Abundance and Dynamic Distribution of Antibiotic Resistance Genes in the Environment Surrounding a Veterinary Antibiotic Manufacturing Site
Abstract
:1. Introduction
2. Results
2.1. Detection of Resistance Genes by qPCR Assay
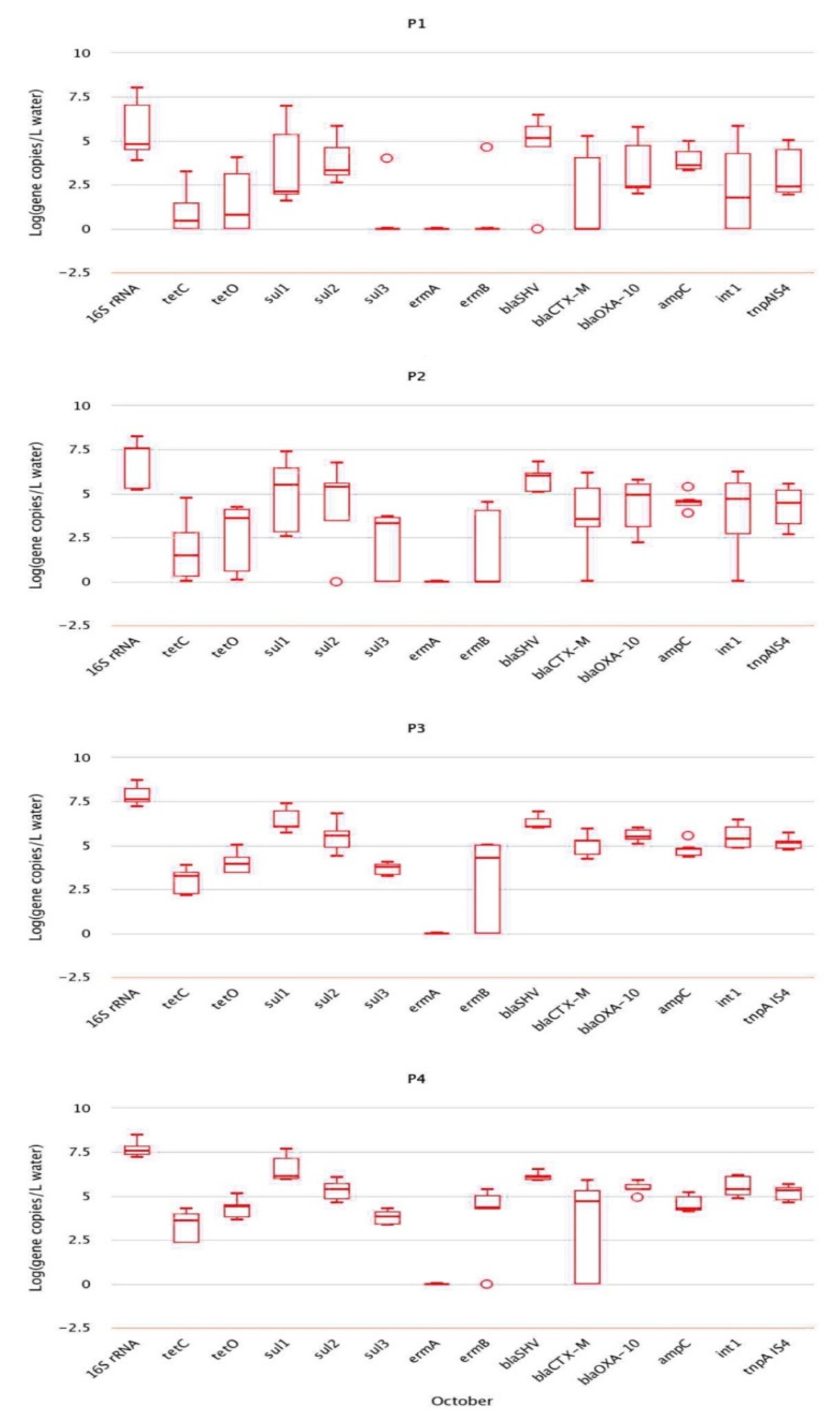
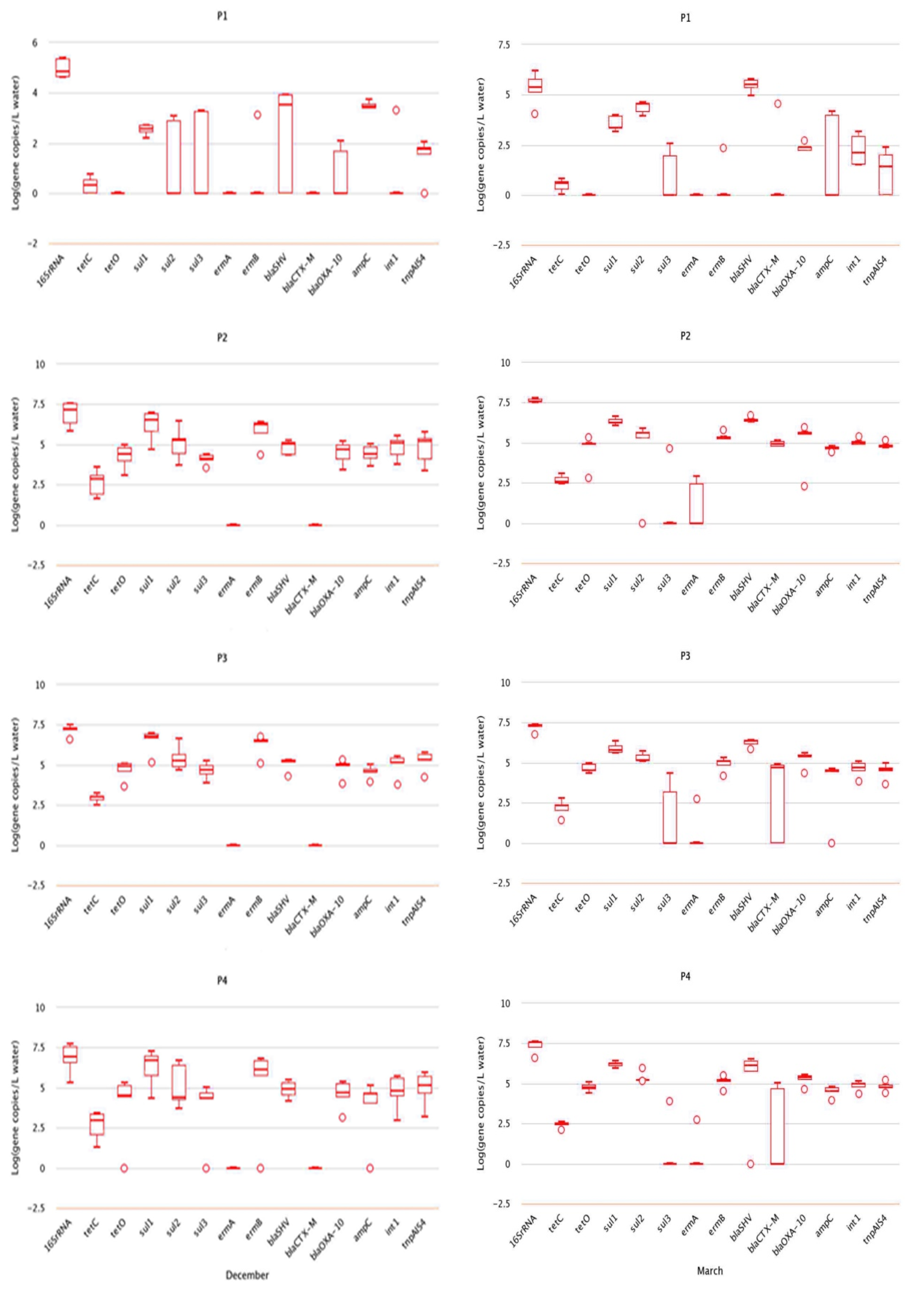
2.2. Analysis of Absolute Abundance of ARGs by qPCR
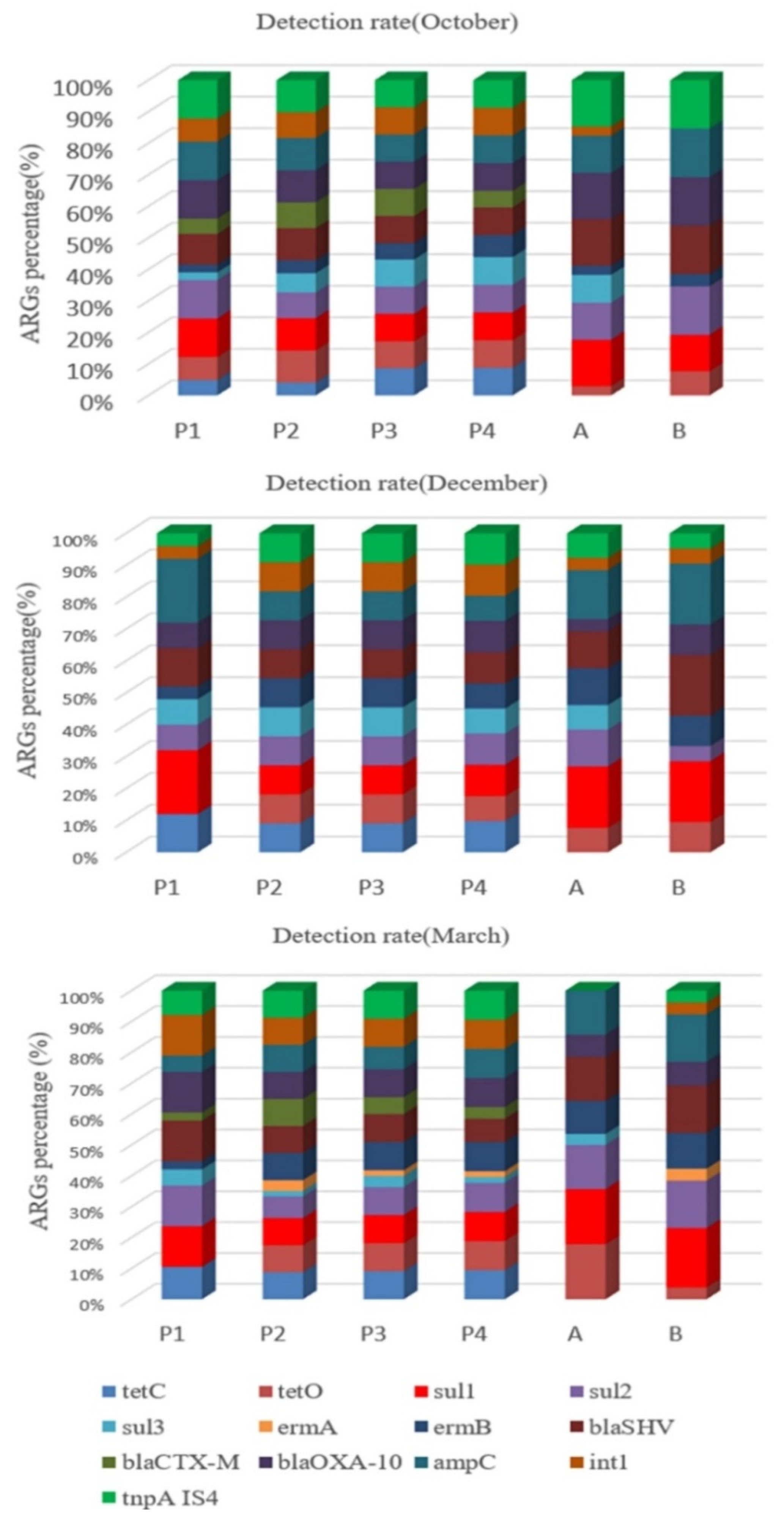
2.3. Relative Abundance of ARGs
2.4. Correlation Analysis of ARGs with Integron intI and Transposon tnpA-IS4
2.5. Soil Moisture Content
3. Discussion
4. Materials and Methods
4.1. Reagents and Instrumentation
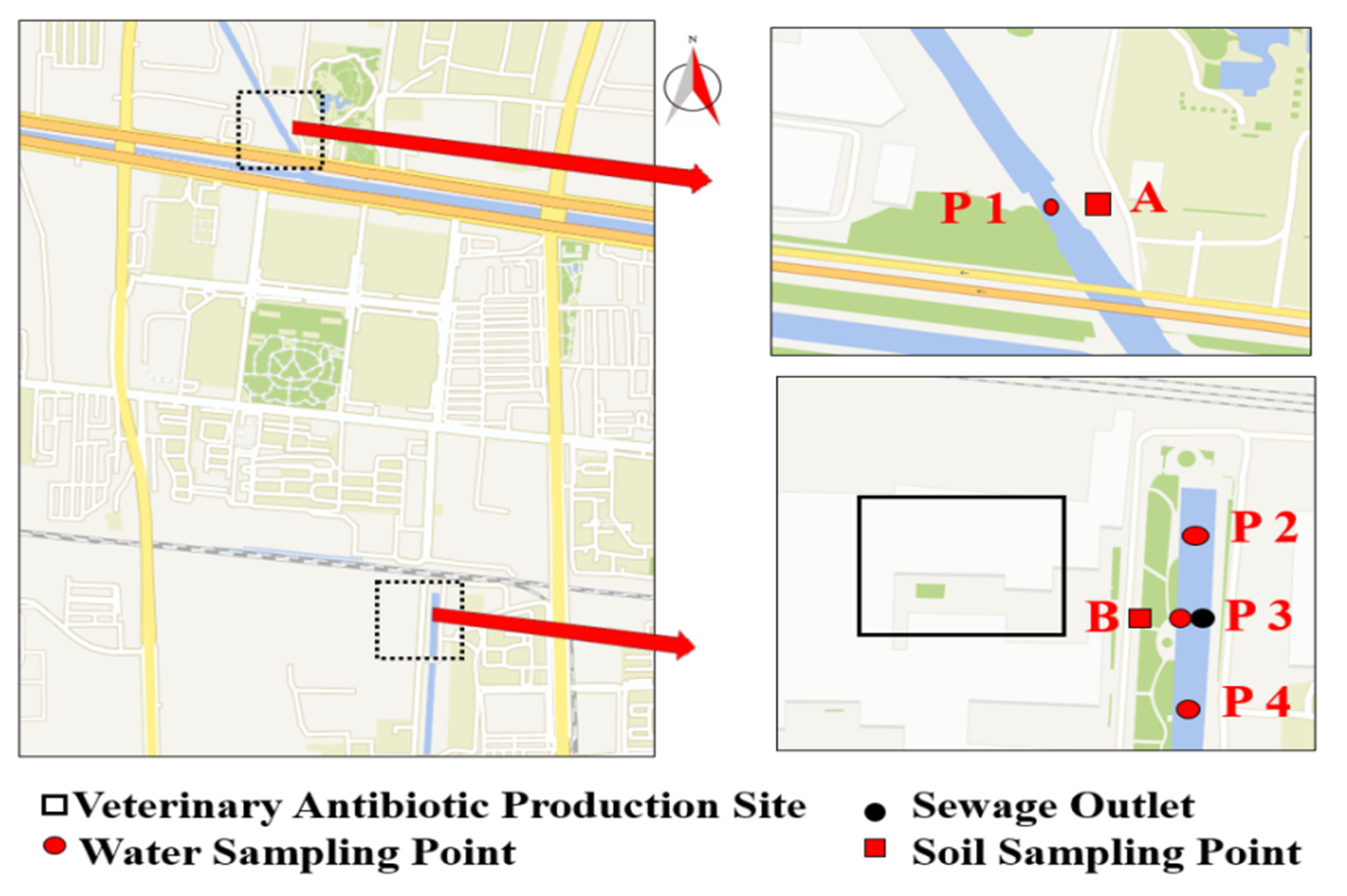
4.2. Sample Collection and Processing
4.3. Selection of 11 Resistance Genes
4.4. Antibiotic Residue Analysis
4.5. DNA Extraction
4.6. Fluorescence Quantitative PCR
4.7. Data Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, L.; Wu, J.; Zhang, C.; Zhang, Y.; Lin, Y.; Luo, Y. Occurrence, distribution, and ecological-health risks of selected antibiotics in coastal waters along the coastline of China. Sci. Total Environ. 2018, 644, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Mao, D.Q.; Michal, R.; Rysz, M.; Zhou, Q.; Zhang, H.; Xu, L.; Alvarez, P.J.J. Trends in Antibiotic Resistance Genes Occurrence in the Haihe River, China. Environ. Sci. Technol. 2010, 19, 7220–7225. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhou, Q.X. Antibiotic resistance genes (ARGs) as emerging pollutants. Acta Sci. Circumstantiae 2008, 28, 1499–1505. [Google Scholar]
- Martinez, J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 2009, 157, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Pruden, A. Balancing water sustainability and public health goals in the face of growing concerns about antibiotic resistance. Environ. Sci. Technol. 2014, 48, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Hu, Y.F.; Luo, M.; Zhou, H.J.; Wang, X.X.; Du, Y.; Li, Z.P.; Xu, J.L.; Zhu, B.L.; Xu, X.B.; et al. MCR-1.6, a New MCR Variant Carried by an IncP Plasmid in a Colistin-Resistant Salmonella enterica Serovar Typhimurium Isolate from a Healthy Individual. Antimicrob. Agents Chemother. 2017, 61, e02632-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruppé, E.; Ghozlane, A.; Ehrlich, S.D. Prediction of the intestinal resistome by a three-dimensional structure-based method. Nat. Microbiol. 2019, 4, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Pruden, A.; Pei, R.; Storteboom, H.; Carlson, K.H. Antibiotic resistance genes as emerging contaminants: Studies in northern Colorado. Environ. Sci. Technol. 2006, 40, 7445–7450. [Google Scholar] [CrossRef] [PubMed]
- Dang, B.J.; Mao, D.Q.; Xu, Y.; Luo, Y. Conjugative multi-resistant plasmids in Haihe River and their impacts on the abundance and spatial distribution of antibiotic resistance genes. Water Res. 2017, 111, 81–91. [Google Scholar] [CrossRef]
- Mendes, R.E.; Deshpande, L.; Rodriguez-Noriega, E.; Ross, J.E.; Jones, R.N.; Morfin-Otero, R. First report of Staphylococcal clinical isolates in Mexico with linezolid resistance caused by cfr: Evidence of in vivo cfr mobilization. J. Clin. Microbiol. 2010, 48, 3041–3043. [Google Scholar] [CrossRef] [Green Version]
- Nõlvak, H.; Truu, M.; Tiirik, K.; Oopkaup, K.; Sildvee, T.; Kaasik, A.; Mander, Ü.; Truu, J. Dynamics of antibiotic resistance genes and their relationships with system treatment efficiency in a horizontal subsurface flow constructed wetland. Sci. Total Environ. 2013, 461–462, 636–644. [Google Scholar] [CrossRef]
- LaPara, T.M.; Burch, T.R.; McNamara, P.J.; Tan, D.T.; Yan, M.; Eichmille, J.J. Tertiary-Treated Municipal Wastewater is a Significant Point Source of Antibiotic Resistance Genes into Duluth-Superior Harbor. Environ. Sci. Technol. 2011, 45, 9543–9549. [Google Scholar] [CrossRef] [Green Version]
- Czekalski1, N.; Berthold, T.; Caucci, S.; Egli, A.; Bürgmann, H. Increased levels of multiresistant bacteria and resistance genes after wastewater treatment and their dissemination into Lake Geneva, Switzerland. Front. Microbiol. 2012, 3, 106. [Google Scholar] [CrossRef] [Green Version]
- Fiorentino, A.; Cesare, A.D.; Eckert, E.M.; Rizzo, L.; Fontaneto, D.; Yang, Y.; Corno, G. Impact of industrial wastewater on the dynamics of antibiotic resistance genes in a full-scale urban wastewater treatment plant. Sci. Total Environ. 2019, 646, 1204–1210. [Google Scholar] [CrossRef]
- Zhang, W.H.; Suyamud, B.; Lohwacharin, J.; Yang, Y.Y. Large-scale pattern of resistance genes and bacterial community in the tap water along the middle and low reaches of the Yangtze River. Ecotoxicol. Environ. Saf. 2021, 208, 111517. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yun, Z.; Ha, U.H.; Lee, S.; Park, H.; Kwon, E.E.; Cho, Y.; Choung, S.; Oh, J.; Medriano, C.A.; et al. Transfer of antibiotic resistance plasmids in pure and activated sludge cultures in the presence of environmentally representative micro-contaminant concentrations. Sci. Total Environ. 2014, 468–469, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Yin, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Chen, Q.L.; Ding, J.; Wang, Y.F.; Cui, H.L.; Zhu, Y.G. Resistance genes in soil ecosystems and planetary health: Progress and prospects. Sci. China Life Sci. 2019, 49, 1652–1663. [Google Scholar] [CrossRef]
- Zammit, I.; Marano, R.B.M.; Vaiano, V.; Cytryn, E.; Rizzo, L. Changes in Antibiotic Resistance Gene Levels in Soil after Irrigation with Treated Wastewater: A Comparison between Heterogeneous Photocatalysis and Chlorination. Environ. Sci. Technol. 2020, 54, 7677–7686. [Google Scholar] [CrossRef]
- Knapp, C.W.; Dolfing, J.; Ehlert, P.A.; Graham, D.W. Evidence of Increasing Antibiotic Resistance Gene Abundances in Archived Soils since 1940. Environ. Sci. Technol. 2010, 44, 580–587. [Google Scholar] [CrossRef]
- Zhu, D.; Xiang, Q.; Yang, X.R.; Ke, X.; O’Connor, P.; Zhu, Y.G. Trophic Transfer of Antibiotic Resistance Genes in a Soil Detritus Food Chain. Environ. Sci. Technol. 2019, 53, 7770–7781. [Google Scholar] [CrossRef]
- Xu, H.; Chen, Z.Y.; Huang, R.Y.; Cui, Y.X.; Li, Q.; Zhao, Y.H.; Wang, X.L.; Mao, D.Q.; Luo, Y.; Ren, H.Q. Antibiotic Resistance Gene-Carrying Plasmid Spreads into the Plant Endophytic Bacteria using Soil Bacteria as Carriers. Environ. Sci. Technol. 2021, 55, 10462–10470. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.G.; He, L.Y.; Ying, A.J.; Zhang, Q.Q.; Liu, Y.S.; Zhao, J.L. China Must Reduce Its Antibiotic Use. Environ. Sci. Technol. 2017, 51, 1072–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joakim Larsson, D.G. Antibiotics in the environment. Upsala J. Med. Sci. 2014, 119, 108–112. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhang, G.D.; Liu, Y.; Lu, S.Y.; Qin, P.; Guo, X.C.; Bi, B.; Wang, L.; Xi, B.D.; Wu, F.C.; et al. Occurrence and fate of antibiotics and antibiotic resistance genes in typical urban water of Beijing, China. Environ. Pollut. 2019, 246, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Bai, X.M.; Li, Y.Z.; Jing, L.J.; Chen, R.H.; Teng, Y.G. Characterization and source-tracking of antibiotic resistomes in the sediments of a peri-urban river. Sci. Total Environ. 2019, 679, 88–96. [Google Scholar] [CrossRef]
- Szekeres, E.; Chiriac, C.M.; Baricz, A.; Szőke-Nagy, T.; Lung, I.; Soran, M.L.; Rudi, K.; Dragos, N.; Coman, C. Investigating antibiotics, antibiotic resistance genes, and microbial contaminants in groundwater in relation to the proximity of urban areas. Environ. Pollut. 2018, 236, 734–744. [Google Scholar] [CrossRef]
- Xie, J.W.; Jin, L.; He, T.T.; Chen, B.W.; Luo, X.S.; Feng, B.H.; Huang, W.; Li, J.; Fu, P.Q.; Li, X.D. Bacteria and antibiotic resistance genes (ARGs) in PM2.5 from China: Implications for human exposure. Environ. Sci. Technol. 2019, 53, 963–972. [Google Scholar] [CrossRef]
- Gaviria-Figueroa, A.; Preisner, E.C.; Hoque, S.; Feigley, C.E.; Norman, R.S. Emission and dispersal of antibiotic resistance genes through bioaerosols generated during the treatment of municipal sewage. Sci. Total Environ. 2019, 686, 402–412. [Google Scholar] [CrossRef]
- Zhou, R.J.; Zeng, S.Z.; Hou, D.W.; Liu, J.; Weng, S.P.; He, J.G.; Huang, Z.J. Occurrence of human pathogenic bacteria carrying antibiotic resistance genes revealed by metagenomic approach: A case study from an aquatic environment. J. Environ. Sci. 2019, 80, 248–256. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, P.; Huang, Y.S.; Yu, K.F.; Chen, H.J.; Cui, K.P.; Huang, Q.L.; Zhang, J.Y.; Gin, K.Y.H.; He, Y.L. Environmental media exert a bottleneck in driving the dynamics of antibiotic resistance genes in modern aquatic environment. Water Res. 2019, 162, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.J.; Zhao, Y.; Yang, K.J.; Chen, J.; Zhou, H.X.; Chen, X.M.; Liu, Q.; Wei, Z.M. Host bacterial community of MGEs determines the risk of horizontal gene transfer during composting of different animal manures. Environ. Pollut. 2019, 250, 166–174. [Google Scholar] [CrossRef] [PubMed]
- González-Plaza, J.J.; Blau, K.; Milaković, M.; Jurina, T.; Smalla, K.; Udiković-Kolić, N. Antibiotic-manufacturing sites are hot-spots for the release and spread of antibiotic resistance genes and mobile genetic elements in receiving aquatic environments. Environ. Int. 2019, 130, 104735. [Google Scholar] [CrossRef]
- Hegstad, K.; Mikalsen, T.; Coque, T.M.; Werner, G.; Sundsfjord, A. Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin. Microbiol. Infect. 2010, 16, 468–469. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Mao, D.; Luo, Y.; Wang, L.; Xu, B.; Xu, L. Occurrence of sulfonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment. Water Res. 2012, 46, 2355–2364. [Google Scholar] [CrossRef]
- Jahnavi, K.; Pooja, S.; Prabhat, K.M. Presence of fluoroquinolone resistance with persistent occurrence of gyrA gene mutations in a municipal wastewater treatment plant in india. Chemosphere 2018, 211, 817–825. [Google Scholar]
- Kristiansson, E.; Fick, J.; Janzon, A.; Grabic, R.; Rutgersson, C.; Weijdegård, B.; Söderström, H.; Larsson, D.J. Pyrosequencing of antibiotic-contaminated river sediments reveals high levels of resistance and gene transfer elements. PLoS ONE 2011, 6, e17038. [Google Scholar] [CrossRef]
- Jérôme, C.; Mercè, B.; Ina, S.; Jürg, B.L.; Eva, S.L. Contribution of different bacterial dispersal sources to lakes: Population and community effects in different seasons. Environ. Microbiol. 2017, 19, 2391–2404. [Google Scholar]
- Son, D.I.; Aleta, P.; Park, M.; Yoon, H.; Cho, K.H.; Kim, Y.M.; Kim, S. Seasonal Changes in Antibiotic Resistance Genes in Rivers and Reservoirs in South Korea. J. Environ. Qual. 2018, 47, 1079–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Zhou, Z.; Wei, Y.; Chen, T.; Feng, W.; Chen, H. High-throughput profiling of seasonal variations of antibiotic resistance gene transport in a peri-urban river. Environ. Int. 2018, 114, 87–94. [Google Scholar] [CrossRef]
- Su, J.Q.; Wei, B.; Xu, C.Y.; Qiao, M.; Zhu, Y.G. Functional metagenomic characterization of antibiotic resistance genes in agricultural soils from China. Environ. Int. 2014, 65, 9–15. [Google Scholar] [CrossRef]
- Chen, J.; Su, Z.; Dai, T.; Huang, B.; Mu, Q.; Zhang, Y.; Wen, D. Occurrence and distribution of antibiotic resistance genes in the sediments of the East China Sea bays. J. Environ. Sci. 2019, 81, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dechesne, A.; He, Z.; Madsen, J.S.; Nesme, J.; Sørensen, S.J.; Smets, B.F. Estimating the transfer range of plasmids encoding antimicrobial resistance in a wastewater treatment plant microbial community. Environ. Sci. Tech. Let. 2018, 5, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, W.; Xu, C.; Wei, B.; Wang, J. Antibiotic resistance genes in lakes from middle and lower reaches of the Yangtze River, China: Effect of land use and sediment characteristics. Chemosphere 2017, 178, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Yang, Y.Q.; Miao, J.J.; Yin, Z.D.; Zhai, Y.J.; Shi, H.M.; Li, Z.N. Determination of sulfa antibiotic residues in river and particulate matter by field-amplified sample injection-capillary zone electrophoresis. Electrophoresis 2020, 41, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Yin, Z.D.; Zhai, Y.J.; Kang, W.J.; Shi, H.M.; Li, Z.N. Magnetic solid-phase extraction of four β-lactams using polypyrrole-coated magnetic nanoparticles from water samples by micellar electrokinetic capillary chromatography analysis. J. Chromatogr. A 2020, 1610, 460541. [Google Scholar] [CrossRef]
- Li, X.H.; Miao, J.J.; Kang, K.; Wang, W.; Shi, H.M. Simultaneous Detection of Thirteen Antibiotics in Solid and Water by solid phase extraction combined with High Performance Capillary Electrophoresis. Ptca Part B Chem. Anal. 2019, 55, 769–777. [Google Scholar]
- Baran, W.; Adamek, E.; Ziemiańska, J.; Sobczak, A. Effects of the presence of sulfonamides in the environment and their influence on human health. J. Hazard. Mater. 2011, 196, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, A.L.; Dai, T.J.; Li, F.F.; Xie, H.; Chen, L.J.; Wen, D.H. Cell-free DNA: A neglected source for antibiotic resistance genes spreading from WWTPs. Environ. Sci. Technol. 2018, 52, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.H.; Li, J.; Sun, Y.X.; Mao, D.Q.; Wang, Q.; Luo, Y. Occurrence of sulfonamide-, tetracycline-, plasmid-mediated quinolone- and macrolide-resistance genes in livestock feedlots in Northern China. Environ. Sci. Pollut. Res. 2015, 22, 6932–6940. [Google Scholar] [CrossRef]
- Lin, H.; Chapman, S.J.; Freitag, T.E.; Kyle, C.; Ma, J.; Yang, Y.; Zhang, Z. Fate of tetracycline and sulfonamide resistance genes in a grassland soil amended with different organic fertilizers. Ecotox. Environ. Saf. 2019, 170, 39–46. [Google Scholar] [CrossRef]
- Pei, R.T.; Kim, S.C.; Carlson, K.H.; Pruden, A. Effect of River Landscape on the sediment concentrations of antibiotics and corresponding antibiotic resistance genes (ARG). Water Res. 2006, 40, 2427–2435. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Johnson, T.A.; Su, J.Q.; Guo, G.X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. USA 2013, 9, 3435–3440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, C.; Yu, Y.; Diao, J.; Gong, X.; Li, J.; Sun, Y. Exploring the persistence and spreading of antibiotic resistance from manure to biocompost, soils and vegetables. Sci. Total. Environ. 2019, 688, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, Z.T.; Michel, F.C.; Wittum, T.; Mark, M. Development and application of real-time PCR assays for quantification of erm genes conferring resistance to macrolides-lincosamides-streptogramin B in livestock manure and manure management systems. Appl. Environ. Microb. 2007, 73, 4407–4416. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Hu, X.; Xu, T.; Zhang, H.; Sheng, D.; Yin, D. Prevalence of antibiotic resistance genes and their relationship with antibiotics in the Huangpu River and the drinking water sources, Shanghai, China. Sci. Total Environ. 2013, 458, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Ma, L.; Li, B.; Jiang, X.T.; Wang, Y.L.; Xia, Y.; Li, A.T.; Zhang, T. Catalogue of antibiotic resistome and host-tracking in drinking water deciphered by a large scale survey. Microbiome 2017, 5, 154. [Google Scholar] [CrossRef] [Green Version]

| Relative Abundance | October | December | March | |||
|---|---|---|---|---|---|---|
| F * | p * | F | p | F | p | |
| RtetC | 0.264 | 0.850 | 1.983 | 0.163 | 0.304 | 0.822 |
| RtetO | 3.270 | 0.053 | 1.533 | 0.259 | 0.978 | 0.404 |
| Rsul1 | 2.843 | 0.071 | 34.329 | 0.000 | 1.922 | 0.167 |
| Rsul2 | 0.954 | 0.440 | 0.125 | 0.944 | 6.919 | 0.004 |
| Rsul3 | 0.747 | 0.548 | 1.727 | 0.215 | 2.985 | 0.261 |
| RermA | - | - | - | - | 0.204 | 0.843 |
| RermB | 3.920 | 0.073 | 6.595 | 0.008 | 0.508 | 0.683 |
| RblaSHV | 2.837 | 0.073 | 3.011 | 0.066 | 26.119 | 0.000 |
| RblaCTX-M | 3.483 | 0.058 | - | - | 37.296 | 0.000 |
| RblaOXA-10 | 0.701 | 0.565 | 25.305 | 0.000 | 1.402 | 0.278 |
| RampC | 3.854 | 0.030 | 13.569 | 0.000 | 14.981 | 0.000 |
| Rint1 | 0.314 | 0.815 | 3.512 | 0.049 | 4.720 | 0.015 |
| RtnpA IS4 | 0.550 | 0.656 | 26.040 | 0.000 | 2.637 | 0.090 |
| Relative Abundance | October | December | March | |||
|---|---|---|---|---|---|---|
| Rint1 | RtnpA IS4 | Rint1 | RtnpA IS4 | Rint1 | RtnpA IS4 | |
| RtetC | 0.74 * | 0.37 | −0.56 * | 0.14 | 0.18 | 0.27 |
| RtetO | 0.56 * | −0.23 | 0.57 * | 0.47 | 0.35 | 0.22 |
| Rsul1 | 0.59 * | −0.33 | −0.21 | 0.95 * | 0.74 * | 0.68 |
| Rsul2 | 0.01 | 0.21 | 0.06 | 0.14 | −0.37 | −0.12 |
| Rsul3 | 0.02 | 0.23 | 0.32 | −0.3 | −0.01 | 0.26 |
| RermA | - | - | - | - | −0.94 | −0.73 |
| RermB | 0.35 | 0.71 | 0.59 * | 0.87 * | 0.56 * | 0.42 |
| RampC | −0.19 | 0.62 * | 0.47 | −0.84 * | −0.45 | 0.59 * |
| RblaSHV | −0.3 | 0.51 * | −0.04 | −0.65 * | −0.50 * | −0.18 |
| RblaCTX-M | 0.24 | 0.37 | - | - | −0.5 | 0.62 |
| RblaOXA-10 | 0.44 | 0.57 * | 0.6 0 * | 0.83 * | 0.44 | 0.25 |
| Sample | October | December | March | |||
|---|---|---|---|---|---|---|
| Point A | Point B | Point A | Point B | Point A | Point B | |
| 1 | 3.33% | - | 1.67% | 10.33% | 2.38% | 4.41% |
| 2 | 3.38% | 4.65% | 4.00% | 13.91% | 1.67% | 5.00% |
| 3 | 1.67% | 6.00% | 4.20% | 15.09% | 2.98% | 3.31% |
| 4 | 2.00% | 5.33% | 14.92% | 14.69% | 1.33% | 11.33% |
| 5 | 4.00% | 10.67% | 4.33% | - | 1.99% | 1.99% |
| Types | ARGs | Primer Sequence | Amplicon Size (bp) | Reference |
|---|---|---|---|---|
| Tetracyclines | tetC | CATATCGCAATACATGCGAAAAA AAAGCCGCGGTAAATAGCAA | 78 | [50] |
| tetO | ACGGAGAGTTTATTGTATACC TGGCGTATCTATAATGTTGAC | 171 | [50] | |
| Sulfonamides | Sul1 | CGCACCGGAAACATCGCTGCAC TGAAGTTCCGCCGCAAGGCTCG | 162 | [51] |
| Sul2 | TCCGGTGGAGGCCGGTATCTGG CGGGAATGCCATCTGCCTTGAG | 190 | [52] | |
| Sul3 | TCCGTTCAGCGAATTGGTGCAG TTCGTTCACGCTTTACACCAGC | 127 | [52] | |
| Macrolides | ermA | GAAATCGGATCAGGAAAAGG AACAGCAAACCCAAAGCTC | 332 | [53] |
| ermB | GATACCGTTTACGAAATTGG GAATCGAGACTTGAGTGTGC | 364 | [53] | |
| β-lactams | blaOXA-10 | CGCAATTATCGGCCTAGAAACT TTGGCTTTCCGTCCCATTT | 71 | [54] |
| blaSHV | GCGAAAGCCAGCTGTCGGGC ATTGGCGGCGCTGTTATCGC | 302 | [55] | |
| blaCTX-M | GGAGGCGTGACGGCTTTT TTCAGTGCGATCCAGACGAA | 63 | [56] | |
| ampC | CAGCCGCTGATGAAAAAATATG CAGCGAGCCCACTTCGA | 147 | [57] | |
| Integron | intI | TCGTGCGTCGCCATCACA GCTTGTTCTACGGCACGTTTGA | 67 | [58] |
| Transposon | tnpA-IS4 | GGGCGGGTCGATTGAAA GTGGGCGGGATCTGCTT | 108 | [54] |
| 16S rRNA | V4 | GTGCCAGCAGCCGCGGTAA GGACTACAAGGGTATCTAAT | 292 | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, J.; Yin, Z.; Yang, Y.; Liang, Y.; Xu, X.; Shi, H. Abundance and Dynamic Distribution of Antibiotic Resistance Genes in the Environment Surrounding a Veterinary Antibiotic Manufacturing Site. Antibiotics 2021, 10, 1361. https://doi.org/10.3390/antibiotics10111361
Miao J, Yin Z, Yang Y, Liang Y, Xu X, Shi H. Abundance and Dynamic Distribution of Antibiotic Resistance Genes in the Environment Surrounding a Veterinary Antibiotic Manufacturing Site. Antibiotics. 2021; 10(11):1361. https://doi.org/10.3390/antibiotics10111361
Chicago/Turabian StyleMiao, Junjie, Zhendong Yin, Yuqin Yang, Yiwen Liang, Xiangdong Xu, and Hongmei Shi. 2021. "Abundance and Dynamic Distribution of Antibiotic Resistance Genes in the Environment Surrounding a Veterinary Antibiotic Manufacturing Site" Antibiotics 10, no. 11: 1361. https://doi.org/10.3390/antibiotics10111361
APA StyleMiao, J., Yin, Z., Yang, Y., Liang, Y., Xu, X., & Shi, H. (2021). Abundance and Dynamic Distribution of Antibiotic Resistance Genes in the Environment Surrounding a Veterinary Antibiotic Manufacturing Site. Antibiotics, 10(11), 1361. https://doi.org/10.3390/antibiotics10111361






