Multiple and High-Risk Clones of Extended-Spectrum Cephalosporin-Resistant and blaNDM-5-Harbouring Uropathogenic Escherichia coli from Cats and Dogs in Thailand
Abstract
:1. Introduction
2. Results
2.1. Numbers and Sources of E. coli
2.2. ExPEC Virulence Genes
2.3. Antimicrobial Resistance Phenotypes
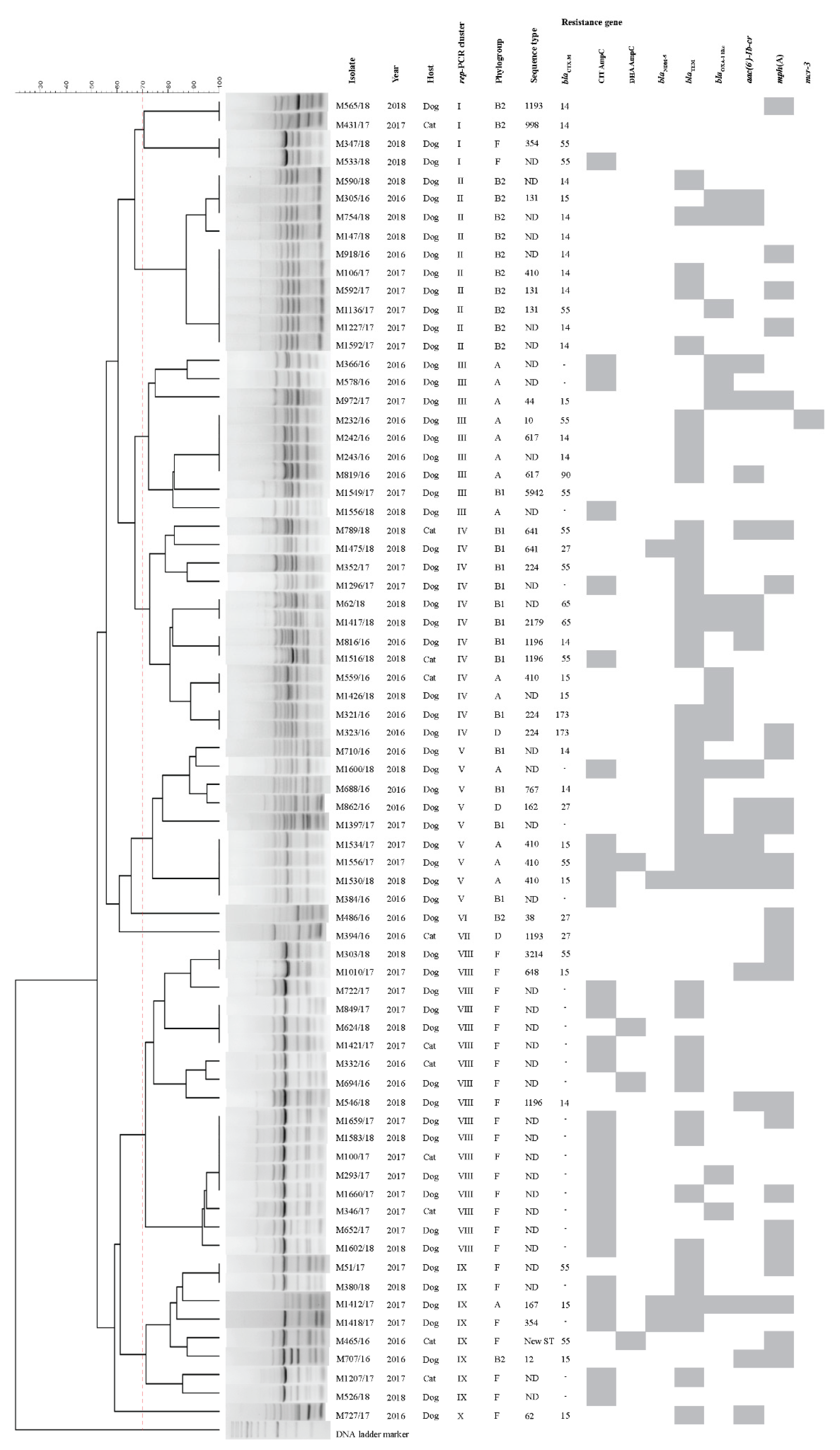
2.4. Antimicrobial Resistance Genes
2.5. Integrase Gene and Plasmid Replicons
2.6. Molecular Characteristics
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates, Species Identification and DNA Isolation
4.2. Antimicrobial Susceptibility Testing
4.3. Detection of Antimicrobial Resistance Genes
4.4. Detection of ExPEC Virulence Genes
4.5. Plasmid Replicon Typing and Integrase Detection
4.6. Molecular Typing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statements
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moyaert, H.; Morrissey, I.; de Jong, A.; El Garch, F.; Klein, U.; Ludwig, C.; Thiry, J.; Youala, M. Antimicrobial susceptibility monitoring of bacterial pathogens isolated from urinary tract infections in dogs and cats across Europe: ComPath results. Microb. Drug Resist. 2017, 23, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; Blondeau, J.; Boothe, D.; Guardabassi, L.G.; Gumley, N.; Papich, M.; Jessen, L.R.; Lappin, M.; Rankin, S.; Westropp, J.L.; et al. International Society for Companion Animal Infectious Diseases (ISCAID) guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Vet. J. 2019, 247, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Ewers, C.; Janssen, T.; Kiessling, S.; Philipp, H.C.; Wieler, L.H. Molecular epidemiology of avian pathogenic Escherichia coli (APEC) isolated from colisepticemia in poultry. Vet. Microbiol. 2004, 104, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Zogg, A.L.; Simmen, S.; Zurfluh, K.; Stephan, R.; Schmitt, S.N.; Nüesch-Inderbinen, M. High prevalence of extended-spectrum β-lactamase producing Enterobacteriaceae among clinical isolates from cats and dogs admitted to a veterinary hospital in Switzerland. Front. Vet. Sci. 2018, 5, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado-Caxito, M.; Benavides, J.A.; Adell, A.D.; Paes, A.C.; Moreno-Switt, A.I. Global prevalence and molecular characterization of extended-spectrum β-lactamase producing-Escherichia coli in dogs and cats—A scoping review and meta-analysis. One Health 2021, 12, 100236. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Andrey, D.O.; Yang, R.-S.; Sands, K.; Tansawai, U.; Li, M.; Portal, E.; Gales, A.C.; Niumsup, P.R.; Sun, J. A Klebsiella pneumoniae strain co-harbouring mcr-1 and mcr-3 from a human in Thailand. J. Antimicrob. Chemother. 2020, 75, 2372–2374. [Google Scholar]
- Alba, P.; Taddei, R.; Cordaro, G.; Fontana, M.C.; Toschi, E.; Gaibani, P.; Marani, I.; Giacomi, A.; Diaconu, E.L.; Iurescia, M.; et al. Carbapenemase IncF-borne blaNDM-5 gene in the E. coli ST167 high-risk clone from canine clinical infection, Italy. Vet. Microbiol. 2021, 256, 109045. [Google Scholar] [CrossRef]
- Endimiani, A.; Brilhante, M.; Bernasconi, O.J.; Perreten, V.; Schmidt, J.S.; Dazio, V.; Nigg, A.; Gobeli Brawand, S.; Kuster, S.P.; Schuller, S.; et al. Employees of Swiss veterinary clinics colonized with epidemic clones of carbapenemase-producing Escherichia coli. J. Antimicrob. Chemother. 2020, 75, 766–768. [Google Scholar] [CrossRef]
- Brilhante, M.; Menezes, J.; Belas, A.; Feudi, C.; Schwarz, S.; Pomba, C.; Perreten, V. OXA-181-producing extraintestinal pathogenic Escherichia coli sequence type 410 isolated from a dog in Portugal. Antimicrob. Agents Chemother. 2020, 64, e02298-19. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.-Y.; Xia, Y.-B.; Guo, Z.-W.; Ma, Z.-B.; Yi, M.-Y.; Lv, L.-C.; Lu, P.-L.; Yan, J.-C.; Huang, J.-W.; et al. Clonal spread of Escherichia coli ST93 carrying mcr-1-harboring IncN1-IncHI2/ST3 plasmid among companion animals, China. Front. Microbiol. 2018, 9, 2989. [Google Scholar] [CrossRef]
- Hong, J.S.; Song, W.; Park, H.-M.; Oh, J.-Y.; Chae, J.-C.; Shin, S.; Jeong, S.H. Clonal spread of extended-spectrum cephalosporin-resistant Enterobacteriaceae between companion animals and humans in South Korea. Front. Microbiol. 2019, 10, 1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirth, T.; Falush, D.; Lan, R.; Colles, F.; Mensa, P.; Wieler, L.H.; Karch, H.; Reeves, P.R.; Maiden, M.C.; Ochman, H.; et al. Sex and virulence in Escherichia coli: An evolutionary perspective. Mol. Microbiol. 2006, 60, 1136–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bubpamala, J.; Khuntayaporn, P.; Thirapanmethee, K.; Montakantikul, P.; Santanirand, P.; Chomnawang, M.T. Phenotypic and genotypic characterizations of extended-spectrum β-lactamase-producing Escherichia coli in Thailand. Infect. Drug Resist. 2018, 11, 2151. [Google Scholar] [CrossRef] [Green Version]
- Kidsley, A.K.; White, R.T.; Beatson, S.A.; Saputra, S.; Schembri, M.A.; Gordon, D.; Johnson, J.R.; O’Dea, M.; Mollinger, J.L.; Abraham, S.; et al. Companion animals are spillover hosts of the multidrug-resistant human extraintestinal Escherichia coli pandemic clones ST131 and ST1193. Front. Microbiol. 2020, 11, 1968. [Google Scholar] [CrossRef] [PubMed]
- Marrs, C.F.; Zhang, L.; Foxman, B. Escherichia coli mediated urinary tract infections: Are there distinct uropathogenic E. coli (UPEC) pathotypes? FEMS Microbiol. Lett. 2005, 252, 183–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Anjos Borges, L.G.; Dalla Vechia, V.; Corcao, G. Characterisation and genetic diversity via REP-PCR of Escherichia coli isolates from polluted waters in southern Brazil. FEMS Microbiol. Ecol. 2003, 45, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Bevan, E.R.; Jones, A.M.; Hawkey, P.M. Global epidemiology of CTX-M β-lactamases: Temporal and geographical shifts in genotype. J. Antimicrob. Chemother. 2017, 72, 2145–2155. [Google Scholar] [CrossRef] [Green Version]
- Nebbia, P.; Tramuta, C.; Odore, R.; Nucera, D.; Zanatta, R.; Robino, P. Genetic and phenotypic characterisation of Escherichia coli producing cefotaximase-type extended-spectrum β-lactamases: First evidence of the ST131 clone in cats with urinary infections in Italy. J. Feline Med. Surg. 2014, 16, 966–971. [Google Scholar] [CrossRef]
- Timofte, D.; Maciuca, I.E.; Williams, N.J.; Wattret, A.; Schmidt, V. Veterinary hospital dissemination of CTX-M-15 extended-spectrum β-lactamase–producing Escherichia coli ST410 in the United Kingdom. Microb. Drug Resist. 2016, 22, 609–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-H.; Kuan, N.-L.; Yeh, K.-S. Characteristics of extended-spectrum β-lactamase–producing Escherichia coli from dogs and cats admitted to a veterinary teaching hospital in Taipei, Taiwan from 2014 to 2017. Front. Vet. Sci. 2020, 7, 395. [Google Scholar] [CrossRef] [PubMed]
- Cormier, A.; Zhang, P.L.; Chalmers, G.; Weese, J.S.; Deckert, A.; Mulvey, M.; McAllister, T.; Boerlin, P. Diversity of CTX-M-positive Escherichia coli recovered from animals in Canada. Vet. Microbiol. 2019, 231, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Schmiedel, J.; Falgenhauer, L.; Domann, E.; Bauerfeind, R.; Prenger-Berninghoff, E.; Imirzalioglu, C.; Chakraborty, T. Multiresistant extended-spectrum β-lactamase-producing Enterobacteriaceae from humans, companion animals and horses in central Hesse, Germany. BMC Microbiol. 2014, 14, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, H.; Zweifel, C.; Wittenbrink, M.M.; Stephan, R. ESBL-producing uropathogenic Escherichia coli isolated from dogs and cats in Switzerland. Vet. Microbiol. 2013, 162, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Dahmen, S.; Haenni, M.; Châtre, P.; Madec, J.-Y. Characterization of blaCTX-M IncFII plasmids and clones of Escherichia coli from pets in France. J. Antimicrob. Chemother. 2013, 68, 2797–2801. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, B.W.; Nayak, R.; Foley, S.L.; Boothe, D.M. Chromosomal and plasmid-mediated fluoroquinolone resistance mechanisms among broad-spectrum-cephalosporin-resistant Escherichia coli isolates recovered from companion animals in the USA. J. Antimicrob. Chemother. 2013, 68, 1019–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, I.; Chenouf, N.S.; Cunha, R.; Martins, C.; Pimenta, P.; Pereira, A.; Martínez-Álvarez, S.; Ramos, S.; Silva, V.; Igrejas, G. Antimicrobial resistance genes and diversity of clones among ESBL-and acquired AmpC-producing Escherichia coli isolated from fecal samples of healthy and sick cats in Portugal. Antibiotics 2021, 10, 262. [Google Scholar] [CrossRef]
- Carvalho, I.; Cunha, R.; Martins, C.; Martínez-Álvarez, S.; Safia Chenouf, N.; Pimenta, P.; Pereira, A.R.; Ramos, S.; Sadi, M.; Martins, Â. Antimicrobial resistance genes and diversity of clones among faecal ESBL-producing Escherichia coli isolated from healthy and sick dogs living in Portugal. Antibiotics 2021, 10, 1013. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.D.; Peak, L.; Tyson, G.H.; Reimschuessel, R.; Ceric, O.; Rankin, S.C. New Delhi Metallo-β-Lactamase-5–Producing Escherichia coli in Companion Animals, United States. Emerg. Infect. Dis. 2020, 26, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giufrè, M.; Errico, G.; Accogli, M.; Monaco, M.; Villa, L.; Distasi, M.A.; Del Gaudio, T.; Pantosti, A.; Carattoli, A.; Cerquetti, M. Emergence of NDM-5-producing Escherichia coli sequence type 167 clone in Italy. Int. J. Antimicrob. Agents 2018, 52, 76–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Gong, L.; Walsh, T.R.; Lan, R.; Wang, T.; Zhang, J.; Mai, W.; Ni, N.; Lu, J.; Xu, J.; et al. Infection by and dissemination of NDM-5-producing Escherichia coli in China. J. Antimicrob. Chemother. 2016, 71, 563–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubin, J.E.; Pitout, J.D. Extended-spectrum β-lactamase, carbapenemase and AmpC producing Enterobacteriaceae in companion animals. Vet. Microbiol. 2014, 170, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Khine, N.O.; Lugsomya, K.; Kaewgun, B.; Honhanrob, L.; Pairojrit, P.; Jermprasert, S.; Prapasarakul, N. Multidrug resistance and virulence factors of Escherichia coli harboring plasmid-mediated colistin resistance: Mcr-1 and mcr-3 genes in contracted pig farms in Thailand. Front. Vet. Sci. 2020, 7, 582899. [Google Scholar] [CrossRef] [PubMed]
- Manges, A.R.; Geum, H.M.; Guo, A.; Edens, T.J.; Fibke, C.D.; Pitout, J.D. Global extraintestinal pathogenic Escherichia coli (ExPEC) lineages. Clin. Microbiol. Rev. 2019, 32, e00135-18. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wakeham, D.; Brouwers, H.J.; Cobbold, R.N.; Abraham, S.; Mollinger, J.L.; Johnson, J.R.; Chapman, T.A.; Gordon, D.M.; Barrs, V.R.; et al. Human-associated fluoroquinolone-resistant Escherichia coli clonal lineages, including ST354, isolated from canine feces and extraintestinal infections in Australia. Microbes Infect. 2015, 17, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Schaufler, K.; Semmler, T.; Wieler, L.H.; Wöhrmann, M.; Baddam, R.; Ahmed, N.; Müller, K.; Kola, A.; Fruth, A.; Ewers, C.; et al. Clonal spread and interspecies transmission of clinically relevant ESBL-producing Escherichia coli of ST410 another successful pandemic clone? FEMS Microbiol. Ecol. 2016, 92, fiv155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, R.C., Jr.; Johnson, J.R.; Stogsdill, P.; Yarmus, L.; Lolans, K.; Quinn, J. Community transmission in the United States of a CTX-M-15-producing sequence type ST131 Escherichia coli strain resulting in death. J. Clin. Microbiol. 2011, 49, 3406. [Google Scholar] [CrossRef] [Green Version]
- Platell, J.L.; Cobbold, R.N.; Johnson, J.R.; Trott, D.J. Clonal group distribution of fluoroquinolone-resistant Escherichia coli among humans and companion animals in Australia. J. Antimicrob. Chemother. 2010, 65, 1936–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falgenhauer, L.; Imirzalioglu, C.; Ghosh, H.; Gwozdzinski, K.; Schmiedel, J.; Gentil, K.; Bauerfeind, R.; Kämpfer, P.; Seifert, H.; Michael, G.B.; et al. Circulation of clonal populations of fluoroquinolone-resistant CTX-M-15-producing Escherichia coli ST410 in humans and animals in Germany. Int. J. Antimicrob. Agents 2016, 47, 457–465. [Google Scholar] [CrossRef]
- Ewers, C.; Bethe, A.; Stamm, I.; Grobbel, M.; Kopp, P.A.; Guerra, B.; Stubbe, M.; Doi, Y.; Zong, Z.; Kola, A.; et al. CTX-M-15-D-ST648 Escherichia coli from companion animals and horses: Another pandemic clone combining multiresistance and extraintestinal virulence? J. Antimicrob. Chemother. 2014, 69, 1224–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamang, M.D.; Nam, H.-M.; Jang, G.-C.; Kim, S.-R.; Chae, M.H.; Jung, S.-C.; Byun, J.-W.; Park, Y.H.; Lim, S.-K. Molecular characterization of extended-spectrum-β-lactamase-producing and plasmid-mediated AmpC β-lactamase-producing Escherichia coli isolated from stray dogs in South Korea. Antimicrob. Agents Chemother. 2012, 56, 2705. [Google Scholar] [CrossRef] [Green Version]
- Harada, K.; Nakai, Y.; Kataoka, Y. Mechanisms of resistance to cephalosporin and emergence of O25b-ST131 clone harboring CTX-M-27 β-lactamase in extraintestinal pathogenic Escherichia coli from dogs and cats in Japan. Microbiol. Immunol. 2012, 56, 480–485. [Google Scholar] [CrossRef]
- Cointe, A.; Birgy, A.; Mariani-Kurkdjian, P.; Liguori, S.; Courroux, C.; Blanco, J.; Delannoy, S.; Fach, P.; Loukiadis, E.; Bidet, P.; et al. Emerging multidrug-resistant hybrid pathotype shiga toxin–producing Escherichia coli O80 and related strains of clonal complex 165, Europe. Emerg. Infect. Dis. 2018, 24, 2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nukui, Y.; Ayibieke, A.; Taniguchi, M.; Aiso, Y.; Shibuya, Y.; Sonobe, K.; Nakajima, J.; Kanehira, S.; Hadano, Y.; Tohda, S.; et al. Whole-genome analysis of EC129, an NDM-5-, CTX-M-14-, OXA-10-and MCR-1-co-producing Escherichia coli ST167 strain isolated from Japan. J. Glob. Antimicrob. Resist. 2019, 18, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Sellera, F.P.; Fernandes, M.R.; Moura, Q.; Garino, F.; Azevedo, S.S.; Lincopan, N. Genomic features of a highly virulent, ceftiofur-resistant, CTX-M-8-producing Escherichia coli ST224 causing fatal infection in a domestic cat. J. Glob. Antimicrob. Resist. 2018, 15, 252–253. [Google Scholar] [CrossRef]
- Graham, E.M.; Taylor, D.J. Bacterial reproductive pathogens of cats and dogs. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 561–582. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI Supplement M100: Wayne, PA, USA, 2021. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 5th ed.; CLSI Supplement VET01S: Wayne, PA, USA, 2020. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, 11th ed.; EUCAST: Växjö, Sweden, 2021; Available online: http://www.eucast.org (accessed on 8 May 2021).
- Dallenne, C.; Da Costa, A.; Decre, D.; Favier, C.; Arlet, G. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.-H.; Wei, S.-Y.; Ma, J.-Y.; Zeng, Z.-L.; Lü, D.-H.; Yang, G.-X.; Chen, Z.-L. Detection and characterisation of CTX-M and CMY-2 β-lactamases among Escherichia coli isolates from farm animals in Guangdong Province of China. Int. J. Antimicrob. Agents 2007, 29, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Voets, G.M.; Fluit, A.; Scharringa, J.; Stuart, J.C.; Leverstein-van Hall, M.A. A set of multiplex PCRs for genotypic detection of extended-spectrum β-lactamases, carbapenemases, plasmid-mediated AmpC β-lactamases and OXA β-lactamases. Int. J. Antimicrob. Agents 2011, 37, 356–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.H.; Robicsek, A.; Jacoby, G.A.; Sahm, D.; Hooper, D.C. Prevalence in the United States of aac (6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob. Agents Chemother. 2006, 50, 3953–3955. [Google Scholar] [CrossRef] [Green Version]
- Ojo, K.; Ulep, C.; Van Kirk, N.; Luis, H.; Bernardo, M.; Leitao, J.; Roberts, M. The mef(A) gene predominates among seven macrolide resistance genes identified in Gram-negative strains representing 13 genera, isolated from healthy Portuguese children. Antimicrob. Agents Chemother. 2004, 48, 3451–3456. [Google Scholar] [CrossRef] [Green Version]
- Karczmarczyk, M.; Martins, M.; McCusker, M.; Mattar, S.; Amaral, L.; Leonard, N.; Aarestrup, F.M.; Fanning, S. Characterization of antimicrobial resistance in Salmonella enterica food and animal isolates from Colombia: Identification of a qnrB19-mediated quinolone resistance marker in two novel serovars. FEMS Microbiol. Lett. 2010, 313, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Cattoir, V.; Poirel, L.; Rotimi, V.; Soussy, C.-J.; Nordmann, P. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J. Antimicrob. Chemother. 2007, 60, 394–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.B.; Park, C.H.; Kim, C.J.; Kim, E.-C.; Jacoby, G.A.; Hooper, D.C. Prevalence of plasmid-mediated quinolone resistance determinants over a 9-year period. Antimicrob. Agents Chemother. 2009, 53, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Guo, Q.; Xu, X.; Wang, X.; Ye, X.; Wu, S.; Hooper, D.C.; Wang, M. New plasmid-mediated quinolone resistance gene, qnrC, found in a clinical isolate of Proteus mirabilis. Antimicrob. Agents Chemother. 2009, 53, 1892–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavaco, L.M.; Hasman, H.; Xia, S.; Aarestrup, F.M. qnrD, a novel gene conferring transferable quinolone resistance in Salmonella enterica serovar Kentucky and Bovismorbificans strains of human origin. Antimicrob. Agents Chemother. 2009, 53, 603–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebelo, A.R.; Bortolaia, V.; Kjeldgaard, J.S.; Pedersen, S.K.; Leekitcharoenphon, P.; Hansen, I.M.; Guerra, B.; Malorny, B.; Borowiak, M.; Hammerl, J.A.; et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Eurosurveillance 2018, 23, 17–00672. [Google Scholar] [CrossRef] [PubMed]
- Borowiak, M.; Baumann, B.; Fischer, J.; Thomas, K.; Deneke, C.; Hammerl, J.A.; Szabo, I.; Malorny, B. Development of a novel mcr-6 to mcr-9 multiplex PCR and assessment of mcr-1 to mcr-9 occurrence in colistin-resistant Salmonella enterica isolates from environment, feed, animals, and food (2011–2018) in Germany. Front. Microbiol. 2020, 11, 80. [Google Scholar] [CrossRef] [Green Version]
- Ewers, C.; Li, G.; Wilking, H.; Kieβling, S.; Alt, K.; Antáo, E.-M.; Laturnus, C.; Diehl, I.; Glodde, S.; Homeier, T.; et al. Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: How closely related are they? Int. J. Med. Microbiol. 2007, 297, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Nam, E.-H.; Ko, S.; Chae, J.-S.; Hwang, C.-Y. Characterization and zoonotic potential of uropathogenic Escherichia coli isolated from dogs. J. Microbiol. Biotechnol. 2013, 23, 422–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carattoli, A.; Bertini, A.; Villa, L.; Falbo, V.; Hopkins, K.L.; Threlfall, E.J. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 2005, 63, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, O.; Juan, C.; Cercenado, E.; Navarro, F.; Bouza, E.; Coll, P.; Pérez, J.; Oliver, A. Molecular epidemiology and mechanisms of carbapenem resistance in Pseudomonas aeruginosa isolates from Spanish hospitals. Antimicrob. Agents Chemother. 2007, 51, 4329–4335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Versalovic, J.; Koeuth, T.; Lupski, R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991, 19, 6823–6831. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nittayasut, N.; Yindee, J.; Boonkham, P.; Yata, T.; Suanpairintr, N.; Chanchaithong, P. Multiple and High-Risk Clones of Extended-Spectrum Cephalosporin-Resistant and blaNDM-5-Harbouring Uropathogenic Escherichia coli from Cats and Dogs in Thailand. Antibiotics 2021, 10, 1374. https://doi.org/10.3390/antibiotics10111374
Nittayasut N, Yindee J, Boonkham P, Yata T, Suanpairintr N, Chanchaithong P. Multiple and High-Risk Clones of Extended-Spectrum Cephalosporin-Resistant and blaNDM-5-Harbouring Uropathogenic Escherichia coli from Cats and Dogs in Thailand. Antibiotics. 2021; 10(11):1374. https://doi.org/10.3390/antibiotics10111374
Chicago/Turabian StyleNittayasut, Naiyaphat, Jitrapa Yindee, Pongthai Boonkham, Teerapong Yata, Nipattra Suanpairintr, and Pattrarat Chanchaithong. 2021. "Multiple and High-Risk Clones of Extended-Spectrum Cephalosporin-Resistant and blaNDM-5-Harbouring Uropathogenic Escherichia coli from Cats and Dogs in Thailand" Antibiotics 10, no. 11: 1374. https://doi.org/10.3390/antibiotics10111374
APA StyleNittayasut, N., Yindee, J., Boonkham, P., Yata, T., Suanpairintr, N., & Chanchaithong, P. (2021). Multiple and High-Risk Clones of Extended-Spectrum Cephalosporin-Resistant and blaNDM-5-Harbouring Uropathogenic Escherichia coli from Cats and Dogs in Thailand. Antibiotics, 10(11), 1374. https://doi.org/10.3390/antibiotics10111374





