Characterization and Molecular Determinants for β-Lactam Specificity of the Multidrug Efflux Pump AcrD from Salmonella typhimurium
Abstract
1. Introduction
2. Results
2.1. Substrate Specificity of St_AcrD
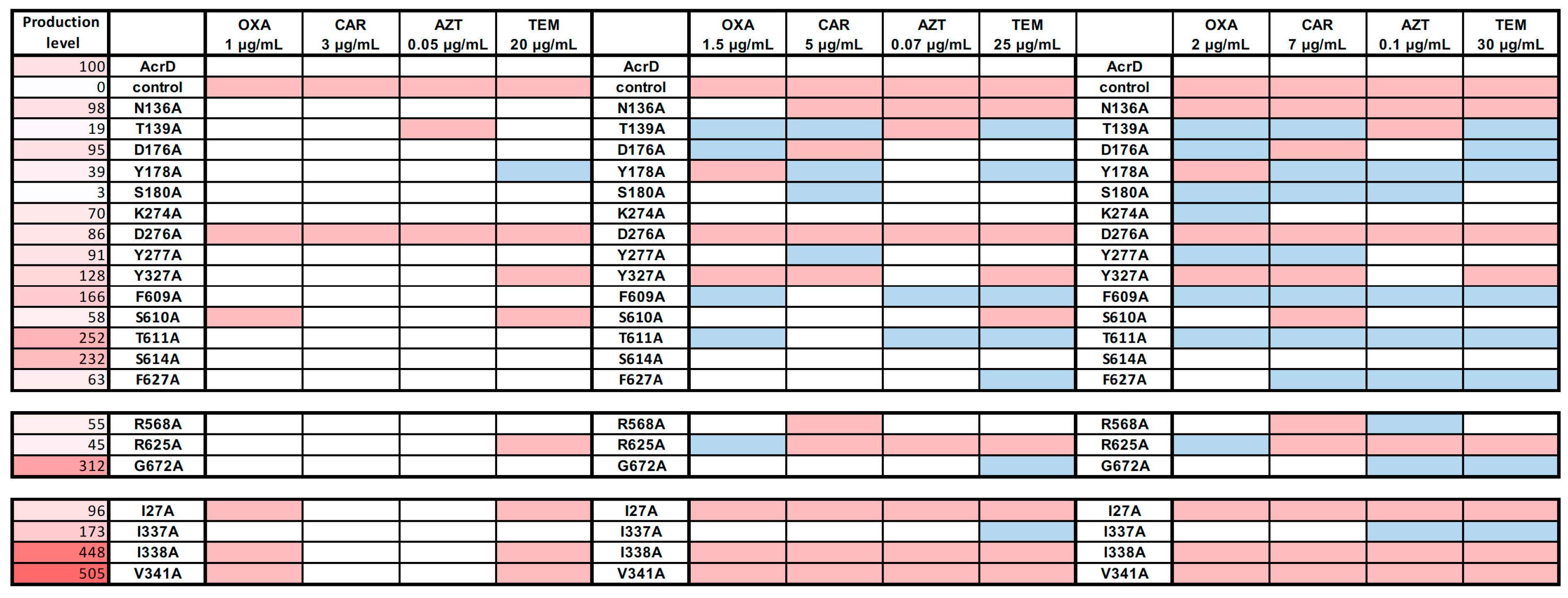
2.2. Site-Directed Mutagenesis of Substrate Binding Pocket Residues
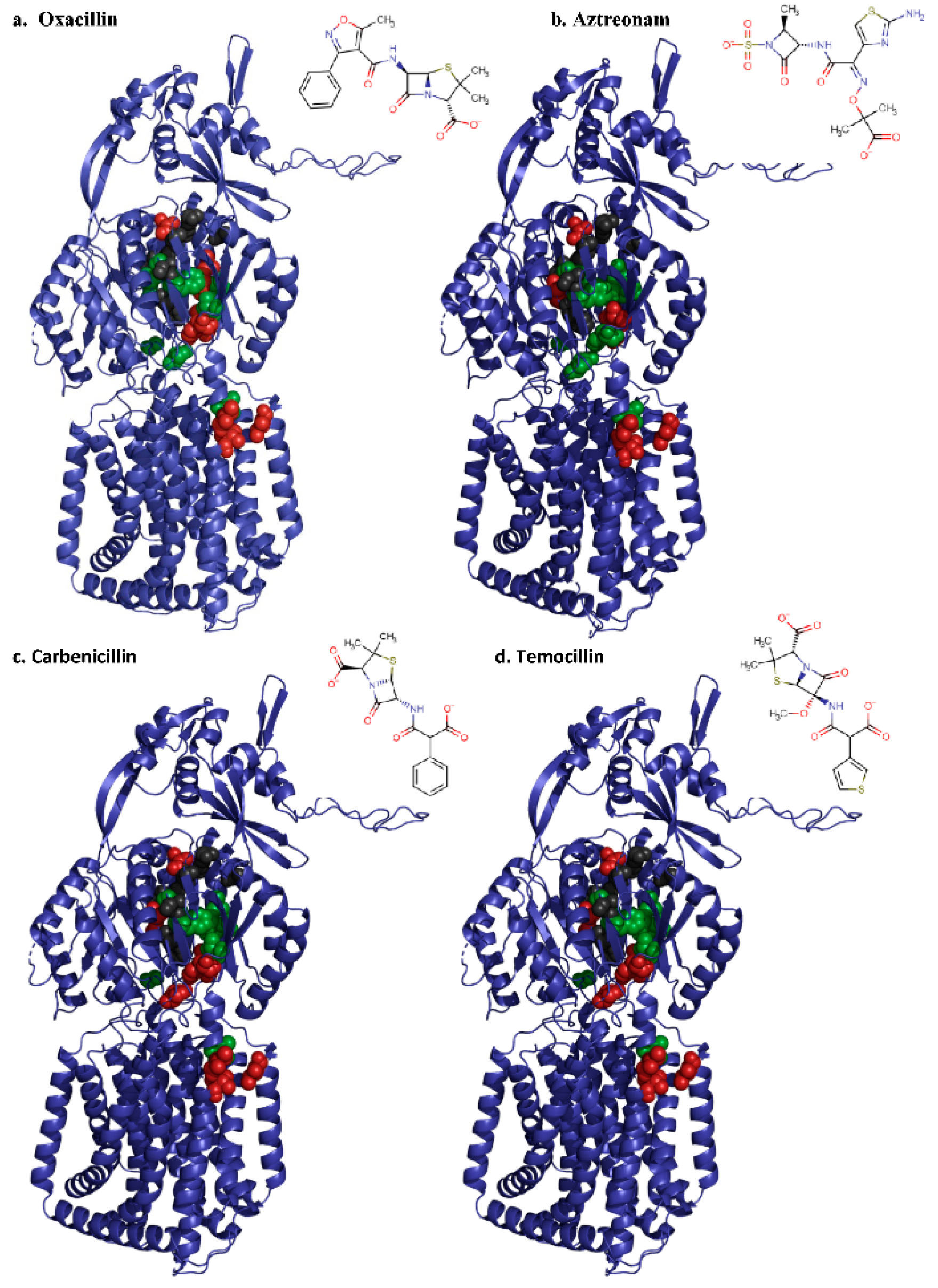
2.2.1. Deep Binding Pocket Variants
2.2.2. Access Pocket Variants
2.2.3. Substitution Variants in the TM1/TM2 Groove
3. Discussion
4. Materials and Methods
4.1. Site-Directed Mutagenesis
4.2. Antimicrobial Susceptibility Testing: Plate Dilution Assay and MIC Determination
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.Z.; Nikaido, H. Efflux-mediated drug resistance in bacteria: An update. Drugs 2009, 69, 1555–1623. [Google Scholar] [CrossRef]
- Li, X.Z.; Plesiat, P.; Nikaido, H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef]
- Colclough, A.L.; Alav, I.; Whittle, E.E.; Pugh, H.L.; Darby, E.M.; Legood, S.W.; McNeil, H.E.; Blair, J.M. RND efflux pumps in Gram-negative bacteria; regulation, structure and role in antibiotic resistance. Future Microbiol. 2020, 15, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Alav, I.; Kobylka, J.; Kuth, M.S.; Pos, K.M.; Picard, M.; Blair, J.M.A.; Bavro, V.N. Structure, assembly, and function of tripartite efflux and type 1 secretion systems in gram-negative bacteria. Chem. Rev. 2021, 121, 5479–5596. [Google Scholar] [CrossRef] [PubMed]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef]
- Kvist, M.; Hancock, V.; Klemm, P. Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl. Environ. Microbiol. 2008, 74, 7376–7382. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lopez, C.R.; Zechiedrich, E.L. Quorum sensing and multidrug transporters in Escherichia coli. Proc. Natl. Acad. Sci. USA 2006, 103, 2386–2391. [Google Scholar] [CrossRef]
- Thanassi, D.G.; Cheng, L.W.; Nikaido, H. Active efflux of bile salts by Escherichia coli. J. Bacteriol. 1997, 179, 2512–2518. [Google Scholar] [CrossRef]
- Wang-Kan, X.; Blair, J.M.A.; Chirullo, B.; Betts, J.; La Ragione, R.M.; Ivens, A.; Ricci, V.; Opperman, T.J.; Piddock, L.J.V. Lack of AcrB efflux function confers loss of virulence on Salmonella enterica Serovar Typhimurium. mBio 2017, 8, e00968-17. [Google Scholar] [CrossRef]
- El Meouche, I.; Dunlop, M.J. Heterogeneity in efflux pump expression predisposes antibiotic-resistant cells to mutation. Science 2018, 362, 686–690. [Google Scholar] [CrossRef]
- Nikaido, H. RND transporters in the living world. Res. Microbiol. 2018, 169, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Elbourne, L.D.; Tetu, S.G.; Hassan, K.A.; Paulsen, I.T. TransportDB 2.0: A database for exploring membrane transporters in sequenced genomes from all domains of life. Nucleic Acids Res. 2017, 45, D320–D324. [Google Scholar] [CrossRef]
- Klenotic, P.A.; Moseng, M.A.; Morgan, C.E.; Yu, E.W. Structural and functional diversity of resistance-nodulation-cell division transporters. Chem. Rev. 2021, 121, 5378–5416. [Google Scholar] [CrossRef] [PubMed]
- Nishino, K.; Latifi, T.; Groisman, E.A. Virulence and drug resistance roles of multidrug efflux systems of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 2006, 59, 126–141. [Google Scholar] [CrossRef] [PubMed]
- McClelland, M.; Sanderson, K.E.; Spieth, J.; Clifton, S.W.; Latreille, P.; Courtney, L.; Porwollik, S.; Ali, J.; Dante, M.; Du, F.; et al. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 2001, 413, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Eaves, D.J.; Ricci, V.; Piddock, L.J. Expression of acrB, acrF, acrD, marA, and soxS in Salmonella enterica serovar Typhimurium: Role in multiple antibiotic resistance. Antimicrob. Agents Chemother. 2004, 48, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Buckner, M.M.; Blair, J.M.; La Ragione, R.M.; Newcombe, J.; Dwyer, D.J.; Ivens, A.; Piddock, L.J. Beyond antimicrobial resistance: Evidence for a distinct role of the AcrD Efflux pump in Salmonella biology. mBio 2016, 7, e01916-16. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef]
- Blair, J.M.; Smith, H.E.; Ricci, V.; Lawler, A.J.; Thompson, L.J.; Piddock, L.J. Expression of homologous RND efflux pump genes is dependent upon AcrB expression: Implications for efflux and virulence inhibitor design. J. Antimicrob. Chemother. 2015, 70, 424–431. [Google Scholar] [CrossRef]
- Yamasaki, S.; Nagasawa, S.; Hayashi-Nishino, M.; Yamaguchi, A.; Nishino, K. AcrA dependency of the AcrD efflux pump in Salmonella enterica serovar Typhimurium. J. Antibiot. 2011, 64, 433–437. [Google Scholar] [CrossRef]
- Kobayashi, N.; Tamura, N.; van Veen, H.W.; Yamaguchi, A.; Murakami, S. beta-Lactam selectivity of multidrug transporters AcrB and AcrD resides in the proximal binding pocket. J. Biol. Chem. 2014, 289, 10680–10690. [Google Scholar] [CrossRef] [PubMed]
- Aires, J.R.; Nikaido, H. Aminoglycosides are captured from both periplasm and cytoplasm by the AcrD multidrug efflux transporter of Escherichia coli. J. Bacteriol. 2005, 187, 1923–1929. [Google Scholar] [CrossRef]
- Rosenberg, E.Y.; Ma, D.; Nikaido, H. AcrD of Escherichia coli is an aminoglycoside efflux pump. J. Bacteriol. 2000, 182, 1754–1756. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.K.; Vargiu, A.V.; Malloci, G.; Dreier, J.; Ruggerone, P. Molecular rationale behind the differential substrate specificity of bacterial RND multi-drug transporters. Sci. Rep. 2017, 7, 8075. [Google Scholar] [CrossRef] [PubMed]
- Oswald, C.; Tam, H.K.; Pos, K.M. Transport of lipophilic carboxylates is mediated by transmembrane helix 2 in multidrug transporter AcrB. Nat. Commun. 2016, 7, 13819. [Google Scholar] [CrossRef]
- Rahman, M.M.; Matsuo, T.; Ogawa, W.; Koterasawa, M.; Kuroda, T.; Tsuchiya, T. Molecular cloning and characterization of all RND-type efflux transporters in Vibrio cholerae non-O1. Microbiol. Immunol. 2007, 51, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, J.A.; Schuster, S.; Seeger, M.A.; Fahnrich, E.; Pos, K.M.; Kern, W.V. Site-directed mutagenesis reveals putative substrate binding residues in the Escherichia coli RND efflux pump AcrB. J. Bacteriol. 2008, 190, 8225–8229. [Google Scholar] [CrossRef]
- Eicher, T.; Cha, H.J.; Seeger, M.A.; Brandstätter, L.; El-Delik, J.; Bohnert, J.A.; Kern, W.V.; Verrey, F.; Grutter, M.G.; Diederichs, K.; et al. Transport of drugs by the multidrug transporter AcrB involves an access and a deep binding pocket that are separated by a switch-loop. Proc. Natl. Acad. Sci. USA 2012, 109, 5687–5692. [Google Scholar] [CrossRef]
- Lu, W.; Zhong, M.; Chai, Q.; Wang, Z.; Yu, L.; Wei, Y. Functional relevance of AcrB Trimerization in pump assembly and substrate binding. PLoS ONE 2014, 9, e89143. [Google Scholar] [CrossRef][Green Version]
- Tam, H.K.; Foong, W.E.; Oswald, C.; Herrmann, A.; Zeng, H.; Pos, K.M. Allosteric drug transport mechanism of multidrug transporter AcrB. Nat. Commun. 2021, 12, 3889. [Google Scholar] [CrossRef] [PubMed]
- Geertsma, E.R.; Groeneveld, M.; Slotboom, D.J.; Poolman, B. Quality control of overexpressed membrane proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 5722–5727. [Google Scholar] [CrossRef] [PubMed]
- Ruggerone, P.; Murakami, S.; Pos, K.M.; Vargiu, A.V. RND efflux pumps: Structural information translated into function and inhibition mechanisms. Curr. Top. Med. Chem. 2013, 13, 3079–3100. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H.; Basina, M.; Nguyen, V.; Rosenberg, E.Y. Multidrug efflux pump AcrAB of Salmonella typhimurium excretes only those beta-lactam antibiotics containing lipophilic side chains. J. Bacteriol. 1998, 180, 4686–4692. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.J.; Muller, R.T.; Pos, K.M. Switch-loop flexibility affects transport of large drugs by the promiscuous AcrB multidrug efflux transporter. Antimicrob. Agents Chemother. 2014, 58, 4767–4772. [Google Scholar] [CrossRef]
- Nakashima, R.; Sakurai, K.; Yamasaki, S.; Nishino, K.; Yamaguchi, A. Structures of the multidrug exporter AcrB reveal a proximal multisite drug-binding pocket. Nature 2011, 480, 565–569. [Google Scholar] [CrossRef] [PubMed]
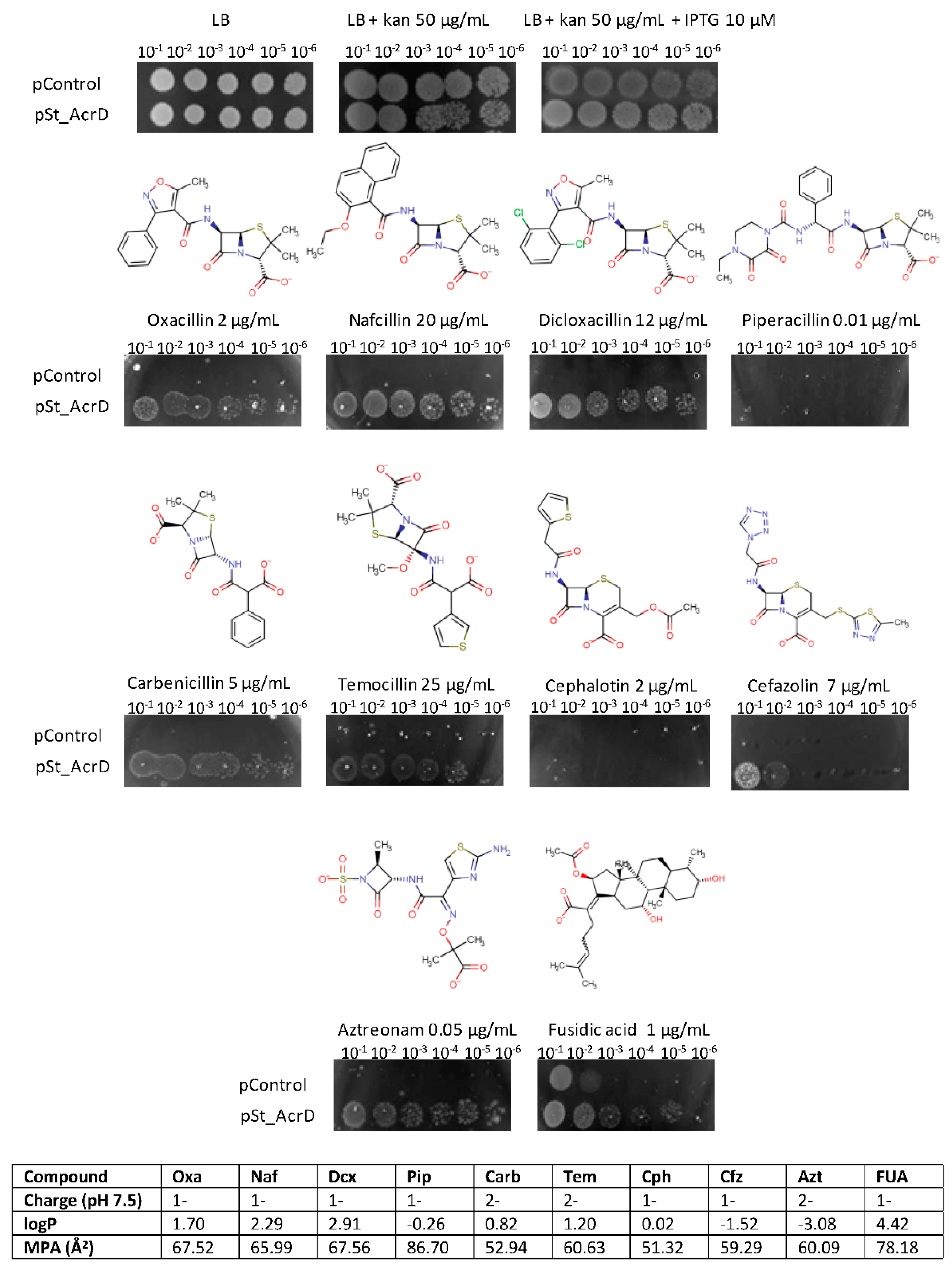
| MIC (µg/mL) | ||
|---|---|---|
| Antibiotic | pControl | pSt_AcrD |
| Ampicillin | 0.75 | 1 |
| Temocillin | 0.75 | 6 |
| Piperacillin | 0.047 | 0.064 |
| Oxacillin | 0.125 | 2 |
| Penicillin | 8 | 8 |
| Mecillinam | 0.023 | 0.032 |
| Aztreonam | 0.032 | 0.25 |
| Cefotaxime | <0.016 | <0.016 |
| Cefoxitin | 0.5 | 1 |
| Cefepime | ≤0.016 | 0.016 |
| Ceftriaxone | 0.023 | 0.016 |
| Cefuroxime | 0.125 | 0.125 |
| Ceftaroline | ≤0.016 | 0.016 |
| Ceftazidime | 0.047 | 0.047 |
| Cefazolin | 2 | 16 |
| Cephalotin | 2 | 12 |
| Cefpodoxime | 0.064 | 0.094 |
| Imipenem | 0.125 | 0.25 |
| Doripenem | 0.032 | 0.064 |
| Meropenem | 0.023 | 0.023 |
| Tetracycline | 0.25 | 0.25 |
| Fusidic acid | 2 | 4 |
| Erythromycin | 0.75 | 1.5 |
| Chloramphenicol | 0.5 | 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuesta Bernal, J.; El-Delik, J.; Göttig, S.; Pos, K.M. Characterization and Molecular Determinants for β-Lactam Specificity of the Multidrug Efflux Pump AcrD from Salmonella typhimurium. Antibiotics 2021, 10, 1494. https://doi.org/10.3390/antibiotics10121494
Cuesta Bernal J, El-Delik J, Göttig S, Pos KM. Characterization and Molecular Determinants for β-Lactam Specificity of the Multidrug Efflux Pump AcrD from Salmonella typhimurium. Antibiotics. 2021; 10(12):1494. https://doi.org/10.3390/antibiotics10121494
Chicago/Turabian StyleCuesta Bernal, Jenifer, Jasmin El-Delik, Stephan Göttig, and Klaas M. Pos. 2021. "Characterization and Molecular Determinants for β-Lactam Specificity of the Multidrug Efflux Pump AcrD from Salmonella typhimurium" Antibiotics 10, no. 12: 1494. https://doi.org/10.3390/antibiotics10121494
APA StyleCuesta Bernal, J., El-Delik, J., Göttig, S., & Pos, K. M. (2021). Characterization and Molecular Determinants for β-Lactam Specificity of the Multidrug Efflux Pump AcrD from Salmonella typhimurium. Antibiotics, 10(12), 1494. https://doi.org/10.3390/antibiotics10121494






