Assessment of Micafungin Dosage Regimens in Patients with Cancer Using Pharmacokinetic/Pharmacodynamic Modeling and Monte Carlo Simulation
Abstract
1. Introduction
2. Methods and Patients
2.1. Study Design and Subjects
2.2. Micafungin Dosing and Blood Sampling
2.3. Analytical Assay
2.4. Population Pharmacokinetic Modeling
2.5. Covariate Model
2.6. Model Diagnostics
2.7. Monte Carlo Simulations of Plasma Concentrations and PTA
3. Results
3.1. Study Cohort
3.2. Population Pharmacokinetic Modeling
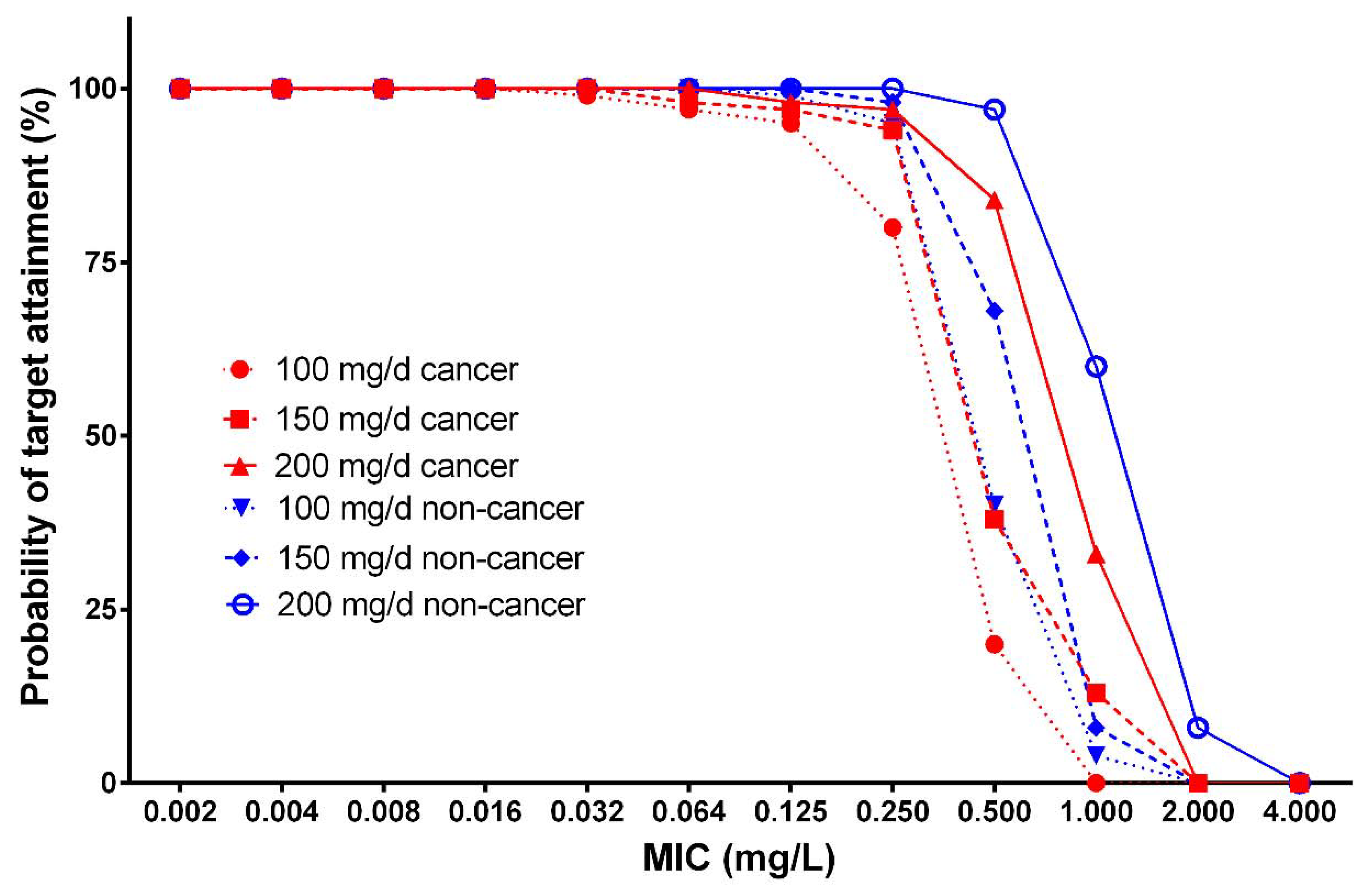
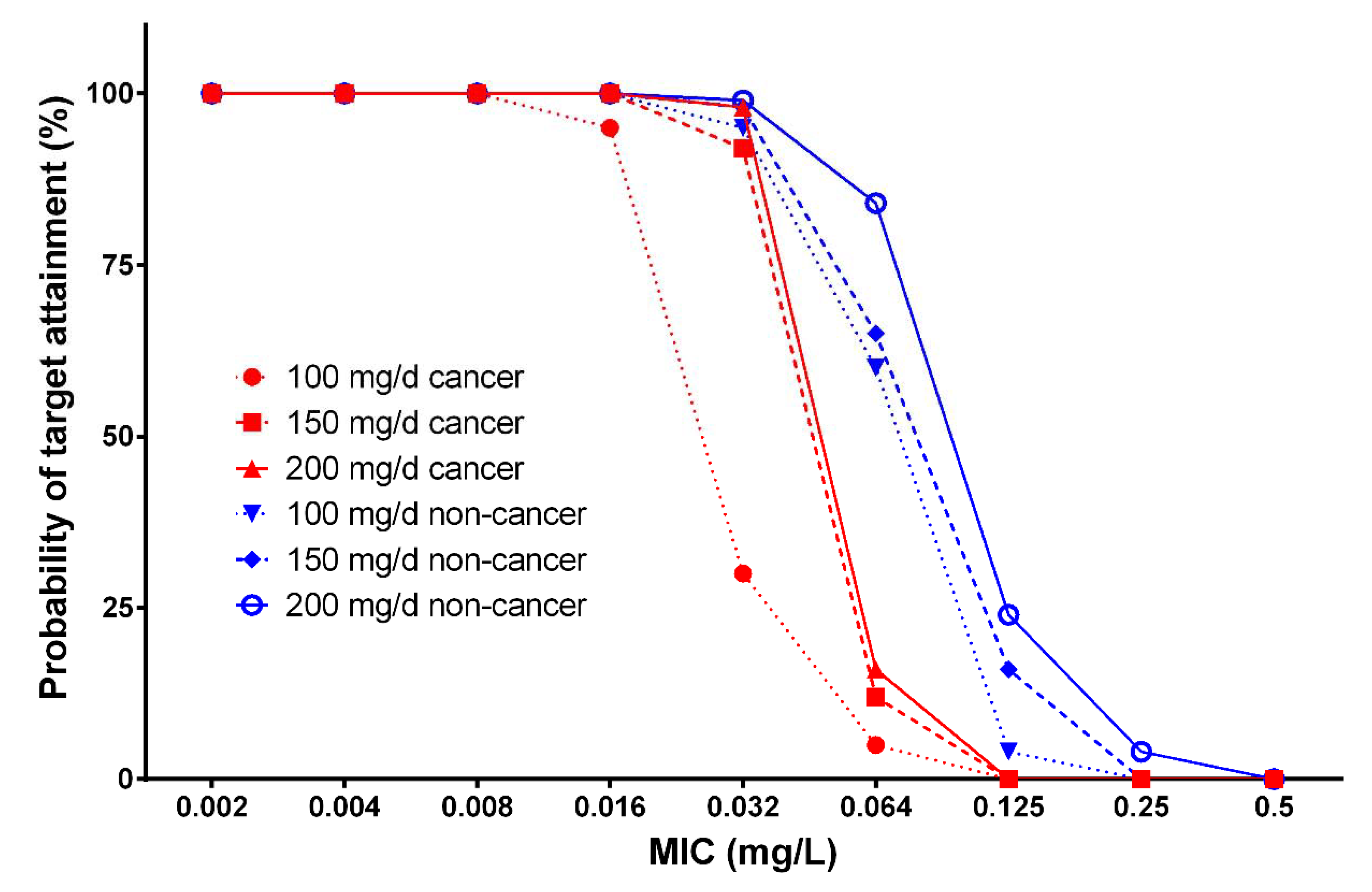
3.3. Monte Carlo Simulations of Plasma Concentrations and Probability of Target Attainment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Crawford, J.; Dale, D.C.; Lyman, G.H. Chemotherapy-induced neutropenia: Risks, consequences, and new directions for its management. Cancer 2004, 100, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Dale, D.C.; Crawford, J.; Cosler, L.E.; Lyman, G.H. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 2006, 106, 2258–2266. [Google Scholar] [CrossRef] [PubMed]
- Padilla, G.; Ropka, M.E. Quality of life and chemotherapy-induced neutropenia. Cancer Nurs. 2005, 28, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Schelenz, S.; Giles, D.; Abdallah, S. Epidemiology, management and economic impact of febrile neutropenia in oncology patients receiving routine care at a regional UK cancer centre. Ann. Oncol. 2012, 23, 1889–1893. [Google Scholar] [CrossRef]
- Culakova, E.; Thota, R.; Poniewierski, M.S.; Kuderer, N.M.; Wogu, A.F.; Dale, D.C.; Crawford, J.; Lyman, G.H. Patterns of chemotherapy-associated toxicity and supportive care in US oncology practice: A nationwide prospective cohort study. Cancer Med. 2014, 3, 434–444. [Google Scholar] [CrossRef]
- Flowers, C.R.; Seidenfeld, J.; Bow, E.J.; Karten, C.; Gleason, C.; Hawley, D.K.; Kuderer, N.M.; Langston, A.A.; Marr, K.A.; Rolston, K.V.; et al. Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2013, 31, 794–810. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A.; Hinedi, K.; Khairallah, H.; Saadeh, B.; Abbasi, S.; Noureen, M.; Raza, S.; Alkhatti, A. Epidemiology and source of infection in patients with febrile neutropenia: A ten-year longitudinal study. J. Infect. Public Health 2019, 12, 364–366. [Google Scholar] [CrossRef]
- Chindaprasirt, J.; Wanitpongpun, C.; Limpawattana, P.; Thepsuthammarat, K.; Sripakdee, W.; Wirasorn, K.; Sookprasert, A. Mortality, length of stay, and cost associated with hospitalized adult cancer patients with febrile neutropenia. Asian Pac. J. Cancer Prev. 2013, 14, 1115–1119. [Google Scholar] [CrossRef]
- Holland, T.; Fowler, V.G., Jr.; Shelburne, S.A., 3rd. Invasive gram-positive bacterial infection in cancer patients. Clin. Infect. Dis. 2014, 59 (Suppl. 5), S331–S334. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin. Infect. Dis. 2003, 36, 1103–1110. [Google Scholar] [CrossRef]
- Cornely, O.A.; Gachot, B.; Akan, H.; Bassetti, M.; Uzun, O.; Kibbler, C.; Marchetti, O.; De Burghgraeve, P.; Ramadan, S.; Pylkkanen, L.; et al. Epidemiology and outcome of fungemia in a cancer Cohort of the Infectious Diseases Group (IDG) of the European Organization for Research and Treatment of Cancer (EORTC 65031). Clin. Infect. Dis. 2015, 61, 324–331. [Google Scholar] [CrossRef]
- Hachem, R.; Hanna, H.; Kontoyiannis, D.; Jiang, Y.; Raad, I. The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer 2008, 112, 2493–2499. [Google Scholar] [CrossRef]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.A.H.; Wingard, J.R. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 52, 427–431. [Google Scholar] [CrossRef]
- Klastersky, J.; De Naurois, J.; Rolston, K.; Rapoport, B.; Maschmeyer, G.; Aapro, M.; Herrstedt, J. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann. Oncol. 2016, 27, v111–v811. [Google Scholar] [CrossRef]
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.; et al. Outpatient Management of Fever and Neutropenia in Adults Treated for Malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1443–1453. [Google Scholar] [CrossRef]
- de la Torre, P.; Reboli, A.C. Micafungin: An evidence-based review of its place in therapy. Core Evid. 2014, 9, 27–39. [Google Scholar]
- Kofla, G.; Ruhnke, M. Pharmacology and metabolism of anidulafungin, caspofungin and micafungin in the treatment of invasive candidosis: Review of the literature. Eur. J. Med. Res. 2011, 16, 159–166. [Google Scholar] [CrossRef]
- Eschenauer, G.; Depestel, D.D.; Carver, P.L. Comparison of echinocandin antifungals. Ther. Clin. Risk Manag. 2007, 3, 71–97. [Google Scholar] [CrossRef]
- Theuretzbacher, U. Pharmacokinetics/pharmacodynamics of echinocandins. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 805–812. [Google Scholar] [CrossRef]
- Wasmann, R.E.; Muilwijk, E.W.; Burger, D.M.; Verweij, P.E.; Knibbe, C.A.; Bruggemann, R.J. Clinical Pharmacokinetics and Pharmacodynamics of Micafungin. Clin. Pharmacokinet 2018, 57, 267–286. [Google Scholar] [CrossRef]
- Franken, L.G.; de Winter, B.M.; Van Esch, H.J.; van Zuylen, L.; Baar, F.P.M.; Tibboel, D.; Mathôt, R.A.A.; van Gelder, T.; Koch, B.C.P. Pharmacokinetic considerations and recommendations in palliative care, with focus on morphine, midazolam and haloperidol. Expert Opin. Drug Metab. Toxicol. 2016, 12, 669–680. [Google Scholar] [CrossRef]
- Grau, S.; Luque, S.; Campillo, N.; Samso, E.; Rodriguez, U.; Garcia-Bernedo, C.A.; Salas, E.; Sharma, R.; Hope, W.W.; Roberts, J.A. Plasma and peritoneal fluid population pharmacokinetics of micafungin in post-surgical patients with severe peritonitis. J. Antimicrob. Chemother. 2015, 70, 2854–2861. [Google Scholar] [CrossRef]
- Jullien, V.; Azoulay, E.; Schwebel, C.; Le Saux, T.; Charles, P.E.; Cornet, M.; Souweine, B.; Klouche, K.; Jaber, S.; Trouillet, J.L.; et al. Population pharmacokinetics of micafungin in ICU patients with sepsis and mechanical ventilation. J. Antimicrob. Chemother. 2017, 72, 181–189. [Google Scholar] [CrossRef]
- Martial, L.C.; Ter Heine, R.; Schouten, J.A.; Hunfeld, N.G.; Van Leeuwen, H.J.; Verweij, P.E.; de Lange, D.W.; Pickkers, P.; Brüggemann, R.J. Population Pharmacokinetic Model and Pharmacokinetic Target Attainment of Micafungin in Intensive Care Unit Patients. Clin. Pharmacokinet 2017, 56, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Goutelle, S.; Jelliffe, R.W.; Golden, J.A.; Little, E.A.; DeVoe, C.; Mickiene, D.; Hayes, M.; Conte, J.E., Jr. Intrapulmonary pharmacokinetics and pharmacodynamics of micafungin in adult lung transplant patients. Antimicrob. Agents Chemother. 2010, 54, 3451–3459. [Google Scholar] [CrossRef] [PubMed]
- Martens-Lobenhoffer, J.; Rupprecht, V.; Bode-Boger, S.M. Determination of micafungin and anidulafungin in human plasma: UV- or mass spectrometric quantification? J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Lavielle, M.; Mentre, F. Estimation of population pharmacokinetic parameters of saquinavir in HIV patients with the MONOLIX software. J. Pharmacokinet. Pharmacodyn. 2007, 34, 229–249. [Google Scholar] [CrossRef]
- Alqahtani, S.A.; Alsultan, A.S.; Alqattan, H.M.; Eldemerdash, A.; Albacker, T.B. Population Pharmacokinetic Model for Vancomycin Used in Open Heart Surgery: Model-Based Evaluation of Standard Dosing Regimens. Antimicrob. Agents Chemother 2018, 62, e00088-18. [Google Scholar] [CrossRef]
- Alqahtani, S.A.; Alsultan, A.S.; Alqattan, H.M.; Eldemerdash, A.; Albacker, T.B. Population Pharmacokinetic Model-Based Evaluation of Standard Dosing Regimens for Cefuroxime Used in Coronary Artery Bypass Graft Surgery with Cardiopulmonary Bypass. Antimicrob. Agents Chemother. 2018, 62, e02241-17. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Findlay, M.; Osterwalder, B.; Kocha, W.; Dalley, D.; Pazdur, R.; Cassidy, J.; Dirix, L.; Twelves, C.; Allman, D.; et al. Capecitabine, an oral fluoropyrimidine carbamate with substantial activity in advanced colorectal cancer: Results of a randomized phase II study. J. Clin. Oncol. 2000, 18, 1337–1345. [Google Scholar] [CrossRef]
- Andes, D.; Ambrose, P.G.; Hammel, J.P.; Van Wart, S.A.; Iyer, V.; Reynolds, D.K.; Buell, D.N.; Kovanda, L.L.; Bhavnani, S.M. Use of pharmacokinetic-pharmacodynamic analyses to optimize therapy with the systemic antifungal micafungin for invasive candidiasis or candidemia. Antimicrob. Agents Chemother. 2011, 55, 2113–2121. [Google Scholar] [CrossRef]
- Bertino, J.S., Jr.; Booker, L.A.; Franck, P.; Rybicki, B. Gentamicin pharmacokinetics in patients with malignancies. Antimicrob. Agents Chemother. 1991, 35, 1501–1503. [Google Scholar] [CrossRef][Green Version]
- Bury, D.; Ter Heine, R.; van de Garde, E.M.W.; Nijziel, M.R.; Grouls, R.J.; Deenen, M.J. The effect of neutropenia on the clinical pharmacokinetics of vancomycin in adults. Eur. J. Clin. Pharmacol. 2019, 75, 921–928. [Google Scholar] [CrossRef]
- Romano, S.; Fdez de Gatta, M.M.; Calvo, M.V.; Caballero, D.; Dominguez-Gil, A.; Lanao, J.M. Population pharmacokinetics of amikacin in patients with haematological malignancies. J. Antimicrob. Chemother. 1999, 44, 235–242. [Google Scholar] [CrossRef]
- Gumbo, T.; Hiemenz, J.; Ma, L.; Keirns, J.J.; Buell, D.N.; Drusano, G.L. Population pharmacokinetics of micafungin in adult patients. Diagn. Microbiol. Infect. Dis. 2008, 60, 329–331. [Google Scholar] [CrossRef]
- Hall, R.G.; Swancutt, M.A.; Gumbo, T. Fractal geometry and the pharmacometrics of micafungin in overweight, obese, and extremely obese people. Antimicrob. Agents Chemother. 2011, 55, 5107–5112. [Google Scholar] [CrossRef]
- Maseda, E.; Grau, S.; Luque, S.; Castillo-Mafla, M.P.; Suárez-de-la-Rica, A.; Montero-Feijoo, A.; Salgado, P.; Gimenez, M.J.; García-Bernedo, C.A.; Gilsanz, F.; et al. Population pharmacokinetics/pharmacodynamics of micafungin against Candida species in obese, critically ill, and morbidly obese critically ill patients. Crit. Care 2018, 22, 94. [Google Scholar] [CrossRef]
- Tabata, K.; Katashima, M.; Kawamura, A.; Kaibara, A.; Tanigawara, Y. Population pharmacokinetic analysis of micafungin in Japanese patients with fungal infections. Drug Metab. Pharmacokinet. 2006, 21, 324–331. [Google Scholar] [CrossRef][Green Version]
- Zomp, A.; Bookstaver, P.B.; Ahmed, Y.; Turner, J.E.; King, C. Micafungin therapy in a critically ill, morbidly obese patient. J. Antimicrob. Chemother. 2011, 66, 2678–2680. [Google Scholar] [CrossRef]
- Kunishima, S.; Taniguchi, H.; Yamaguchi, A.; Koh, T.; Yamagishi, H. Changes in hepatic parenchymal blood flow with colorectal metastases: Increase in arterial and decrease in portal blood flow. Hepatogastroenterology 2003, 50, 1457–1462. [Google Scholar]
- Shuto, K.; Mori, M.; Kosugi, C.; Narushima, K.; Nakabayashi, S.; Fujisiro, T.; Sato, A.; Hayano, K.; Shimizu, H.; Koda, K. Hepatic blood flow by perfusion computed tomography as an imaging biomarker for patients with gastric cancer. Oncol. Lett. 2019, 17, 3267–3276. [Google Scholar] [CrossRef]


| Characteristics | Patients with Cancer (n = 10) | Patients without Cancer (n = 9) | p Value |
|---|---|---|---|
| Age, years, mean (SD) | 47.3 (12.3) | 51.1 (19.1) | 0.25 |
| Sex, % male/female | 60/40 | 67/33 | 0.35 |
| Weight, kg, mean (SD) | 63.4 (18.2) | 69.8 (15.7) | 0.23 |
| Height, cm, mean (SD) | 162.2 (9.9) | 163.1 (7.3) | 0.78 |
| Serum creatinine, mmol/L, mean (SD) | 74.7 (43.4) | 63.6 (35.8) | 0.16 |
| CLCr, mL/min, mean (SD) | 103 (58.8) | 99 (69.3) | 0.97 |
| Albumin, mean (SD) | 25.6 (5.8) | 22.6 (3.7) | 0.12 |
| AST, mean (SD) | 34.2 (9.3) | 37.7 (15.4) | 0.14 |
| ALT, mean (SD) | 26.3 (11.3) | 28.3 (6.7) | 0.23 |
| Total bilirubin, mean (SD) | 26.5 (3.8) | 20.5 (16.4) | 0.31 |
| SOFA score | 7 (5.5) | 8 (6.5) | 0.25 |
| Parameter | Patients with Cancer | Patients without Cancer | |||
|---|---|---|---|---|---|
| Estimate | RSE (%) | Estimate | RSE (%) | p Values | |
| CL (L/h) | 1.2 | 11.6 | 0.6 | 14 | 0.012 |
| V1 (L) | 10.7 | 23.6 | 12 | 22.2 | 0.65 |
| Q (L/h) | 0.144 | 14 | 0.188 | 10 | 0.56 |
| V2 (L) | 3.5 | 16 | 2.77 | 12.5 | 0.73 |
| IIV ** for CL (%) | 34.1 | 14.8 | 11.8 | 18 | |
| IIV for V1 (%) | 7.6 | 5.2 | 7.6 | 20 | |
| IIV for Q (%) | 32.2 | 18 | 20.4 | 13 | |
| IIV for V2 (%) | 36.8 | 15 | 32.1 | 22 | |
| Residual error | |||||
| a | 0.21 | 10.7 | 0.15 | 9.2 | |
| b | 0.22 | 4.5 | 0.18 | 13.6 | |
| Micafungin Dose (mg) | Body Weight (kg) | MIC Breakpoint (mg/L) | |||
|---|---|---|---|---|---|
| Patients with Cancer | Patients without Cancer | ||||
| Candida spp. | C. parapsilosis | Candida spp. | C. parapsilosis | ||
| 100 | 50 | 0.032 | 0.25 | 0.032 | 0.5 |
| 70 | 0.016 | 0.125 | 0.032 | 0.25 | |
| 100 | 0.016 | 0.064 | 0.016 | 0.125 | |
| 150 | 50 | 0.032 | 0.25 | 0.064 | 0.5 |
| 70 | 0.032 | 0.25 | 0.032 | 0.25 | |
| 100 | 0.016 | 0.125 | 0.032 | 0.25 | |
| 200 | 50 | 0.032 | 0.25 | 0.064 | 0.5 |
| 70 | 0.032 | 0.25 | 0.032 | 0.5 | |
| 100 | 0.016 | 0.125 | 0.032 | 0.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, S.; Alfarhan, A.; Alsultan, A.; Alsarhani, E.; Alsubaie, A.; Asiri, Y. Assessment of Micafungin Dosage Regimens in Patients with Cancer Using Pharmacokinetic/Pharmacodynamic Modeling and Monte Carlo Simulation. Antibiotics 2021, 10, 1363. https://doi.org/10.3390/antibiotics10111363
Alqahtani S, Alfarhan A, Alsultan A, Alsarhani E, Alsubaie A, Asiri Y. Assessment of Micafungin Dosage Regimens in Patients with Cancer Using Pharmacokinetic/Pharmacodynamic Modeling and Monte Carlo Simulation. Antibiotics. 2021; 10(11):1363. https://doi.org/10.3390/antibiotics10111363
Chicago/Turabian StyleAlqahtani, Saeed, Asma Alfarhan, Abdullah Alsultan, Emad Alsarhani, Abdulaziz Alsubaie, and Yousif Asiri. 2021. "Assessment of Micafungin Dosage Regimens in Patients with Cancer Using Pharmacokinetic/Pharmacodynamic Modeling and Monte Carlo Simulation" Antibiotics 10, no. 11: 1363. https://doi.org/10.3390/antibiotics10111363
APA StyleAlqahtani, S., Alfarhan, A., Alsultan, A., Alsarhani, E., Alsubaie, A., & Asiri, Y. (2021). Assessment of Micafungin Dosage Regimens in Patients with Cancer Using Pharmacokinetic/Pharmacodynamic Modeling and Monte Carlo Simulation. Antibiotics, 10(11), 1363. https://doi.org/10.3390/antibiotics10111363






