Levofloxacin Versus Ciprofloxacin-Based Prophylaxis during the Pre-Engraftment Phase in Allogeneic Hematopoietic Stem Cell Transplant Pediatric Recipients: A Single-Center Retrospective Matched Analysis
Abstract
:1. Introduction
2. Results
2.1. Patients and Clinical Characteristics
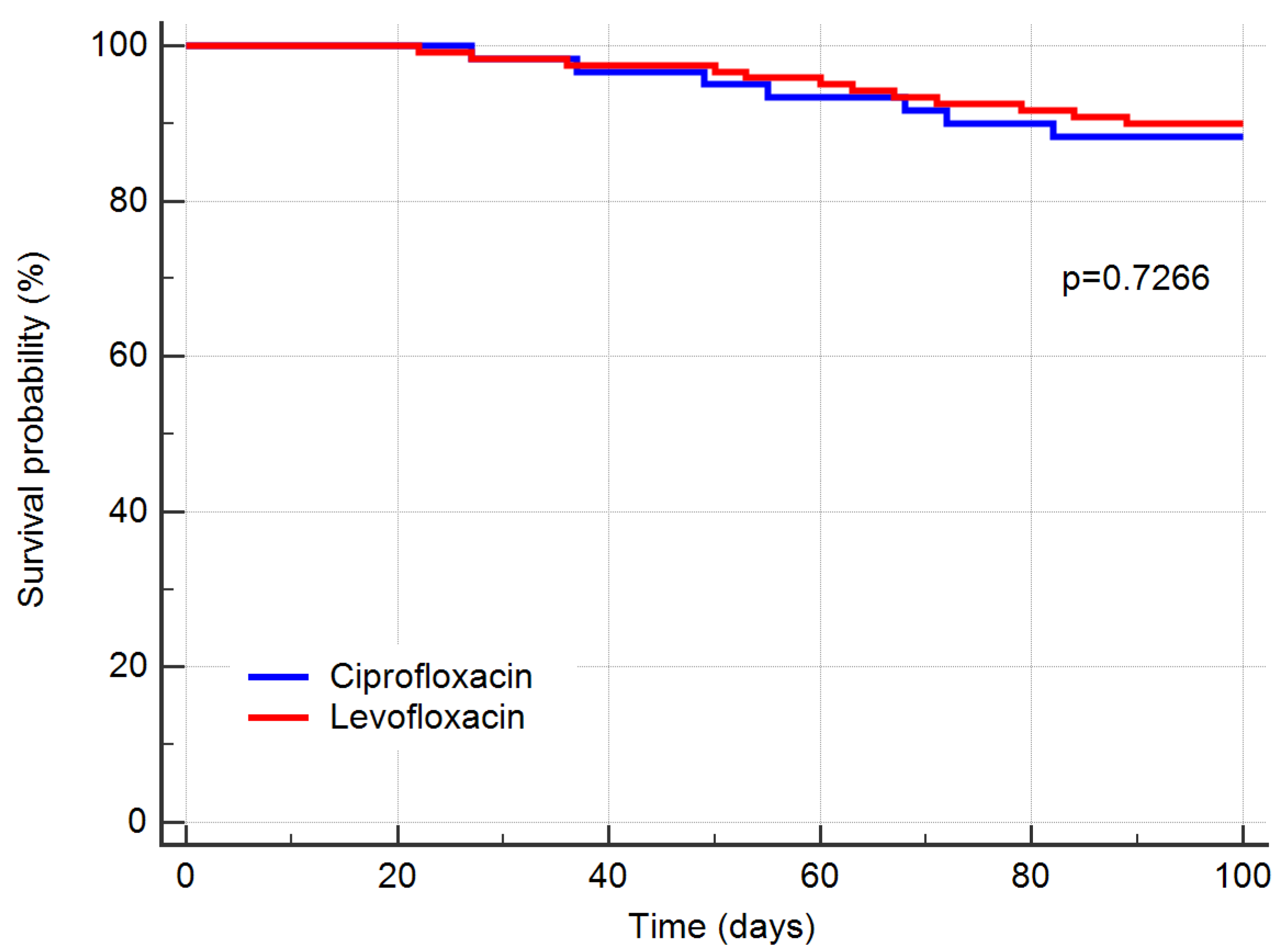
2.2. Treatment Outcomes
3. Discussion
4. Materials and Methods
4.1. Study Design and Patients
4.2. Febrile Neutropenia Treatment Protocol
4.3. Outcomes
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atilla, E.; Atilla, P.A.; Bozdağ, S.C.; Demirer, T. A review of infectious complications after haploidentical hematopoietic stem cell transplantations. Infection 2017, 45, 403–411. [Google Scholar] [CrossRef]
- Collin, B.A.; Leather, H.L.; Wingard, J.R.; Ramphal, R. Evolution, incidence, and susceptibility of bacterial bloodstream isolates from 519 bone marrow transplant patients. Clin. Infect. Dis. 2001, 33, 947–953. [Google Scholar] [CrossRef] [Green Version]
- Gudiol, C.; Garcia-Vidal, C.; Arnan, M.; Sánchez-Ortega, I.; Patiño, B.; Duarte, R.; Carratalà, J. Etiology, clinical features and outcomes of pre-engraftment and post-engraftment bloodstream infection in hematopoietic SCT recipients. Bone Marrow Transplant. 2014, 49, 824–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikulska, M.; Del Bono, V.; Raiola, A.M.; Bruno, B.; Gualandi, F.; Occhini, D.; di Grazia, C.; Frassoni, F.; Bacigalupo, A.; Viscoli, C. Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: Reemergence of Gram-negative rods and increasing antibiotic resistance. Biol. Blood Marrow Transplant. 2009, 15, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, B.; Noda, A.; Godbout, E.; Stevens, M.; Noda, C. Levofloxacin for Antibacterial Prophylaxis in Pediatric Patients with Acute Myeloid Leukemia or Undergoing Hematopoietic Stem Cell Transplantation. J. Pediatric Pharmacol. Ther. 2020, 25, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Dandoy, C.E.; Kelley, T.; Gaur, A.H.; Nagarajan, R.; Demmel, K.; Alonso, P.B.; Guinipero, T.; Savelli, S.; Hakim, H.; Owings, A.; et al. Outcomes after bloodstream infection in hospitalized pediatric hematology/oncology and stem cell transplant patients. Pediatric Blood Cancer 2019, 66, e27978. [Google Scholar] [CrossRef] [PubMed]
- Oltolini, C.; Greco, R.; Galli, L.; Clerici, D.; Lorentino, F.; Xue, E.; Lupo Stanghellini, M.T.; Giglio, F.; Uhr, L.; Ripa, M.; et al. Infections after Allogenic Transplant with Post-Transplant Cyclophosphamide: Impact of Donor HLA Matching. Biol. Blood Marrow Transplant. 2020, 26, 1179–1188. [Google Scholar] [CrossRef]
- Zheng, C.; Tang, B.; Zhu, X.; Zhang, X.; Zhang, L.; Geng, L.; Liu, H.; Sun, Z. Pre-engraftment bloodstream infections in acute leukemia patients undergoing unrelated cord blood transplantation following intensified myeloablative conditioning without ATG. Ann. Hematol. 2017, 96, 115–124. [Google Scholar] [CrossRef]
- Girmenia, C.; Rossolini, G.M.; Piciocchi, A.; Bertaina, A.; Pisapia, G.; Pastore, D.; Sica, S.; Severino, A.; Cudillo, L.; Ciceri, F.; et al. Infections by carbapenem-resistant Klebsiella pneumoniae in SCT recipients: A nationwide retrospective survey from Italy. Bone Marrow Transplant. 2015, 50, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.H.; Wang, Y.; Mo, X.D.; Sun, Y.Q.; Wang, F.R.; Fu, H.X.; Chen, Y.; Han, T.T.; Kong, J.; Cheng, Y.F.; et al. Incidence, Risk Factors, Microbiology and Outcomes of Pre-engraftment Bloodstream Infection After Haploidentical Hematopoietic Stem Cell Transplantation and Comparison With HLA-identical Sibling Transplantation. Clin. Infect. Dis. 2018, 67, S162–S173. [Google Scholar] [CrossRef]
- Girmenia, C.; Bertaina, A.; Piciocchi, A.; Perruccio, K.; Algarotti, A.; Busca, A.; Cattaneo, C.; Raiola, A.M.; Guidi, S.; Iori, A.P.; et al. Incidence, Risk Factors and Outcome of Pre-engraftment Gram-Negative Bacteremia After Allogeneic and Autologous Hematopoietic Stem Cell Transplantation: An Italian Prospective Multicenter Survey. Clin. Infect. Dis. 2017, 65, 1884–1896. [Google Scholar] [CrossRef]
- Herbers, A.; Haan, A.; van der Velden, W.; Donnelly, J.; Blijlevens, N. Mucositis not neutropenia determines bacteremia among hematopoietic stem cell transplant recipients. Transpl. Infect. Dis. 2014, 16, 279–285. [Google Scholar] [CrossRef]
- Modi, A.; Rybicki, L.; Majhail, N.S.; Mossad, S.B. Severity of acute gastrointestinal graft-vs-host disease is associated with incidence of bloodstream infection after adult allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2020, 22, e13217. [Google Scholar] [CrossRef] [Green Version]
- Gafter-Gvili, A.; Fraser, A.; Paul, M.; Leibovici, L. Meta-analysis: Antibiotic prophylaxis reduces mortality in neutropenic patients. Ann. Intern. Med. 2005, 142, 979–995. [Google Scholar] [CrossRef]
- Owattanapanich, W.; Chayakulkeeree, M. Efficacy of levofloxacin as an antibacterial prophylaxis for acute leukemia patients receiving intensive chemotherapy: A systematic re-view and meta-analysis. Hematology 2019, 24, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Egan, G.; Robinson, P.D.; Martinez, J.P.D.; Alexander, S.; Ammann, R.A.; Dupuis, L.L.; Fisher, B.T.; Lehrnbecher, T.; Phillips, B.; Cabral, S.; et al. Efficacy of antibiotic prophylaxis in patients with cancer and hematopoietic stem cell transplantation recipients: A systematic review of randomized trials. Cancer Med. 2019, 8, 4536–4546. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, M.; Averbuch, D.; Tissot, F.; Cordonnier, C.; Akova, M.; Calandra, T.; Ceppi, M.; Bruzzi, P.; Viscoli, C. Fluoroquinolone prophylaxis in haematological cancer patients with neutropenia: ECIL critical appraisal of previous guidelines. J. Infect. 2018, 76, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Lehrnbecher, T.; Averbuch, D.; Castagnola, E.; Cesaro, S.; Ammann, R.A.; Garcia-Vidal, C.; Kanerva, J.; Lanternier, F.; Mesini, A.; Mikulska, M.; et al. 8th European Conference on Infections in Leukaemia: 2020 guidelines for the use of antibiotics in paediatric patients with cancer or post-haematopoietic cell transplantation. Lancet Oncol. 2021, 22, e270–e280. [Google Scholar] [CrossRef]
- Yeh, T.C.; Liu, H.C.; Hou, J.Y.; Chen, K.H.; Huang, T.H.; Chang, C.Y.; Liang, D.C. Severe infections in children with acute leukemia undergoing intensive chemotherapy can successfully be pre-vented by ciprofloxacin, voriconazole, or micafungin prophylaxis. Cancer 2014, 120, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.A.; Yousif, D.; Abbassi, M.; Elborai, Y.; Elhaddad, A. Prophylactic levofloxacin in pediatric neutropenic patients during autologous hematopoietic stem cell transplantation. Clin. Transplant. 2015, 29, 1112–1118. [Google Scholar] [CrossRef]
- Yousef, A.A.; Fryer, C.J.; Chedid, F.D.; Abbas, A.A.; Felimban, S.K.; Khattab, T.M. A pilot study of prophylactic ciprofloxacin during delayed intensification in children with acute lymphoblastic leukemia. Pediatric Blood Cancer 2004, 43, 637–643. [Google Scholar] [CrossRef]
- Wolf, J.; Tang, L.; Flynn, P.M.; Pui, C.H.; Gaur, A.H.; Sun, Y.; Inaba, H.; Stewart, T.; Hayden, R.T.; Hakim, H.; et al. Levofloxacin Prophylaxis During Induction Therapy for Pediatric Acute Lymphoblastic Leukemia. Clin. Infect. Dis. 2017, 65, 1790–1798. [Google Scholar] [CrossRef] [Green Version]
- Alexander, S.; Fisher, B.T.; Gaur, A.H.; Dvorak, C.C.; Luna, D.V.; Dang, H.; Chen, L.; Green, M.; Nieder, M.L.; Fisher, B.; et al. Effect of Levofloxacin Prophylaxis on Bacteremia in Children with Acute Leukemia or Undergoing Hematopoietic Stem Cell Transplantation: A Randomized Clinical Trial. JAMA 2018, 320, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Schimpff, S.C.; Greene, W.H.; Young, V.M.; Wiernik, P.H. Significance of Pseudomonas aeruginosa in the patient with leukemia or lymphoma. J. Infect. Dis. 1974, 130, S24–S31. [Google Scholar] [CrossRef]
- Bodey, G.P.; Jadeja, L.; Elting, L. Pseudomonas bacteremia: Retrospective analysis of 410 episodes. Arch. Intern. Med. 1985, 145, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, M.; Rampazzo, R.; Malena, M.; Lazzarini, L.; Todeschini, G.; Messori, A.; Concia, E. Prophylaxis with fluoroquinolones for bacterial infections in neutropenic patients: A meta-analysis. Clin. Infect. Dis. 1996, 23, 795–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gafter-Gvili, A.; Fraser, A.; Paul, M.; van de Wetering, M.; Kremer, L.; Leibovici, L. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst. Rev. 2005, 19, CD004386. [Google Scholar] [CrossRef] [Green Version]
- Copeland, V.; McLaughlin, M.; Trifilio, S. Ciprofloxacin vs levofloxacin for prophylaxis during hematopoietic stem-cell transplantation. Clin. Transplant. 2018, 32, e13145. [Google Scholar] [CrossRef]
- Rambaran, K.A.; Seifert, C.F. Ciprofloxacin vs. levofloxacin for prophylaxis in recipients of hematopoietic stem cell transplantation. J. Oncol. Pharm. Pract. 2019, 25, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.C.; Riva, E.; Mosquera, R.; Galeano, S.; Pierri, S.; Bello, L.; Caneiro, A.; Gai, R.; Miller, A.; Muxi, P. Comparison of two different anti-infectious approaches after high-dose chemotherapy and autologous stem cell transplantation for hematologic malignancies in a 12-year period in British Hospital, Uruguay. Ann. Hematol. 2020, 99, 877–884. [Google Scholar] [CrossRef]
- Guthrie, K.A.; Yong, M.; Frieze, D.; Corey, L.; Fredricks, D.N. The impact of a change in antibacterial prophylaxis from ceftazidime to levofloxacin in allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2010, 45, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Bucaneve, G.; Micozzi, A.; Menichetti, F.; Martino, P.; Dionisi, M.S.; Martinelli, G.; Allione, B.; D’Antonio, D.; Buelli, M.; Nosari, A.M.; et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N. Engl. J. Med. 2005, 353, 977–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, M.; Landsburg, D.; Pegues, D.; Bilker, W.; Gilmar, C.; Kucharczuk, C.; Gorman, T.; Bink, K.; Moore, A.; Fitzpatrick, R.; et al. Fluoroquinolone Prophylaxis Is Highly Effective for the Prevention of Central Line-Associated Bloodstream Infections in Autologous Stem Cell Transplant Patients. Biol. Blood Marrow Transplant. 2019, 25, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Carlone, G.; Simeone, R.; Baraldo, M.; Maestro, A.; Zanon, D.; Barbi, E.; Maximova, N. Area-under-the-Curve-Based Mycophenolate Mofetil Dosage May Contribute to Decrease the Incidence of Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation in Pediatric Patients. J. Clin. Med. 2021, 10, 406. [Google Scholar] [CrossRef]
- Maximova, N.; Schillani, G.; Simeone, R.; Maestro, A.; Zanon, D. Comparison of Efficacy and Safety of Caspofungin Versus Micafungin in Pediatric Allogeneic Stem Cell Transplant Recipients: A Retrospective Analysis. Adv. Ther. 2017, 34, 1184–1199. [Google Scholar] [CrossRef] [PubMed]
- Haeusler, G.M.; Phillips, R.S.; Lehrnbecher, T.; Thursky, K.A.; Sung, L.; Ammann, R.A. Core outcomes and definitions for pediatric fever and neutropenia research: A consensus statement from an international panel. Pediatric Blood Cancer 2015, 62, 483–489. [Google Scholar] [CrossRef]
- Goldstein, B.; Giroir, B.; Randolph, A. International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatric Crit. Care Med. 2005, 6, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CDC/NHSN Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-Central Line-Associated Bloodstream Infection). Centers for Disease Control and Prevention, and the National Healthcare Safety Network. 2018. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf (accessed on 13 December 2021).

| Baseline Characteristics | Ciprofloxacin Group | Levofloxacin Group | p-Value |
|---|---|---|---|
| Number of patients (%) | 60 (33.3) | 120 (66.7) | - |
| Gender, male/female, number (%) | 39/21 (65/35) | 76/44 (64.3/36.7) | 0.8704 |
| Age, median (IQR), years | 8.5 (4–13) | 8.0 (4–13) | 0.9854 |
| Primary diagnosis, number (%): | |||
| acute lymphoblastic leukemia | 30 (50.0) | 57 (47.5) | 0.7548 |
| acute myeloid leukemia | 11 (18.3) | 14 (11.7) | 0.2555 |
| myelodysplastic syndrome | 1 (1.7) | 5 (4.2) | 0.6625 |
| solid tumor | 13 (21.7) | 28 (23.3) | 0.8525 |
| non-malignant disease | 5 (8.3) | 16 (13.3) | 0.4608 |
| Allogeneic transplant, number (%) | 60 (100) | 120 (100) | - |
| Myeloablative conditioning, number (%): | 60 (100) | 120 (100) | - |
| chemotherapy-based | 36 (60.0) | 67 (55.8) | 0.6341 |
| TBI-based | 24 (40.0) | 53 (44.2) | 0.6344 |
| ATG use, number (%) | 39 (65.0) | 81 (67.5) | 0.7403 |
| Graft cell dose, median (IQR) | |||
| CD34 + cells × 106/kg | 8.6 (5.7–11.1) | 7.5 (5.9–10.5) | 0.3973 |
| TNC × 108/kg | 5.4 (4.5–8.2) | 5.6 (4.8–8.1) | 0.8156 |
| Duration of neutropenia, median (IQR), days | 18 (15–20) | 16 (13–19.7) | 0.0779 |
| Duration of aplasia, median (IQR), days | 11 (10–12) | 10 (9–11) | <0.001 |
| Duration of prophylaxis, median (IQR), days | 10 (7.2–14) | 12 (8–17) | 0.3475 |
| Supportive care interventions, number (%): | |||
| prophylactic G-CSF | 16 (26.7) | 28 (23.3) | 0.7133 |
| steroids for >10 days consecutively | 25 (41.7) | 47 (39.2) | 0.7498 |
| steroids ≥ 2 mg/kg >7 days consecutively | 16 (26.7) | 29 (24.2) | 0.7184 |
| Acute GVHD grade II-IV, number (%): | 32 (53.3) | 9 (7.5) | <0.0001 |
| Length of stay, median (IQR), days | 43.5 (38–48) | 42 (37–48) | 0.6236 |
| Readmission,* number (%) | 22 (36.6) | 37 (30.8) | 0.501 |
| Infection-related readmission,* number (%) | 9 (15.0) | 11 (9.2) | 0.314 |
| Outcomes | Ciprofloxacin Group (n = 60) | Levofloxacin Group (n = 120) | p-Value |
|---|---|---|---|
| Febrile neutropenia, number (%) | 22 (36.7) | 40 (33.3) | 0.7397 |
| Bloodstream infection, number (%): | 17 (28.3) | 18 (15.0) | <0.05 |
| at the first episode of febrile neutropenia | 9 (15.0) | 6 (5.0) | <0.05 |
| within 30 days of transplantation | 15 (25.0) | 14 (11.7) | <0.05 |
| before neutrophil engraftment | 12 (20.0) | 11 (9.2) | 0.0567 |
| associated with severe sepsis | 5 (8.3) | 4 (3.3) | 0.1624 |
| Gram-positive bacteremia | 12 (20.0) | 10 (8.3) | <0.05 |
| Gram-negative bacteremia | 7 (11.7) | 9 (7.5) | 0.408 |
| Polymicrobial | 2 (3.3) | 2 (1.7) | 0.6016 |
| CLABSI | 5 (8.3) | 6 (5.0) | 0.51 |
| Clinically documented infection, number (%) | 32 (53.3) | 35 (29.2) | <0.05 |
| Invasive fungal infection, number (%) | 8 (13.3) | 9 (7.5) | 0.783 |
| Clostridium difficile infection, number (%) | 9 (15.0) | 3 (2.5) | <0.05 |
| Overall antibiotic exposure, median (IQR), days: | |||
| within day + 30 | 21 (16–25) | 13 (9–19) | <0.0001 |
| within day + 100 | 38 (34.5–41.5) | 31 (31–33) | <0.05 |
| 90-day overall mortality, number (%): | 6 (1.0) | 10 (8.3) | 1 |
| infection-related | 2 (3.3) | 4 (3.3) | 1 |
| bacteria-related | 1 (1.7) | 2 (1.7) | 1 |
| 30-day overall mortality, number (%): | 1 (1.7) | 2 (1.7) | 1 |
| infection-related | 1 (1.7) | 1 (0.8) | 1 |
| bacteria-related | 1 (1.7) | 1 (0.8) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Servidio, A.G.; Simeone, R.; Zanon, D.; Barbi, E.; Maximova, N. Levofloxacin Versus Ciprofloxacin-Based Prophylaxis during the Pre-Engraftment Phase in Allogeneic Hematopoietic Stem Cell Transplant Pediatric Recipients: A Single-Center Retrospective Matched Analysis. Antibiotics 2021, 10, 1523. https://doi.org/10.3390/antibiotics10121523
Servidio AG, Simeone R, Zanon D, Barbi E, Maximova N. Levofloxacin Versus Ciprofloxacin-Based Prophylaxis during the Pre-Engraftment Phase in Allogeneic Hematopoietic Stem Cell Transplant Pediatric Recipients: A Single-Center Retrospective Matched Analysis. Antibiotics. 2021; 10(12):1523. https://doi.org/10.3390/antibiotics10121523
Chicago/Turabian StyleServidio, Alessia G., Roberto Simeone, Davide Zanon, Egidio Barbi, and Natalia Maximova. 2021. "Levofloxacin Versus Ciprofloxacin-Based Prophylaxis during the Pre-Engraftment Phase in Allogeneic Hematopoietic Stem Cell Transplant Pediatric Recipients: A Single-Center Retrospective Matched Analysis" Antibiotics 10, no. 12: 1523. https://doi.org/10.3390/antibiotics10121523
APA StyleServidio, A. G., Simeone, R., Zanon, D., Barbi, E., & Maximova, N. (2021). Levofloxacin Versus Ciprofloxacin-Based Prophylaxis during the Pre-Engraftment Phase in Allogeneic Hematopoietic Stem Cell Transplant Pediatric Recipients: A Single-Center Retrospective Matched Analysis. Antibiotics, 10(12), 1523. https://doi.org/10.3390/antibiotics10121523







