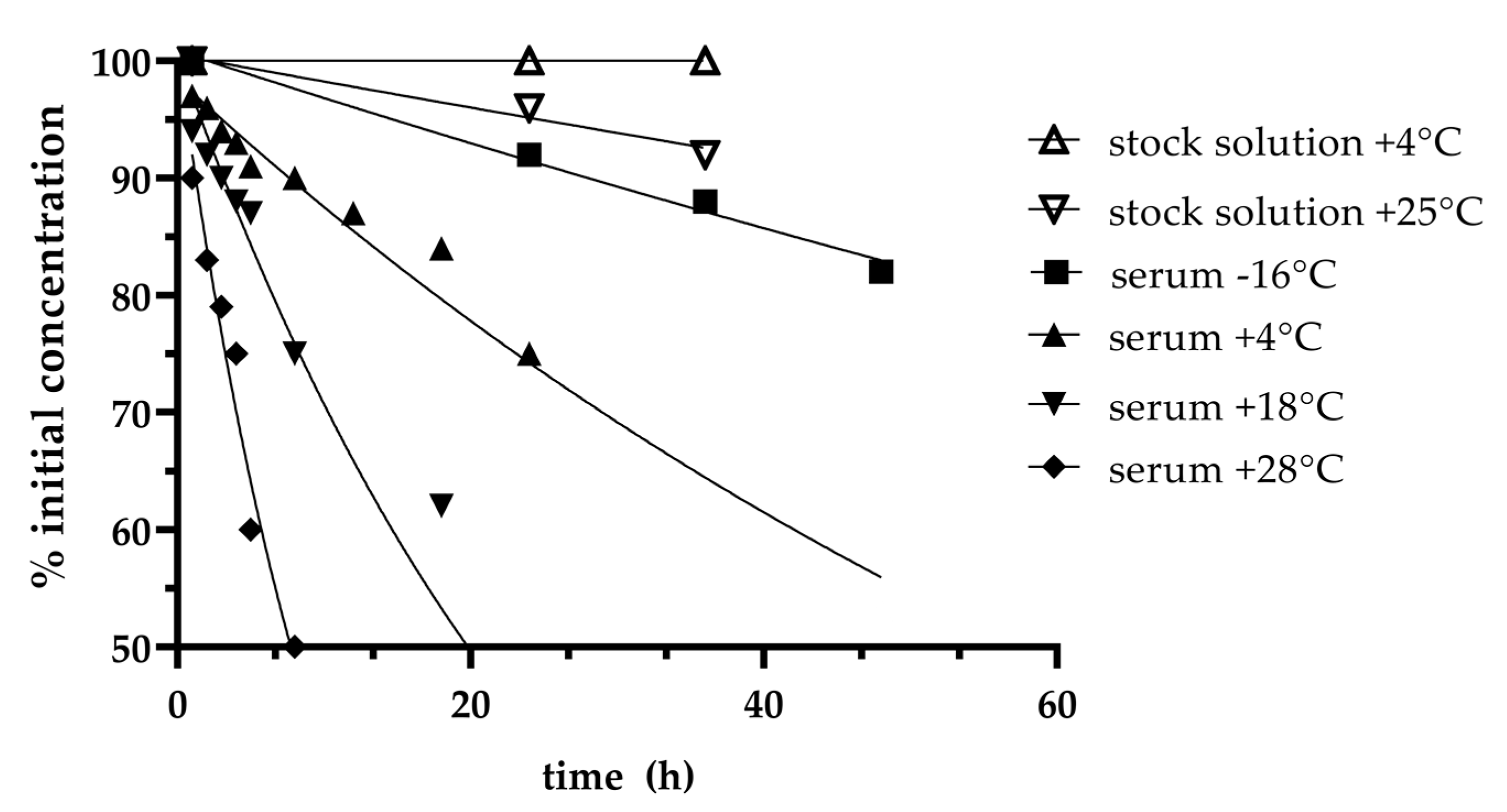
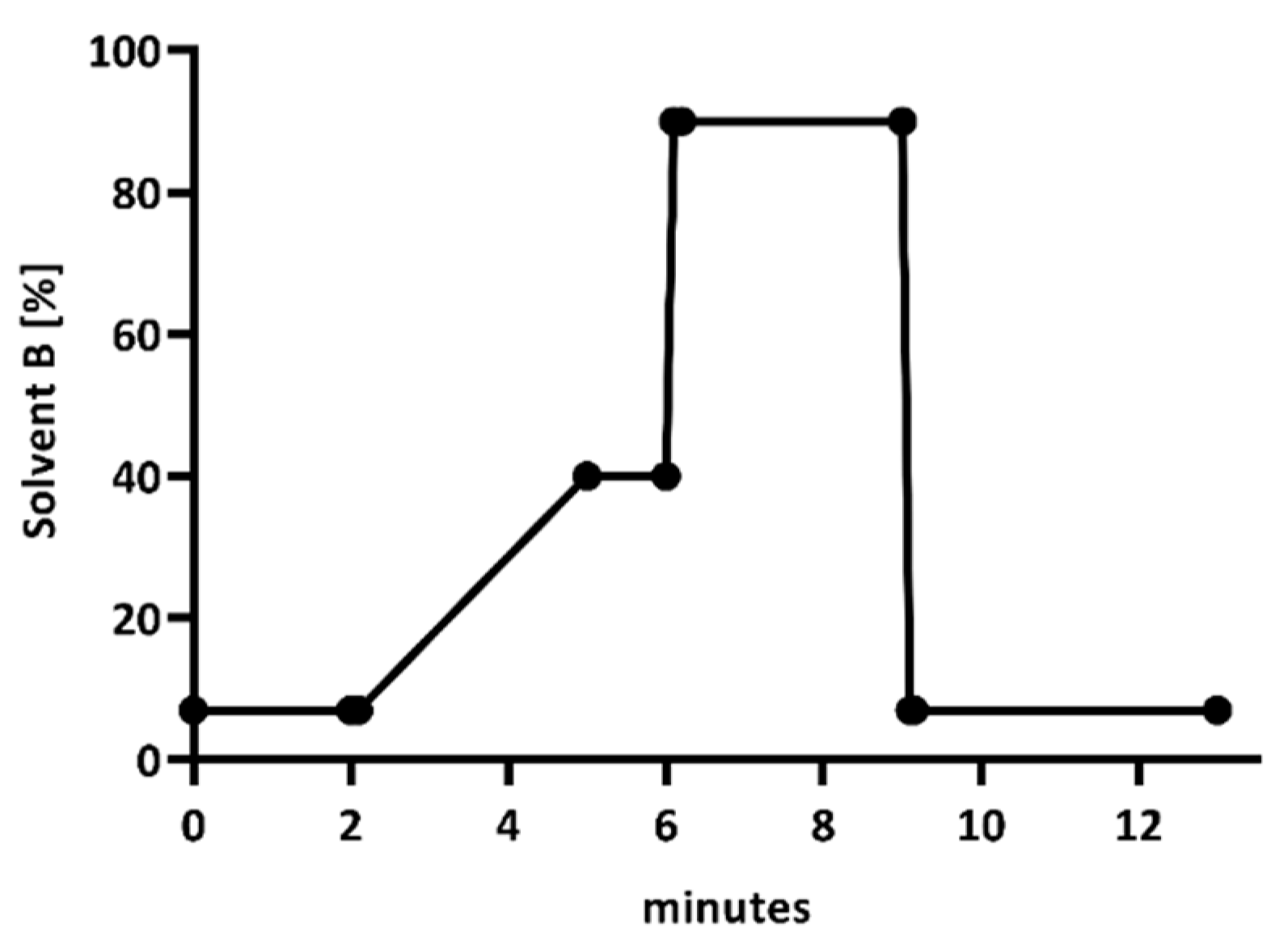

| Low Concentration (32 mg/L) | High Concentration (160 mg/L) | |
|---|---|---|
| Intraday precision (RSD) (%) | 3.3 | 3.8 |
| Interday precision (RSD) (%) | 4.0 | 4.0 |
| Accuracy (Bias) (%) | −5.3 | 8.2 |
| Stability | ||
| 20 °C (h) | 2 | |
| 2–8 °C (h) | 8 | |
| −20 °C (h) | 28 | |
| −80 °C (days) | 31 | |
| Patient | Age (y) | Weight (kg) | eGFR×n | Dose (mg/d) | Cmin (mg/L) | Infection | Pathogen |
|---|---|---|---|---|---|---|---|
| 1 | 69 | 60 | 67 | 3000 | 70 | HAP | P. aeruginosa |
| 22 | 2000 | 49 | |||||
| 2 | 75 | 69 | CRRT | 6000 | 12 | HAP, sepsis | A. baumannii |
| 3 | 58 | 85 | CRRT | 6000 | 18 | HAP, sepsis | A. baumannii |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmer, J.; Röhr, A.C.; Kluge, S.; Faller, J.; Frey, O.R.; Wichmann, D.; König, C. Validation and Application of an HPLC-UV Method for Routine Therapeutic Drug Monitoring of Cefiderocol. Antibiotics 2021, 10, 242. https://doi.org/10.3390/antibiotics10030242
Zimmer J, Röhr AC, Kluge S, Faller J, Frey OR, Wichmann D, König C. Validation and Application of an HPLC-UV Method for Routine Therapeutic Drug Monitoring of Cefiderocol. Antibiotics. 2021; 10(3):242. https://doi.org/10.3390/antibiotics10030242
Chicago/Turabian StyleZimmer, Julia, Anka C. Röhr, Stefan Kluge, Jonas Faller, Otto R. Frey, Dominic Wichmann, and Christina König. 2021. "Validation and Application of an HPLC-UV Method for Routine Therapeutic Drug Monitoring of Cefiderocol" Antibiotics 10, no. 3: 242. https://doi.org/10.3390/antibiotics10030242
APA StyleZimmer, J., Röhr, A. C., Kluge, S., Faller, J., Frey, O. R., Wichmann, D., & König, C. (2021). Validation and Application of an HPLC-UV Method for Routine Therapeutic Drug Monitoring of Cefiderocol. Antibiotics, 10(3), 242. https://doi.org/10.3390/antibiotics10030242






