Clonal Dissemination of Plasmid-Mediated Carbapenem and Colistin Resistance in Refugees Living in Overcrowded Camps in North Lebanon
Abstract
1. Introduction
2. Materials and Methods
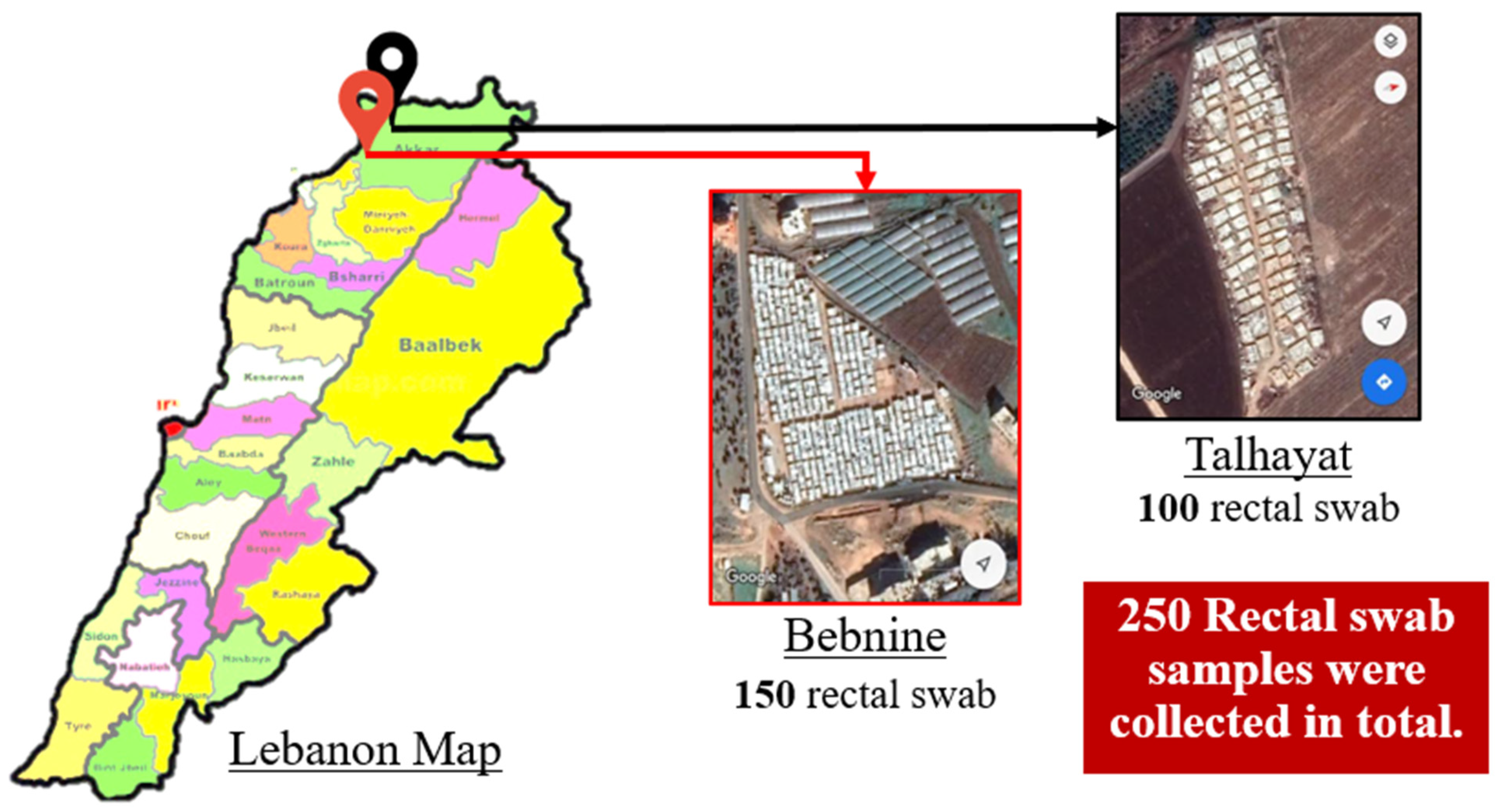
2.1. Study Design
2.2. Bacterial Identification
2.3. Antibiotic Susceptibility Profile
2.4. Phenotypic Detection of Carbapenemase Activity
2.5. DNA Extraction
2.6. Molecular Identification of Carbapenem and Colistin Resistance Genes
2.7. Multilocus Sequence Typing (MLST)
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of Antimicrobial Resistance in the Environment and Its Relevance to Environmental Regulators. Front. Microbiol. 2016, 7, 1728. [Google Scholar] [CrossRef]
- Davies, J. Origins and Evolution of Antibiotic Resistance. Microbiología 1996, 12, 9–16. [Google Scholar] [CrossRef]
- Kadri, S.S. Key Takeaways from the U.S. CDC’s 2019 Antibiotic Resistance Threats Report for Frontline Providers. Crit. Care Med. 2020, 48, 939–945. [Google Scholar] [CrossRef]
- WHO. Library Cataloguing-In-Publication Data Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015; ISBN 9789241509763. [Google Scholar]
- Mellouk, F.Z.; Bakour, S.; Meradji, S.; Al-Bayssari, C.; Bentakouk, M.C.; Zouyed, F.; Djahoudi, A.; Boutefnouchet, N.; Rolain, J.M. First Detection of VIM-4-Producing Pseudomonas Aeruginosa and OXA-48-Producing Klebsiella Pneumoniae in Northeastern (Annaba, Skikda) Algeria. Microb. Drug Resist. 2017, 23, 335–344. [Google Scholar] [CrossRef]
- Carmeli, Y.; Akova, M.; Cornaglia, G.; Daikos, G.L.; Garau, J.; Harbarth, S.; Rossolini, G.M.; Souli, M.; Giamarellou, H. Controlling the Spread of Carbapenemase-Producing Gram-Negatives: Therapeutic Approach and Infection Control. Clin. Microbiol. Infect. 2010, 16, 102–111. [Google Scholar] [CrossRef]
- Taggar, G.; Rheman, M.A.; Boerlin, P.; Diarra, M.S. Molecular Epidemiology of Carbapenemases in Enterobacteriales from Humans, Animals, Food and the Environment. Antibiotics 2020, 9, 693. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Naas, T.; Poirel, L. Global Spread of Carbapenemase Producing Enterobacteriaceae. Emerg. Infect. Dis. 2011, 17, 1791–1798. [Google Scholar] [CrossRef]
- Souli, M.; Galani, I.; Giamarellou, H. Emergence of Extensively Drug-Resistant and Pandrug-Resistant Gram-Negative Bacilli in Europe. Euro Surveill. 2008, 13, 19045. [Google Scholar] [CrossRef] [PubMed]
- Giamarellou, H.; Poulakou, G. Multidrug-Resistant Gram-Negative Infections What Are the Treatment Options? Drugs 2009, 69, 1879–1901. [Google Scholar] [CrossRef]
- Tilahun, M.; Kassa, Y.; Gedefie, A.; Belete, M.A. Emerging Carbapenem-Resistant Enterobacteriaceae Infection, Its Epidemiology and Novel Treatment Options: A Review. Infect. Drug Resist. 2021, 14, 4363–4374. [Google Scholar] [CrossRef] [PubMed]
- Karki, D.; Dhungel, B.; Bhandari, S.; Kunwar, A.; Joshi, P.R.; Shrestha, B.; Rijal, K.R.; Ghimire, P.; Banjara, M.R. Antibiotic Resistance and Detection of Plasmid Mediated Colistin Resistance Mcr-1 Gene among Escherichia Coli and Klebsiella Pneumoniae Isolated from Clinical Samples. Gut Pathog. 2021, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Adhikari, N.; Shah, A.K.; Lamichhane, I.; Dhungel, B.; Shrestha, U.T.; Adhikari, B.; Banjara, M.R.; Ghimire, P.; Rijal, K.R. Detection of NDM-1 and VIM Genes in Carbapenem-Resistant Klebsiella Pneumoniae Isolates from a Tertiary Health-Care Center in Kathmandu, Nepal. Chemotherapy 2021, 66, 199–209. [Google Scholar] [CrossRef]
- Magi, G.; Tontarelli, F.; Caucci, S.; di Sante, L.; Brenciani, A.; Morroni, G.; Giovanetti, E.; Menzo, S.; Mingoia, M. High Prevalence of Carbapenem-Resistant Klebsiella Pneumoniae ST307 Recovered from Fecal Samples in an Italian Hospital. Future Microbiol. 2021, 16, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Mairi, A.; Pantel, A.; Sotto, A.; Lavigne, J.P.; Touati, A. OXA-48-like Carbapenemases Producing Enterobacteriaceae in Different Niches. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 587–604. [Google Scholar] [CrossRef]
- Jeannot, K.; Bolard, A.; Plésiat, P. Resistance to Polymyxins in Gram-Negative Organisms. Int. J. Antimicrob. Agents 2017, 49, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kasiakou, S.K.; Saravolatz, L.D. Colistin: The Revival of Polymyxins for the Management of Multidrug-Resistant Gram-Negative Bacterial Infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef]
- Ko, K.S.; Suh, J.Y.; Kwon, K.T.; Jung, S.I.; Park, K.H.; Kang, C.I.; Chung, D.R.; Peck, K.R.; Song, J.H. High Rates of Resistance to Colistin and Polymyxin B in Subgroups of Acinetobacter Baumannii Isolates from Korea. J. Antimicrob. Chemother. 2007, 60, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.Y.; Son, J.S.; Chung, D.R.; Peck, K.R.; Ko, K.S.; Song, J.H. Nonclonal Emergence of Colistin-Resistant Klebsiella Pneumoniae Isolates from Blood Samples in South Korea. Antimicrob. Agents Chemother. 2010, 54, 560–562. [Google Scholar] [CrossRef]
- Baron, S.; Hadjadj, L.; Rolain, J.M.; Olaitan, A.O. Molecular Mechanisms of Polymyxin Resistance: Knowns and Unknowns. Int. J. Antimicrob. Agents 2016, 48, 583–591. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Morand, S.; Rolain, J.M. Mechanisms of Polymyxin Resistance: Acquired and Intrinsic Resistance in Bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef] [PubMed]
- Skiada, A.; Markogiannakis, A.; Plachouras, D.; Daikos, G.L. Adaptive Resistance to Cationic Compounds in Pseudomonas Aeruginosa. Int. J. Antimicrob. Agents 2011, 37, 187–193. [Google Scholar] [CrossRef]
- National Health Statistics Report in Lebanon in Collaboration with Saint Joseph University of Beirut (USJ), World Health Organisation (WHO) and Ministry Of Public Health in LEBANON. National Health Statistics Report in Lebanon-Edition; Ministry Of Public Health in LEBANON: Baabda, Lebanon, 2012. [Google Scholar]
- Saleh, S.; Ammar, W.; Natafgi, N.; Mourad, Y.; Dimassi, H.; Harb, H. Association Entre La Pluralité Des Payeurs, Les Coûts, Les Revenus et La Rentabilité: Étude Transversale Dans Des Hôpitaux Libanais. East. Mediterr. Health J. 2015, 21, 381–388. [Google Scholar] [CrossRef]
- Isenring, E.; Fehr, J.; Gültekin, N.; Schlagenhauf, P. Infectious Disease Profiles of Syrian and Eritrean Migrants Presenting in Europe: A Systematic Review. Travel Med. Infect. Dis. 2018, 25, 65–76. [Google Scholar] [CrossRef]
- Abbara, A.; Rawson, T.M.; Karah, N.; El-Amin, W.; Hatcher, J.; Tajaldin, B.; Dar, O.; Dewachi, O.; Abu Sitta, G.; Uhlin, B.E.; et al. A Summary and Appraisal of Existing Evidence of Antimicrobial Resistance in the Syrian Conflict. Int. J. Infect. Dis. 2018, 75, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Ruppé, E.; Andremont, A. Causes, Consequences, and Perspectives in the Variations of Intestinal Density of Colonization of Multidrug-Resistant Enterobacteria. Front. Microbiol. 2013, 4, 129. [Google Scholar] [CrossRef]
- Bardet, L.; le Page, S.; Leangapichart, T.; Rolain, J.M. LBJMR Medium: A New Polyvalent Culture Medium for Isolating and Selecting Vancomycin and Colistin-Resistant Bacteria. BMC Microbiol. 2017, 17, 220. [Google Scholar] [CrossRef] [PubMed]
- Bakour, S.; Garcia, V.; Loucif, L.; Brunel, J.M.; Gharout-Sait, A.; Touati, A.; Rolain, J.M. Rapid Identification of Carbapenemase-Producing Enterobacteriaceae, Pseudomonas Aeruginosa and Acinetobacter Baumannii Using a Modified Carba NP Test. New Microbes New Infect. 2015, 7, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Yong, D.; Lee, K.; Yum, J.H.; Shin, H.B.; Rossolini, G.M.; Chong, Y. Imipenem-EDTA Disk Method for Differentiation of Metallo-β-Lactamase-Producing Clinical Isolates of Pseudomonas Spp. and Acinetobacter Spp. J. Clin. Microbiol. 2002, 40, 3798–3801. [Google Scholar] [CrossRef]
- Christophy, R.; Osman, M.; Mallat, H.; Achkar, M.; Ziedeh, A.; Moukaddem, W.; Dabboussi, F.; Hamze, M. Prevalence, Antibiotic Susceptibility and Characterization of Antibiotic Resistant Genes among Carbapenem-Resistant Gram-Negative Bacilli and Yeast in Intestinal Flora of Cancer Patients in North Lebanon. J. Infect. Public Health 2017, 10, 716–720. [Google Scholar] [CrossRef]
- Mesli, E.; Berrazeg, M.; Drissi, M.; Bekkhoucha, S.N.; Rolain, J.M. Prevalence of Carbapenemase-Encoding Genes Including New Delhi Metallo-β-Lactamase in Acinetobacter Species, Algeria. Int. J. Infect. Dis. 2013, 17, 739–743. [Google Scholar] [CrossRef]
- Rebelo, A.R.; Bortolaia, V.; Kjeldgaard, J.S.; Pedersen, S.K.; Leekitcharoenphon, P.; Hansen, I.M.; Guerra, B.; Malorny, B.; Borowiak, M.; Hammerl, J.A.; et al. Multiplex PCR for Detection of Plasmid-Mediated Colistin Resistance Determinants, Mcr-1, Mcr-2, Mcr-3, Mcr-4 and Mcr-5 for Surveillance Purposes. Eurosurveillance 2018, 23. [Google Scholar] [CrossRef]
- Nabti, L.Z.; Sahli, F.; Ngaiganam, E.P.; Radji, N.; Mezaghcha, W.; Lupande-Mwenebitu, D.; Baron, S.A.; Rolain, J.M.; Diene, S.M. Development of Real-Time PCR Assay Allowed Describing the First Clinical Klebsiella Pneumoniae Isolate Harboring Plasmid-Mediated Colistin Resistance Mcr-8 Gene in Algeria. J. Glob. Antimicrob. Resist. 2020, 20, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Jayol, A.; Poirel, L.; Dortet, L.; Nordmann, P. National Survey of Colistin Resistance Among-Producing Enterobacteriaceae and outbreak Caused by Colistin-Resistant OXA-48-Producing Pneumoniae, France, 2014. Eurosurveillance 2017, 22, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, A.O.; Thongmalayvong, B.; Akkhavong, K.; Somphavong, S.; Paboriboune, P.; Khounsy, S.; Morand, S.; Rolain, J.-M. Clonal Transmission of a Colistinresistant Coli from a domesticated Pig to a Human in Laos. J. Antimicrob. Chemother. 2015, 70, 3404–3405. [Google Scholar]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Choi, Q.; Kwon, G.C.; Koo, S.H. Emergence and Transmission of New Delhi Metallo-Beta-Lactamase-5-Producing Escherichia Coli Sequence Type 361 in a Tertiary Hospital in South Korea. J. Clin. Lab. Anal. 2020, 34, e23041. [Google Scholar] [CrossRef]
- Kanamori, H.; Parobek, C.M.; Juliano, J.J.; Johnson, J.R.; Johnston, B.D.; Johnson, T.J.; Weber, D.J.; Rutala, W.A.; Anderson, D.J. Genomic Analysis of Multidrug-Resistant Escherichia Coli from North Carolina Community Hospitals: Ongoing Circulation of CTX-M-Producing ST131-H30Rx and ST131-H30R1 Strains. Antimicrob. Agents Chemother. 2017, 61, e00912-17. [Google Scholar] [CrossRef]
- Meng, Q.; Bai, X.; Zhao, A.; Lan, R.; Du, H.; Wang, T.; Shi, C.; Yuan, X.; Bai, X.; Ji, S.; et al. Characterization of Shiga Toxin-Producing Escherichia Coli Isolated from Healthy Pigs in China. BMC Microbiol. 2014, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Bleichenbacher, S.; Stevens, M.J.A.; Zurfluh, K.; Perreten, V.; Endimiani, A.; Stephan, R.; Nüesch-Inderbinen, M. Environmental Dissemination of Carbapenemase-Producing Enterobacteriaceae in Rivers in Switzerland. Environ. Pollut. 2020, 265, 115081. [Google Scholar] [CrossRef]
- Dandachi, I.; Chabou, S.; Daoud, Z.; Rolain, J.M. Prevalence and Emergence of Extended-Spectrum Cephalosporin-, Carbapenem- and Colistin-Resistant Gram Negative Bacteria of Animal Origin in the Mediterranean Basin. Front. Microbiol. 2018, 9, 2299. [Google Scholar] [CrossRef]
- Scott, J.R.; Hinds, J.; Gould, K.A.; Millar, E.V.; Reid, R.; Santosham, M.; O’Brien, K.L.; Hanage, W.P. Nontypeable Pneumococcal Isolates among Navajo and White Mountain Apache Communities: Are These Really a Cause of Invasive Disease? J. Infect. Dis. 2012, 206, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Li, Y.; Hao, C. Association between Virulence Profile and Fluoroquinolone Resistance in Escherichia Coli Isolated from Dogs and Cats in China. J. Infect. Dev. Ctries. 2017, 11, 306–313. [Google Scholar] [CrossRef]
- Zhang, L.P.; Xue, W.C.; Meng, D.Y. First Report of New Delhi Metallo-β-Lactamase 5 (NDM-5)-Producing Escherichia Coli from Blood Cultures of Three Leukemia Patients. Int. J. Infect. Dis. 2016, 42, 45–46. [Google Scholar] [CrossRef]
- Patil, S.; Min, J.; Feiqiu, W. Molecular Characterization of Co-Existence of MCR-1 and NDM-1 in Extended-Spectrum β-Lactamase-Producing Escherichia Coli ST648 Isolated from a Colonized Patient in China. Jundishapur J. Microbiol. 2019, 12, 91272. [Google Scholar] [CrossRef]
- Mizuno, Y.; Yamaguchi, T.; Matsumoto, T. A First Case of New Delhi Metallo-β-Lactamase-7 in an Escherichia Coli ST648 Isolate in Japan. J. Infect. Chemother. 2014, 20, 814–816. [Google Scholar] [CrossRef]
- El-Herte, R.I.; Araj, G.F.; Matar, G.M.; Baroud, M.; Kanafani, Z.A.; Kanj, S.S. Detection of Carbapenem-Resistant Escherichia Coli and Klebsiella Pneumoniae Producing NDM-1 in Lebanon. J. Infect. Dev. Ctries. 2012, 6, 457–461. [Google Scholar] [CrossRef]
- Baroud, M.; Dandache, I.; Araj, G.F.; Wakim, R.; Kanj, S.; Kanafani, Z.; Khairallah, M.; Sabra, A.; Shehab, M.; Dbaibo, G.; et al. Underlying Mechanisms of Carbapenem Resistance in Extended-Spectrum β-Lactamase-Producing Klebsiella Pneumoniae and Escherichia Coli Isolates at a Tertiary Care Centre in Lebanon: Role of OXA-48 and NDM-1 Carbapenemases. Int. J. Antimicrob. Agents 2013, 41, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Oteo, J.; Hernández, J.M.; Espasa, M.; Fleites, A.; Sáez, D.; Bautista, V.; Pérez-vázquez, M.; Fernández-garcía, M.D.; Delgado-iribarren, A.; Sánchez-romero, I.; et al. Emergence of OXA-48-Producing Klebsiella Pneumoniae and the Novel Carbapenemases OXA-244 and OXA-245 in Spain. J. Antimicrob. Chemother. 2013, 68, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi, D.; Moubareck, C.A.; Aires, J.; Adaime, A.; Barakat, A.; Fayad, N.; Hakime, N.; Houmani, M.; Itani, T.; Najjar, Z.; et al. Countrywide Spread of OXA-48 Carbapenemase in Lebanon: Surveillance and Genetic Characterization of Carbapenem-Non-Susceptible Enterobacteriaceae in 10 Hospitals over a One-Year Period. Int. J. Infect. Dis. 2014, 29, e139–e144. [Google Scholar] [CrossRef]
- Matar, G.M.; Cuzon, G.; Araj, G.F.; Naas, T.; Corkill, J.; Kattar, M.M.; Nordmann, P. Oxacillinase-mediated resistance to carbapenems in Klebsiella pneumoniae from Lebanon. Clin. Microbiol. Infect. 2008, 9, 887–888. [Google Scholar] [CrossRef]
- Al-Bayssari, C.; Olaitan, A.O.; Leangapichart, T.; Okdah, L.; Dabboussi, F.; Hamze, M.; Rolain, J.M. Whole-Genome Sequence of a BlaOXA-48-Harboring Raoultella Ornithinolytica Clinical Isolate from Lebanon. Antimicrob. Agents Chemother. 2016, 60, 2548–2550. [Google Scholar] [CrossRef]
- Moubareck, C.A.; Mouftah, S.F.; Pál, T.; Ghazawi, A.; Halat, D.H.; Nabi, A.; AlSharhan, M.A.; AlDeesi, Z.O.; Peters, C.C.; Celiloglu, H.; et al. Clonal Emergence of Klebsiella Pneumoniae ST14 Co-Producing OXA-48-Type and NDM Carbapenemases with High Rate of Colistin Resistance in Dubai, United Arab Emirates. Int. J. Antimicrob. Agents 2018, 52, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Giske, C.G.; Fröding, I.; Hasan, C.M.; Turlej-Rogacka, A.; Toleman, M.; Livermore, D.; Woodford, N.; Walsh, T.R. Diverse Sequence Types of Klebsiella Pneumoniae Contribute to the Dissemination of Bla NDM-1 in India, Sweden, and the United Kingdom. Antimicrob. Agents Chemother. 2012, 56, 2735–2738. [Google Scholar] [CrossRef]
- Poirel, L.; Al Maskari, Z.; Al Rashdi, F.; Bernabeu, S.; Nordmann, P. NDM-1-Producing Klebsiella Pneumoniae Isolated in the Sultanate of Oman. J. Antimicrob. Chemother. 2011, 66, 304–306. [Google Scholar] [CrossRef]
- Morris, D.; O’Connor, M.; Izdebski, R.; Corcoran, M.; Ludden, C.E.; McGrath, E.; Buckley, V.; Cryan, B.; Gniadkowski, M.; Cormican, M. Dissemination of Clonally Related Multidrug-Resistant Klebsiella Pneumoniae in Ireland. Epidemiol. Infect. 2016, 144, 443–448. [Google Scholar] [CrossRef]
- Paskova, V.; Medvecky, M.; Skalova, A.; Chudejova, K.; Bitar, I.; Jakubu, V.; Bergerova, T.; Zemlickova, H.; Papagiannitsis, C.C.; Hrabak, J. Characterization of NDM-Encoding Plasmids from Enterobacteriaceae Recovered from Czech Hospitals. Front. Microbiol. 2018, 9, 1549. [Google Scholar] [CrossRef]
- Musila, L.; Kyany’a, C.; Maybank, R.; Stam, J.; Oundo, V.; Sang, W. Detection of Diverse Carbapenem and Multidrug Resistance Genes and High-Risk Strain Types among Carbapenem Non-Susceptible Clinical Isolates of Target Gram-Negative Bacteria in Kenya. PLoS ONE 2021, 16, e246937. [Google Scholar] [CrossRef] [PubMed]
- Torres-González, P.; Bobadilla-Del Valle, M.; Tovar-Calderón, E.; Leal-Vega, F.; Hernández-Cruz, A.; Martínez-Gamboa, A.; Niembro-Ortega, M.D.; Sifuentes-Osornio, J.; Ponce-De-León, A. Outbreak Caused by Enterobacteriaceae Harboring NDM-1 Metallo-β-Lactamase Carried in an IncFII Plasmid in a Tertiary Care Hospital in Mexico City. Antimicrob. Agents Chemother. 2015, 59, 7080–7083. [Google Scholar] [CrossRef] [PubMed]
- Cristóvão, F.; Alonso, C.A.; Igrejas, G.; Sousa, M.; Silva, V.; Pereira, J.E.; Lozano, C.; Cortés-Cortés, G.; Torres, C.; Poeta, P. Clonal Diversity of Extended-Spectrum Beta-Lactamase Producing Escherichia Coli Isolates in Fecal Samples of Wild Animals. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef]
- Van Hoek, A.H.A.M.; Veenman, C.; Florijn, A.; Huijbers, P.M.C.; Graat, E.A.M.; de Greeff, S.; Dierikx, C.M.; van Duijkeren, E. Longitudinal Study of ESBL Escherichia Coli Carriage on an Organic Broiler Farm. J. Antimicrob. Chemother. 2018, 73, 3298–3304. [Google Scholar] [CrossRef] [PubMed]
- Dhaouadi, S.; Soufi, L.; Hamza, A.; Fedida, D.; Zied, C.; Awadhi, E.; Mtibaa, M.; Hassen, B.; Cherif, A.; Torres, C.; et al. Co-Occurrence of Mcr-1 Mediated Colistin Resistance and β-Lactamase-Encoding Genes in Multidrug-Resistant Escherichia Coli from Broiler Chickens with Colibacillosis in Tunisia. J. Glob. Antimicrob. Resist. 2020, 22, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.R.; McCulloch, J.A.; Vianello, M.A.; Moura, Q.; Pérez-Chaparro, P.J.; Esposito, F.; Sartori, L.; Dropa, M.; Matté, M.H.; Lira, D.P.A.; et al. First Report of the Globally Disseminated IncX4 Plasmid Carrying the Mcr-1 Gene in a Colistin-Resistant Escherichia Coli Sequence Type 101 Isolate from a Human Infection in Brazil. Antimicrob. Agents Chemother. 2016, 60, 6415–6417. [Google Scholar] [CrossRef]
- Kalantar-Neyestanaki, D.; Mansouri, S.; Kandehkar Ghahraman, M.R.; Tabatabaeifar, F.; Hashemizadeh, Z. Dissemination of Different Sequence Types Lineages Harboring BlaCTX-M-15 among Uropathogenic Escherichia Coli in Kerman, Iran. Iran. J. Basic Med Sci. 2020, 23, 1551–1557. [Google Scholar] [CrossRef]
- Al-Mir, H.; Osman, M.; Azar, N.; Madec, J.-Y.; Hamze, M.; Haenni, M. Emergence of Clinical Mcr-1-Positive Escherichia Coli in Lebanon Running Title: Mcr-1-Positive E. Coli in Lebanese Patients. J. Glob. Antimicrob. Resist. 2019, 19, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Chaalal, N.; Touati, A.; Yahiaoui-Martinez, A.; Aissa, M.A.; Sotto, A.; Lavigne, J.-P.; Pantel, A. Colistin-Resistant Enterobacterales Isolated from Chicken Meat in Western Algeria. Microb. Drug Resist. 2021. [Google Scholar] [CrossRef]
- Elmonir, W.; Abd El-Aziz, N.K.; Tartor, Y.H.; Moustafa, S.M.; Abo Remela, E.M.; Eissa, R.; Saad, H.A.; Tawab, A.A. Emergence of Colistin and Carbapenem Resistance in Extended-Spectrum β-Lactamase Producing Klebsiella Pneumoniae Isolated from Chickens and Humans in Egypt. Biology 2021, 10, 373. [Google Scholar] [CrossRef]
- Okdah, L.; Leangapichart, T.; Hadjadj, L.; Olaitan, A.O.; Al-Bayssari, C.; Rizk, R.; Hammoud, M.; Diene, S.M.; Rolain, J.M. First Report of Colistin-Resistant Klebsiella Pneumoniae Clinical Isolates in Lebanon. J. Glob. Antimicrob. Resist. 2017, 9, 15–16. [Google Scholar] [CrossRef]
- Nawfal Dagher, T.; Al-Bayssari, C.; Chabou, S.; Baron, S.; Hadjadj, L.; Diene, S.M.; Azar, E.; Rolain, J.M. Intestinal Carriage of Colistin-Resistant Enterobacteriaceae at Saint Georges Hospital in Lebanon. J. Glob. Antimicrob. Resist. 2020, 21, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Nawfal Dagher, T.; Azar, E.; Al-Bayssari, C.; Chamieh, A.S.; Rolain, J.M. First Detection of Colistin-Resistant Klebsiella Pneumoniae in Association with NDM-5 Carbapenemase Isolated from Clinical Lebanese Patients. Microb. Drug Resist. 2019, 25, 925–930. [Google Scholar] [CrossRef] [PubMed]
| Strain Name | Source | Antibiotic Susceptibility Profile | MAR Index | IMP MIC (μg/mL) | ERT MIC (μg/mL) | Carba NP Test | blaOXA-48 | blaNDM-1 | ST | Accession Number | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Same tent (same family) | E. coli EC-1 | BN | FF, F, AK, CS, CN | 0.68 | 12 | >32 | + | − | + | 361 | OL542491 |
| E. coli EC-2 | BN | FF, F, AK, CS, CN | 0.68 | >32 | >32 | + | − | + | 361 | OL542492 | |
| E. coli EC-3 | BN | FF, F, AK, CS, CN | 0.68 | 16 | >32 | + | − | + | 361 | OL542493 | |
| E. coli EC-4 | BN | FF, F, AK, CS, CN | 0.68 | >32 | >32 | + | − | + | 1294 | OL542494 | |
| E. coli EC-5 | BN | FF, F, AK, CS, CN | 0.68 | >32 | >32 | + | − | + | 1294 | OL542495 | |
| E. coli EC-6 | TL | FF, F, AK, CS, CN | 0.68 | 4 | >32 | + | − | + | 648 | OL542496 | |
| Same tent (same family) | E. cloacae Eclo-1 | BN | DO, CS | 0.87 | 4 | 12 | + | − | + | 182 | OL474360 |
| E. cloacae Eclo-2 | BN | CS | 0.93 | 4 | 8 | + | − | + | 182 | OL474361 | |
| E. cloacae Eclo-3 | TL | CS | 0.93 | 4 | 8 | + | − | + | 1120 | OL474362 | |
| Tents are close (Not the same family) | K. pneumoniae KP-2 | BN | AK, CS, CN | 0.81 | >32 | >32 | + | + | − | 16 | OL542509 |
| K. pneumoniae KP-3 | BN | AK, CS, CN | 0.81 | >32 | >32 | + | + | − | 16 | OL542510 | |
| K. pneumoniae KP-4 | BN | TZP, CS | 0.87 | >32 | >32 | + | + | − | 16 | OL542511 | |
| K. pneumoniae KP-5 | BN | AK, CS, CN | 0.81 | >32 | >32 | + | + | − | 16 | OL542512 | |
| Tents are close (Not the same family) | K. pneumoniae KP-6 | TL | DO, CS | 0.87 | >32 | >32 | + | + | + | 14 | OL542513 OL542506 |
| K. pneumoniae KP-7 | TL | AK, DO, CS | 0.81 | >32 | >32 | + | + | + | 14 | OL542514 OL542507 | |
| K. pneumoniae KP-8 | TL | FF, AK, DO, CS | 0.75 | >32 | >32 | + | + | + | 14 | OL542515 OL542508 |
| Strain Name | Source | Antibiotic Susceptibility Profile | MAR Index | IMP MIC (μg/mL) | ERT MIC (μg/mL) | UMIC Test (μg/mL) | Carba NP Test | blaNDM-1 | mcr-1 | Mutations of the Associated Colistin-Resistance Proteins | ST | Accession Number | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tents are close (Not the same family) | E. coli EC-7 | BN | FEP, TZP, CRO, ERT, IMP, FF, F, AK, CN | 0.43 | − | − | 4 | − | − | + | − | 2001 | OL542497 |
| E. coli EC-8 | BN | FEP, TPZ, CRO, ERT, IMP, FF, F, AK, CN, SXT | 0.37 | − | − | 4 | − | − | + | − | 2001 | OL542498 | |
| E. coli EC-9 | BN | FEP, TPZ, CRO, ERT, IMP, FF, F, AK, CN, SXT | 0.37 | − | − | 4 | − | − | + | − | 2001 | OL542499 | |
| E. coli EC-10 | BN | FEP, TPZ, CRO, ERT, IMP, FF, F, AK, CN | 0.43 | − | − | 4 | − | − | + | − | 101 | OL542500 | |
| E. coli EC-11 | TL | FEP, TPZ, CRO, ERT, IMP, FF, F, AK, CN, SXT | 0.37 | − | − | 4 | − | − | + | − | 4187 | OL542501 | |
| Different tents (Same family) | K. pneumoniae ColiR KP-1 | BN | − | 1 | 4 | 4 | >64 | + | + | − | + | 944 | OL542502 |
| K. pneumoniae ColiR KP-2 | BN | − | 1 | 2 | 4 | >64 | + | + | − | + | 944 | OL542503 | |
| K. pneumoniae ColiR KP-3 | BN | − | 1 | 2 | 4 | >64 | + | + | − | + | 944 | OL542504 | |
| K. pneumoniae ColiR KP-4 | BN | − | 1 | 2 | 4 | >64 | + | + | − | + | 944 | OL542505 |
| Strain Name | Mgrb | PmrA | PmrB | PhoP | PhoQ | Accession Number |
|---|---|---|---|---|---|---|
| Coli R Kp 1 | No mutation | No mutation | C577del | No mutation | No mutation | OL587685 |
| Coli R Kp 2 | No mutation | No mutation | G432_C433insG | No mutation | No mutation | OL587686 |
| Coli R Kp 3 | No mutation | No mutation | No mutation | No mutation | T459_G460insC | OL587683 |
| Coli R Kp 4 | No mutation | No mutation | G623_C624insG | No mutation | T435_A436insT | OL587687 OL587684 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azour, A.; Al-Bayssari, C.; Dagher, T.N.; Fajloun, F.; Fajloun, M.; Rolain, J.-M. Clonal Dissemination of Plasmid-Mediated Carbapenem and Colistin Resistance in Refugees Living in Overcrowded Camps in North Lebanon. Antibiotics 2021, 10, 1478. https://doi.org/10.3390/antibiotics10121478
Azour A, Al-Bayssari C, Dagher TN, Fajloun F, Fajloun M, Rolain J-M. Clonal Dissemination of Plasmid-Mediated Carbapenem and Colistin Resistance in Refugees Living in Overcrowded Camps in North Lebanon. Antibiotics. 2021; 10(12):1478. https://doi.org/10.3390/antibiotics10121478
Chicago/Turabian StyleAzour, Adel, Charbel Al-Bayssari, Tania Nawfal Dagher, Faraj Fajloun, Mark Fajloun, and Jean-Marc Rolain. 2021. "Clonal Dissemination of Plasmid-Mediated Carbapenem and Colistin Resistance in Refugees Living in Overcrowded Camps in North Lebanon" Antibiotics 10, no. 12: 1478. https://doi.org/10.3390/antibiotics10121478
APA StyleAzour, A., Al-Bayssari, C., Dagher, T. N., Fajloun, F., Fajloun, M., & Rolain, J.-M. (2021). Clonal Dissemination of Plasmid-Mediated Carbapenem and Colistin Resistance in Refugees Living in Overcrowded Camps in North Lebanon. Antibiotics, 10(12), 1478. https://doi.org/10.3390/antibiotics10121478






