Antimicrobial Impact of Different Air-Polishing Powders in a Subgingival Biofilm Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Böcher, S.; Wenzler, J.-S.; Falk, W.; Braun, A. Comparison of different laser-based photochemical systems for periodontal treatment. Photodiagnosis Photodyn. Ther. 2019, 27, 433–439. [Google Scholar] [CrossRef]
- Jepsen, S.; Deschner, J.; Braun, A.; Schwarz, F.; Eberhard, J. Calculus removal and the prevention of its formation. Periodontol. 2000 2011, 55, 167–188. [Google Scholar] [CrossRef]
- Laleman, I.; Cortellini, S.; De Winter, S.; Herrero, E.R.; Dekeyser, C.; Quirynen, M.; Teughels, W. Subgingival debridement: End point, methods and how often? Periodontol. 2000 2017, 75, 189–204. [Google Scholar] [CrossRef]
- Slot, D.E.; Kranendonk, A.A.; van der Reijden, W.A.; van Winkelhoff, A.J.; Rosema, N.A.M.; Schulein, W.H.; van der Velden, U.; van der Weijden, F.A. Adjunctive effect of a water-cooled Nd:YAG laser in the treatment of chronic periodontitis. J. Clin. Periodontol. 2011, 38, 470–478. [Google Scholar] [CrossRef]
- Flemmig, T.F.; Hetzel, M.; Topoll, H.; Gerss, J.; Haeberlein, I.; Petersilka, G. Subgingival debridement efficacy of glycine powder air polishing. J. Periodontol. 2007, 78, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Frankenberger, R.; Lohbauer, U.; Tay, F.R.; Taschner, M.; Nikolaenko, S.A. The effect of different air-polishing powders on dentin bonding. J. Adhes. Dent. 2007, 9, 381–389. [Google Scholar]
- Kontturi-Närhi, V.; Markkanen, S.; Markkanen, H. The gingival effects of dental airpolishing as evaluated by scanning electron microscopy. J. Periodontol. 1989, 60, 19–22. [Google Scholar] [CrossRef]
- Kontturi-Närhi, V.; Markkanen, S.; Markkanen, H. Effects of airpolishing on dental plaque removal and hard tissues as evaluated by scanning electron microscopy. J. Periodontol. 1990, 61, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Berkstein, S.; Reiff, R.L.; McKinney, J.F.; Killoy, W.J. Supragingival root surface removal during maintenance procedures utilizing an air-powder abrasive system or hand scaling. An in vitro study. J. Periodontol. 1987, 58, 327–330. [Google Scholar] [CrossRef]
- Gerbo, L.R.; Barnes, C.M.; Leinfelder, K.F. Applications of the air-powder polisher in clinical orthodontics. Am. J. Orthod. Dentofac. Orthop. 1993, 103, 71–73. [Google Scholar] [CrossRef]
- Wennström, J.L.; Dahlén, G.; Ramberg, P. Subgingival debridement of periodontal pockets by air polishing in comparison with ultrasonic instrumentation during maintenance therapy. J. Clin. Periodontol. 2011, 38, 820–827. [Google Scholar] [CrossRef]
- Petersilka, G.J.; Bell, M.; Häberlein, I.; Mehl, A.; Hickel, R.; Flemmig, T.F. In vitro evaluation of novel low abrasive air polishing powders. J. Clin. Periodontol. 2003, 30, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.A., 3rd; Eakle, W.S.; Summitt, J.B. Air abrasion: An old technology reborn. Compend. Contin. Educ. Dent. 1999, 20, 751–754, 756, 758–759, 764. [Google Scholar] [PubMed]
- Kajihara, H.; Suzuki, S.; Minesaki, Y.; Kurashige, H.; Tanaka, T. The effects of air-abrasion on dentin, enamel, and metal bonding. Am. J. Dent. 2004, 17, 161–164. [Google Scholar] [PubMed]
- Pelka, M.; Trautmann, S.; Petschelt, A.; Lohbauer, U. Influence of air-polishing devices and abrasives on root dentin—An in vitro confocal laser scanning microscope study. Quintessence Int. 2010, 41, e141–e148. [Google Scholar] [PubMed]
- Hägi, T.T.; Hofmänner, P.; Salvi, G.E.; Ramseier, C.A.; Sculean, A. Clinical outcomes following subgingival application of a novel erythritol powder by means of air polishing in supportive periodontal therapy: A randomized, controlled clinical study. Quintessence Int. 2013, 44, 753–761. [Google Scholar] [CrossRef]
- Sahrmann, P.; Ronay, V.; Schmidlin, P.R.; Attin, T.; Paqué, F. Three-dimensional defect evaluation of air polishing on extracted human roots. J. Periodontol. 2014, 85, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Kozlovsky, A.; Artzi, Z.; Nemcovsky, C.E.; Hirshberg, A. Effect of air-polishing devices on the gingiva: Histologic study in the canine. J. Clin. Periodontol. 2005, 32, 329–334. [Google Scholar] [CrossRef]
- Agger, M.S.; Hörsted-Bindslev, P.; Hovgaard, O. Abrasiveness of an air-powder polishing system on root surfaces in vitro. Quintessence Int. 2001, 32, 407–411. [Google Scholar] [PubMed]
- Black, R. Technic for nonmechanical preparation of cavities and prophylaxis. J. Am. Dent. Assoc. 1945, 32, 955–965. [Google Scholar] [CrossRef]
- Moëne, R.; Décaillet, F.; Mombelli, A. Subgingivales Airpolishing: Neue Perspektiven für die parodontale Erhaltungsphase. Schweiz. Mon. Zahnmed. 2010, 120, 902–911. [Google Scholar]
- Galloway, S.E.; Pashley, D.H. Rate of removal of root structure by the use of the Prophy-Jet device. J. Periodontol. 1987, 58, 464–469. [Google Scholar] [CrossRef]
- Petersilka, G.J. Subgingival air-polishing in the treatment of periodontal biofilm infections. Periodontol. 2000 2011, 55, 124–142. [Google Scholar] [CrossRef]
- Zhang, J.L.; Yao, J.; Zhuge, J.N.; Zhang, Y.J. Antibacterial activity of erythritol on periodontal pathogen. Shanghai Kou Qiang Yi Xue 2019, 28, 362–367. (in Chinese). [Google Scholar]
- Kruse, A.B.; Maamar, R.; Akakpo, D.L.; Woelber, J.P.; Wittmer, A.; Vach, K.; Ratka-Krüger, P.; Al-Ahmad, A. Effects of subgingival air-polishing with trehalose powder on oral biofilm during periodontal maintenance therapy: A randomized-controlled pilot study. BMC Oral Health 2020, 20, 123. [Google Scholar] [CrossRef] [PubMed]
- Wenzler, J.-S.; Ziebolz, D.; Böcher, S.; Krause, F.; Braun, A. Integration von Pulverstrahlsystemen in die systematische Parodontitistherapie. Quintessenz 2018, 69, 2–13. [Google Scholar]
- Bühler, J.; Amato, M.; Weiger, R.; Walter, C. A systematic review on the effects of air polishing devices on oral tissues. Int. J. Dent. Hyg. 2016, 14, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; van der Weijden, F.; Doerfer, C.; Herrera, D.; Shapira, L.; Polak, D.; Madianos, P.; Louropoulou, A.; Machtei, E.; Donos, N.; et al. Primary prevention of periodontitis: Managing gingivitis. J. Clin. Periodontol. 2015, 42, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Barnes, C.M.; Hayes, E.F.; Leinfelder, K.F. Effects of an airabrasive polishing system on restored surfaces. Gen. Dent. 1987, 35, 186–189. [Google Scholar] [PubMed]
- Homiak, A.W.; Cook, P.A.; DeBoer, J. Effect of hygiene instrumentation on titanium abutments: A scanning electron microscopy study. J. Prosthet. Dent. 1992, 67, 364–369. [Google Scholar] [CrossRef]
- Petersilka, G.; Faggion, C.M., Jr.; Stratmann, U.; Gerss, J.; Ehmke, B.; Haeberlein, I.; Flemmig, T.F. Effect of glycine powder air-polishing on the gingiva. J. Clin. Periodontol. 2008, 35, 324–332. [Google Scholar] [CrossRef]
- Neta, T.; Takada, K.; Hirasawa, M. Low-cariogenicity of trehalose as a substrate. J. Dent. 2000, 28, 571–576. [Google Scholar] [CrossRef]
- Kruse, A.B.; Akakpo, D.L.; Maamar, R.; Woelber, J.P.; Al-Ahmad, A.; Vach, K.; Ratka-Krueger, P. Trehalose powder for subgingival air-polishing during periodontal maintenance therapy: A randomized controlled trial. J. Periodontol. 2019, 90, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Flemmig, T.F.; Arushanov, D.; Daubert, D.; Rothen, M.; Mueller, G.; Leroux, B.G. Randomized controlled trial assessing efficacy and safety of glycine powder air polishing in moderate-to-deep periodontal pockets. J. Periodontol. 2012, 83, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Petersilka, G.J.; Tunkel, J.; Barakos, K.; Heinecke, A.; Häberlein, I.; Flemmig, T.F. Subgingival plaque removal at interdental sites using a low-abrasive air polishing powder. J. Periodontol. 2003, 74, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Petersilka, G.J.; Steinmann, D.; Häberlein, I.; Heinecke, A.; Flemmig, T.F. Subgingival plaque removal in buccal and lingual sites using a novel low abrasive air-polishing powder. J. Clin. Periodontol. 2003, 30, 328–333. [Google Scholar] [CrossRef]
- Sharawy, A.M.; Sabharwal, K.; Socransky, S.S.; Lobene, R.R. A quantitative study of plaque and calculus formation in normal and periodontally involved mouths. J. Periodontol. 1966, 37, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Haffajee, A.D.; Cugini, M.A.; Dibart, S.; Smith, C.; Kent, R.L., Jr.; Socransky, S.S. The effect of SRP on the clinical and microbiological parameters of periodontal diseases. J. Clin. Periodontol. 1997, 24, 324–334. [Google Scholar] [CrossRef]
- Axelsson, P.; Lindhe, J. The significance of maintenance care in the treatment of periodontal disease. J. Clin. Periodontol. 1981, 8, 281–294. [Google Scholar] [CrossRef]
- Armitage, G.C.; Xenoudi, P. Post-treatment supportive care for the natural dentition and dental implants. Periodontol. 2000 2016, 71, 164–184. [Google Scholar] [CrossRef]
- Rohanizadeh, R.; Legeros, R.Z. Ultrastructural study of calculus-enamel and calculus-root interfaces. Arch. Oral Biol. 2005, 50, 89–96. [Google Scholar] [CrossRef]
- White, D.J. Dental calculus: Recent insights into occurrence, formation, prevention, removal and oral health effects of supragingival and subgingival deposits. Eur. J. Oral Sci. 1997, 105, 508–522. [Google Scholar] [CrossRef]
- Atkinson, D.R.; Cobb, C.M.; Killoy, W.J. The effect of an air-powder abrasive system on in vitro root surfaces. J. Periodontol. 1984, 55, 13–18. [Google Scholar] [CrossRef]
- Horning, G.M.; Cobb, C.M.; Killoy, W.J. Effect of an air-powder abrasive system on root surfaces in periodontal surgery. J. Clin. Periodontol. 1987, 14, 213–220. [Google Scholar] [CrossRef]
- Jost-Brinkmann, P.G. The influence of air polishers on tooth enamel. An in-vitro study. J. Orofac. Orthop. 1998, 59, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bühler, J.; Amato, M.; Weiger, R.; Walter, C. A systematic review on the patient perception of periodontal treatment using air polishing devices. Int. J. Dent. Hyg. 2016, 14, 4–14. [Google Scholar] [CrossRef]
- Aoki, A.; Sasaki, K.M.; Watanabe, H.; Ishikawa, I. Lasers in nonsurgical periodontal therapy. Periodontol. 2000 2004, 36, 59–97. [Google Scholar] [CrossRef] [PubMed]
- Umeda, M.; Takeuchi, Y.; Noguchi, K.; Huang, Y.; Koshy, G.; Ishikawa, I. Effects of nonsurgical periodontal therapy on the microbiota. Periodontol. 2000 2004, 36, 98–120. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, P.A.; Edwards, C.A.; de Boever, J.A.; Loesche, W.J. Ultrastructural observations on bacterial invasion in cementum and radicular dentin of periodontally diseased human teeth. J. Periodontol. 1988, 59, 493–503. [Google Scholar] [CrossRef] [Green Version]
- Drago, L.; Del Fabbro, M.; Bortolin, M.; Vassena, C.; de Vecchi, E.; Taschieri, S. Biofilm removal and antimicrobial activity of two different air-polishing powders: An in vitro study. J. Periodontol. 2014, 85, e363–e369. [Google Scholar] [CrossRef]
- Johnson, W.W.; Barnes, C.M.; Covey, D.A.; Walker, M.P.; Ross, J.A. The effects of a commercial aluminum airpolishing powder on dental restorative materials. J. Prosthodont. 2004, 13, 166–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böcher, S. Effektivität von Curcumin als Photosensibilisator bei Bestrahlung mit einem Neuartigen 445-nm-Laser. Ph.D. Thesis, Philipps-Universität Marburg, Marburg, Germany, 2019. [Google Scholar]
- Muhney, K.A.; Dechow, P.C. Patients’ perception of pain during ultrasonic debridement: A comparison between piezoelectric and magnetostrictive scalers. J. Dent. Hyg. 2010, 84, 185–189. [Google Scholar] [PubMed]
- Weaks, L.M.; Lescher, N.B.; Barnes, C.M.; Holroyd, S.V. Clinical evaluation of the Prophy-Jet as an instrument for routine removal of tooth stain and plaque. J. Periodontol. 1984, 55, 486–488. [Google Scholar] [CrossRef] [PubMed]
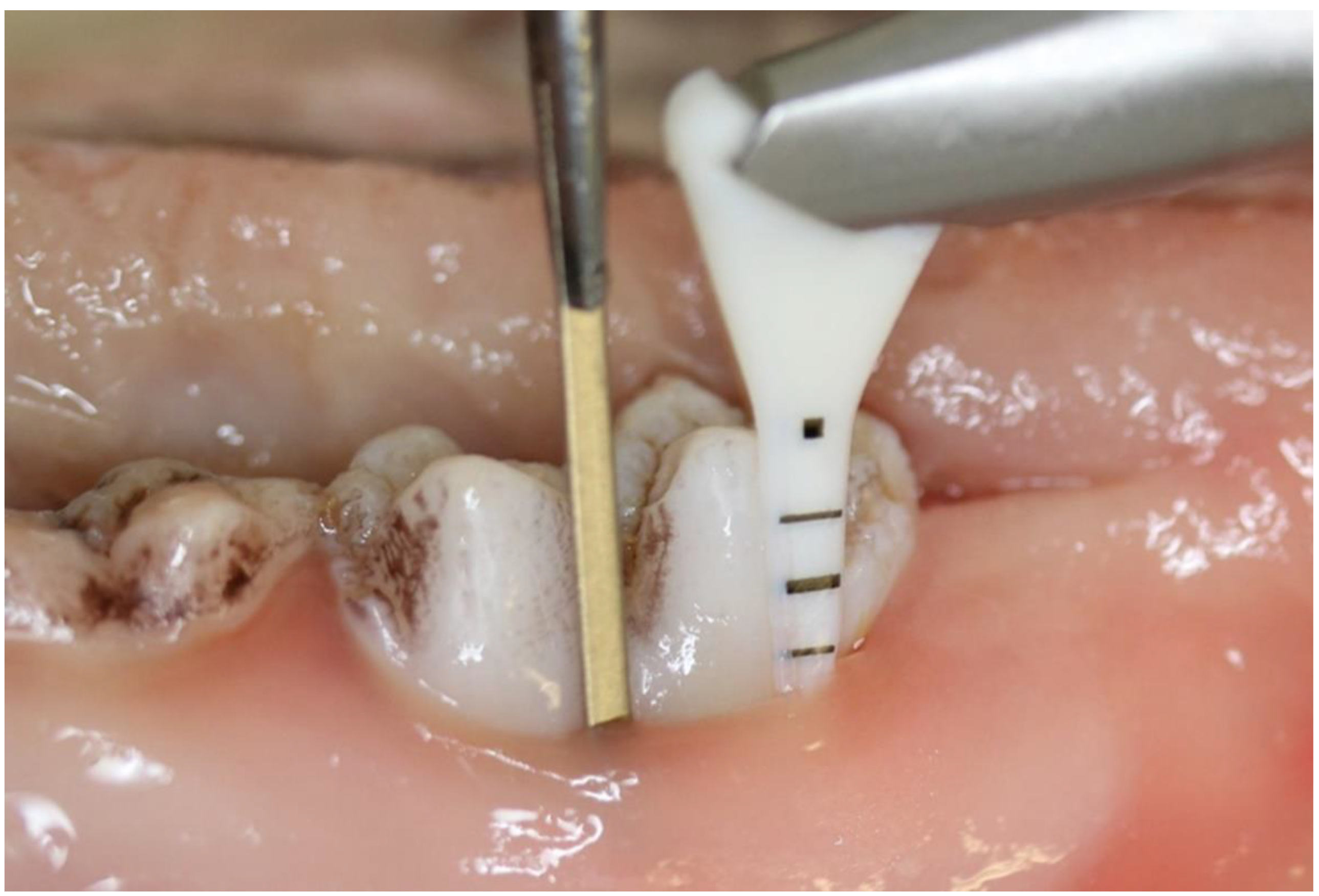
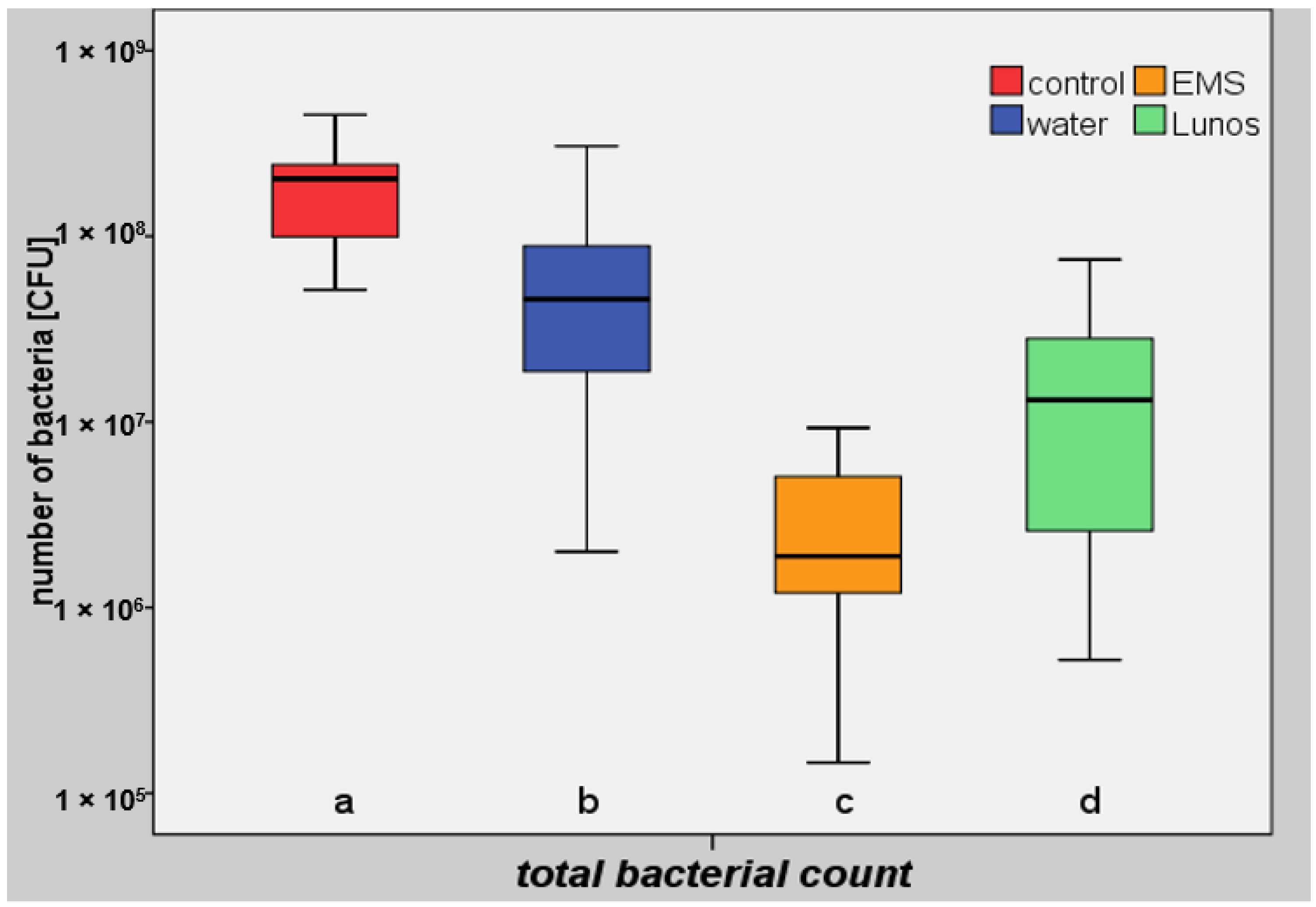

| Group IV (Controls) | Group III (Water) | Group I (Glycine) a | Group II (Trehalose) b | |
|---|---|---|---|---|
| n | 14 | 14 | 14 | 14 |
| Mean value | 2.02 × 108 | 7.25 × 107 | 3.12 × 106 | 2.23 × 107 |
| Standard deviation | 1.19 × 108 | 8.16 × 107 | 2.97 × 106 | 2.53 × 107 |
| Median value | 2.04 × 108 | 4.58 × 107 | 1.96 × 106 | 1.36 × 107 |
| Maximum value | 4.51 × 108 | 3.06 × 108 | 9.30 × 106 | 7.50 × 107 |
| Minimum value | 5.14 × 107 | 2.00 × 106 | 1.46 × 105 | 5.22 × 105 |
| Interquartile range | 1.37 × 108 | 6.17 × 107 | 3.22 × 106 | 2.34 × 107 |
| Percentage reduction | 77.6 | 99.0 | 93.3 |
| Group IV (Controls) | Group III (Water) | Group I (Glycine) a | Group II (Trehalose) b | |
|---|---|---|---|---|
| Controls | 0.001019 | 7.468 × 10−6 | 1.144 × 10−5 | |
| Water | 0.001019 | 3.917 × 10−5 | 0.02158 | |
| Glycine | 7.468 × 10−6 | 3.917 × 10−5 | 0.006702 | |
| Trehalose | 1.144 × 10−5 | 0.02158 | 0.006702 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wenzler, J.-S.; Krause, F.; Böcher, S.; Falk, W.; Birkenmaier, A.; Conrads, G.; Braun, A. Antimicrobial Impact of Different Air-Polishing Powders in a Subgingival Biofilm Model. Antibiotics 2021, 10, 1464. https://doi.org/10.3390/antibiotics10121464
Wenzler J-S, Krause F, Böcher S, Falk W, Birkenmaier A, Conrads G, Braun A. Antimicrobial Impact of Different Air-Polishing Powders in a Subgingival Biofilm Model. Antibiotics. 2021; 10(12):1464. https://doi.org/10.3390/antibiotics10121464
Chicago/Turabian StyleWenzler, Johannes-Simon, Felix Krause, Sarah Böcher, Wolfgang Falk, Axel Birkenmaier, Georg Conrads, and Andreas Braun. 2021. "Antimicrobial Impact of Different Air-Polishing Powders in a Subgingival Biofilm Model" Antibiotics 10, no. 12: 1464. https://doi.org/10.3390/antibiotics10121464
APA StyleWenzler, J.-S., Krause, F., Böcher, S., Falk, W., Birkenmaier, A., Conrads, G., & Braun, A. (2021). Antimicrobial Impact of Different Air-Polishing Powders in a Subgingival Biofilm Model. Antibiotics, 10(12), 1464. https://doi.org/10.3390/antibiotics10121464









