Longitudinal Surveillance and Risk Assessment of Resistance in Escherichia coli to Enrofloxacin from A Large-Scale Chicken Farm in Hebei, China
Abstract
:1. Introduction
2. Results
2.1. The Isolation Rate of E. coli in Four Breeding Cycles
2.2. The Sensitivity of E. coli to Enrofloxacin and Other Antibiotics
2.3. Correlation Analysis among Multiple Antibiotic Resistance
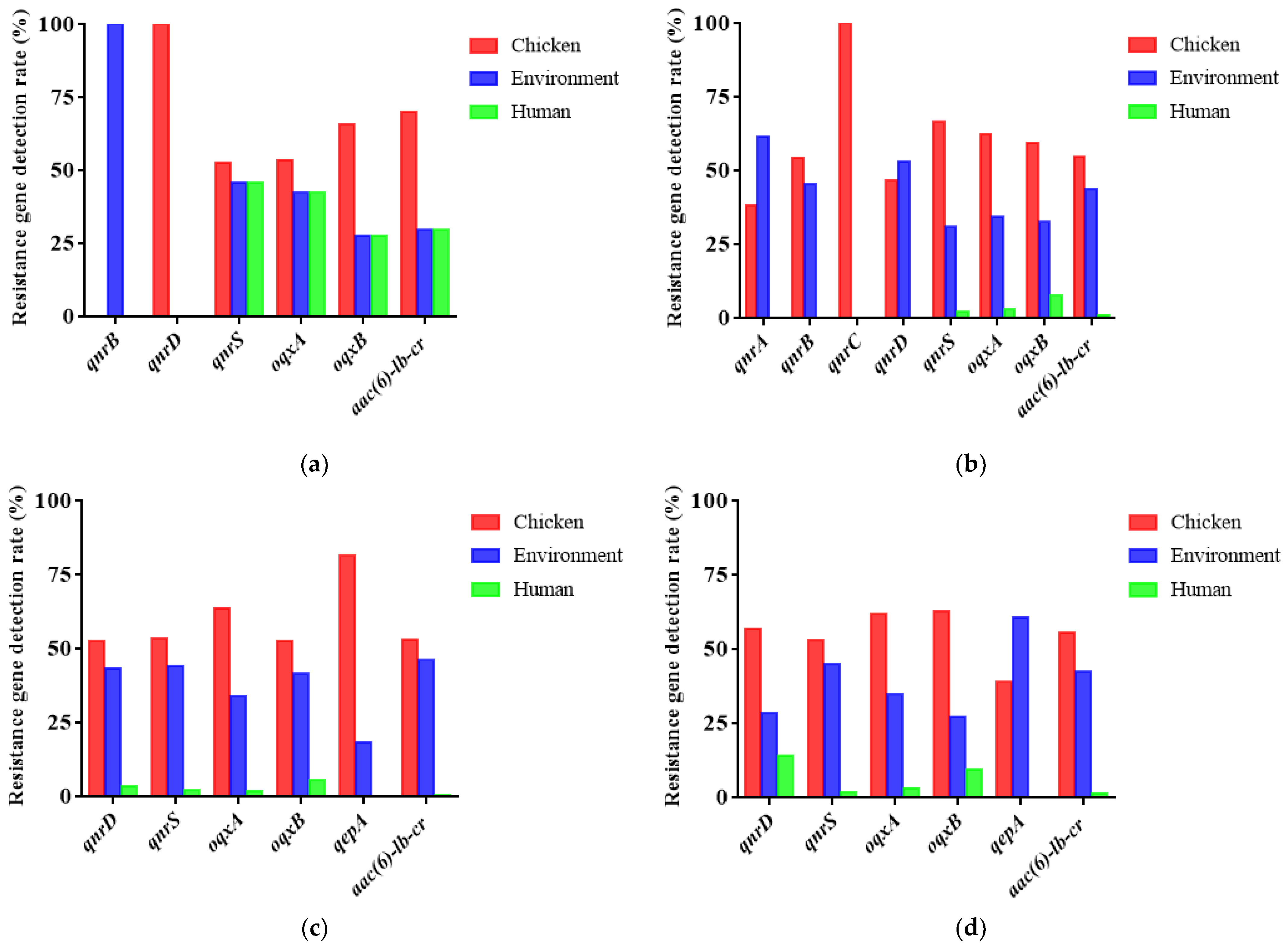
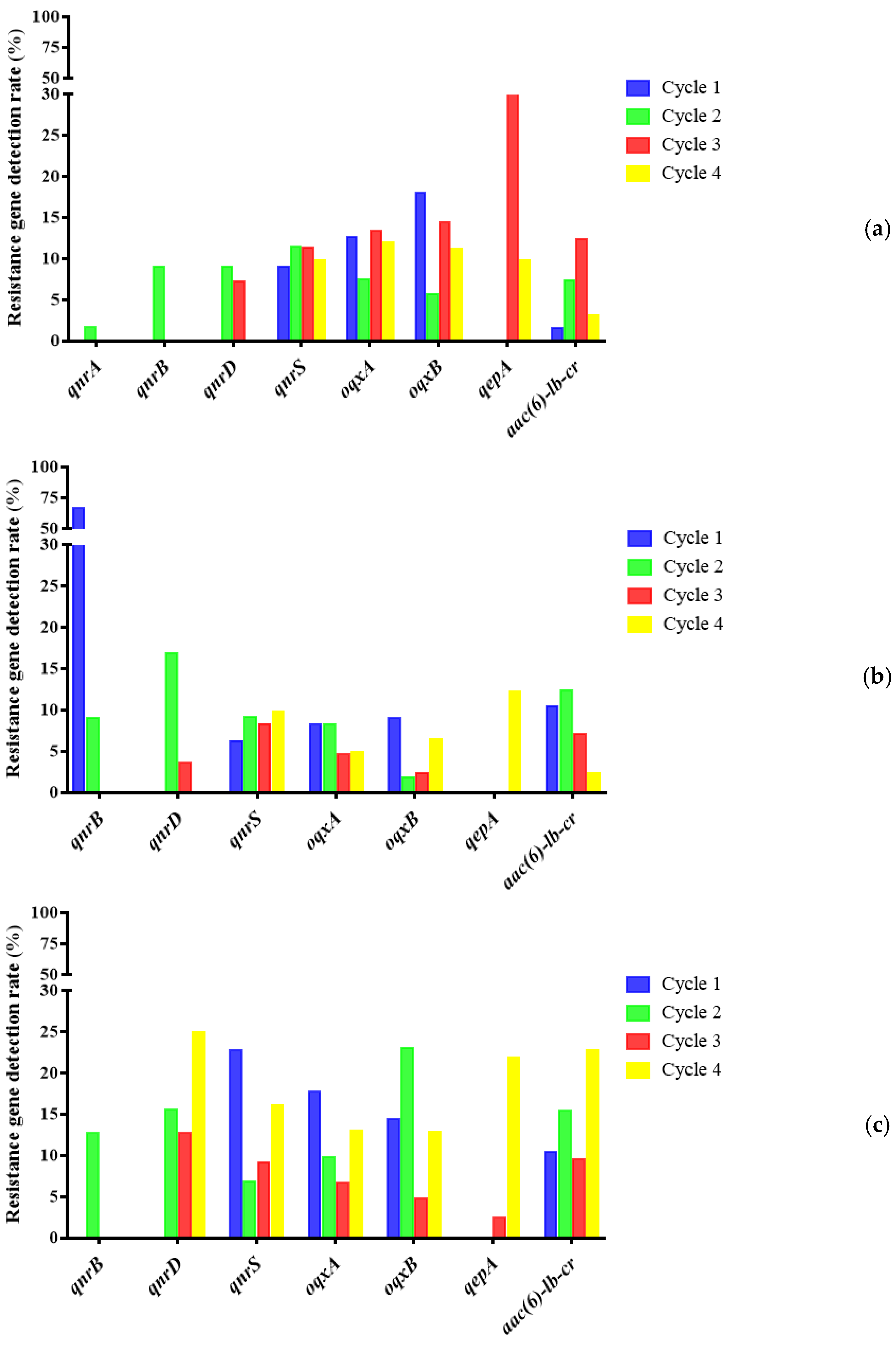
2.4. Detection of Plasmid-Mediated Fluoroquinolone Resistance Genes in E. coli
3. Discussion
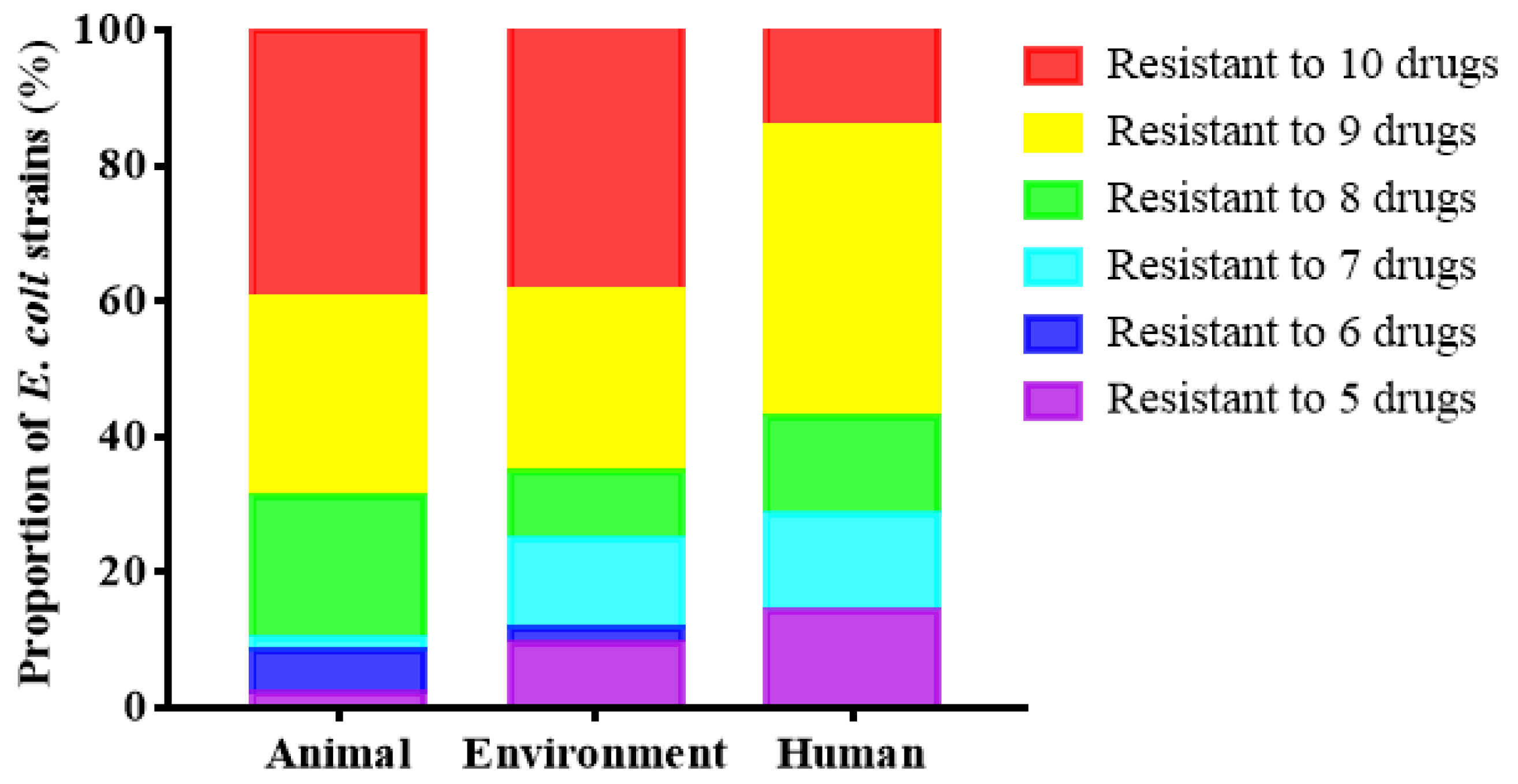
3.1. The Prevalence of E. coli in Broiler Farms
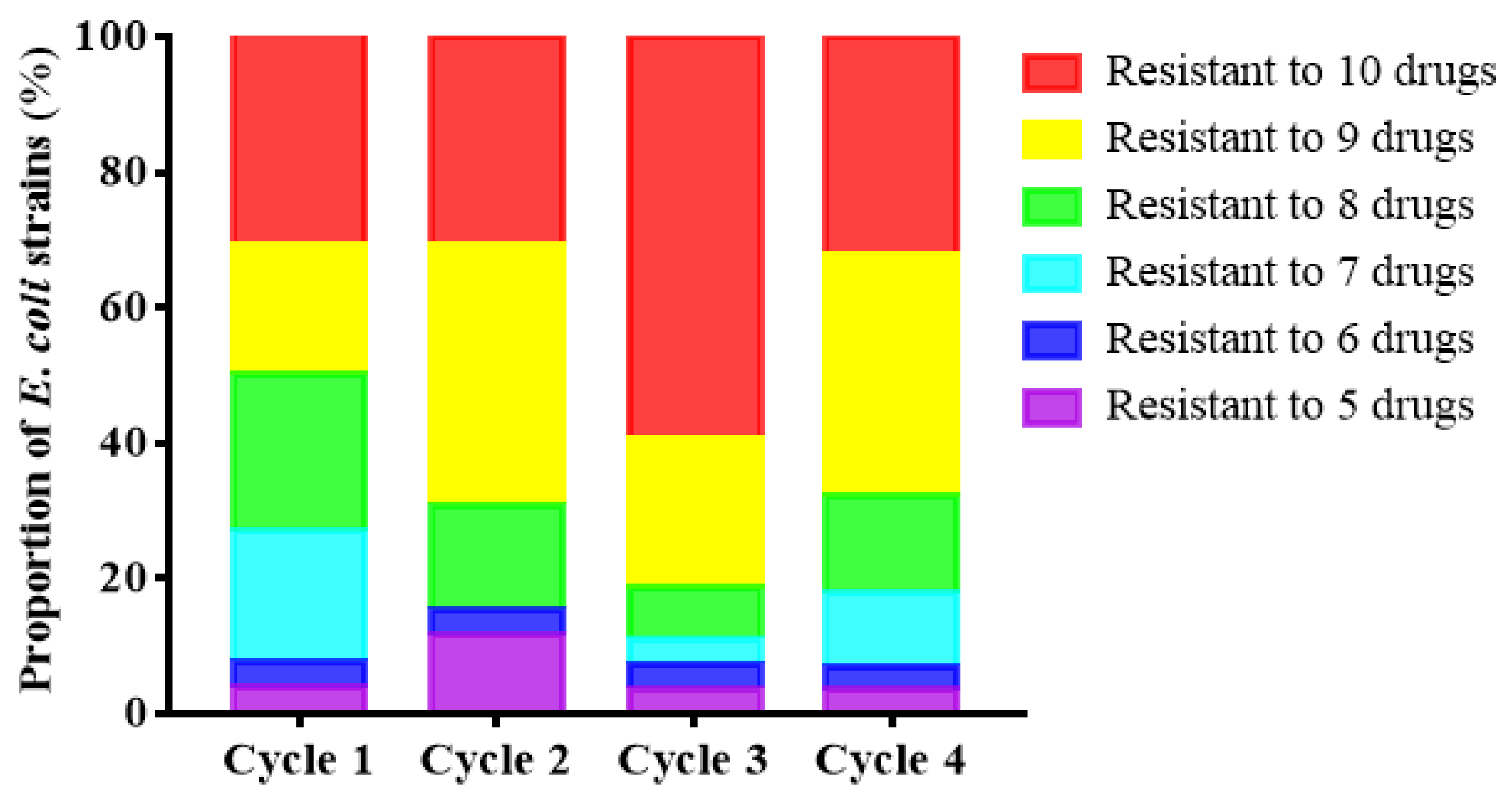
3.2. Epidemic Characteristics of E. coli Resistance and Multi-Drug Resistance in Broiler Farms
3.3. Risk Assessment of Drug Resistance in Broiler Farms Based on SPSS Nonparametric Correlation Analysis
3.4. Epidemic Characteristics of Plasmid-Mediated Fluoroquinolone Resistance Genes in Broiler Farms
4. Materials and Methods
4.1. Collection of Chicken, Environmental, and Human Samples
4.2. Isolation and Identification of E. coli
4.3. Antimicrobial Susceptibility Testing
4.4. Correlation Data Analysis of Multiple Antibiotic Resistance Levels
4.5. Plasmid-Mediated Fluoroquinolone Resistance Gene Detection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmes, A.H.; Moore, L.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.; Piddock, L. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Zucker, B.A.; Trojan, S.; Muller, W. Airborne gram-negative bacterial flora in animal houses. J. Vet. Med. B Infect. Dis. Vet. Public Health 2000, 47, 37–46. [Google Scholar] [CrossRef]
- Tenaillon, O.; Skurnik, D.; Picard, B.; Denamur, E. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 2010, 8, 207–217. [Google Scholar] [CrossRef]
- Riley, L.W. Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clin. Microbiol. Infect. 2014, 20, 380–390. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Hur, H.-G.; Sadowsky, M.; Byappanahalli, M.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications-a review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef] [Green Version]
- Beutin, L.; Steinrück, H.; Krause, G.; Steege, K.; Haby, S.; Hultsch, G.; Appel, B. Comparative evaluation of the Ridascreen® Verotoxin enzyme immunoassay for detection of Shiga-toxin producing strains of Escherichia coli (STEC) from food and other sources. J. Appl. Microbiol. 2007, 102, 630–639. [Google Scholar] [CrossRef]
- Somarelli, J.; Makarewicz, J.; Sia, R.; Simon, R. Wildlife identified as major source of Escherichia coli in agriculturally dominated watersheds by BOX A1R-derived genetic fingerprints. J. Environ. Manag. 2007, 82, 60–65. [Google Scholar] [CrossRef]
- Duan, H.; Chai, T.; Müller, W.; Zucker, B.-A. Concentration of airborne endotoxins and airborne bacteria in Chinese rabbit houses. Berlin Munchener Tierarztliche Wochenschr. 2006, 119, 40–44. [Google Scholar]
- Rice, E.; Johnson, C.; Wild, D.; Reasoner, D. Survival of Escherichia coli O157: H7 in drinking water associated with a waterborne disease outbreak of hemorrhagic colitis. Lett. Appl. Microbiol. 1992, 15, 38–40. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, C.-L.; Chang, S.-K.; Tsai, Y.-L.; Chou, C.-H. Characterization of Escherichia coli Isolated from Day-old Chicken Fluff in Taiwanese Hatcheries. Avian Dis. 2018, 63, 9–16. [Google Scholar] [CrossRef]
- Koutsianos, D.; Athanasiou, L.; Mossialos, D.; Koutoulis, K.C. Colibacillosis in poultry: A disease overview and the new perspectives for its control and prevention. J. Hell. Vet. Med. Soc. 2021, 71, 2425–2436. [Google Scholar] [CrossRef]
- Davis, M.A.; Hancock, D.D.; Rice, D.H.; Call, D.R.; DiGiacomo, R.; Samadpour, M.; Besser, T.E. Feedstuffs as a vehicle of cattle exposure to Escherichia coli O157:H7 and Salmonella enterica. Vet. Microbiol. 2003, 95, 199–210. [Google Scholar] [CrossRef]
- Chuppava, B.; Keller, B.; El-Wahab, A.A.; Sürie, C.; Visscher, C. Resistance Reservoirs and Multi-Drug Resistance of Commensal Escherichia coli From Excreta and Manure Isolated in Broiler Houses with Different Flooring Designs. Front. Microbiol. 2019, 10, 2633. [Google Scholar] [CrossRef] [Green Version]
- Selvam, A.; Xu, D.; Zhao, Z.; Wong, J.W. Fate of tetracycline, sulfonamide and fluoroquinolone resistance genes and the changes in bacterial diversity during composting of swine manure. Bioresour. Technol. 2012, 126, 383–390. [Google Scholar] [CrossRef]
- Colomer-Lluch, M.; Imamovic, L.; Jofre, J.; Muniesa, M. Bacteriophages Carrying Antibiotic Resistance Genes in Fecal Waste from Cattle, Pigs, and Poultry. Antimicrob. Agents Chemother. 2011, 55, 4908–4911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamscher, G.; Sczesny, S.; Höper, H.; Nau, H. Determination of Persistent Tetracycline Residues in Soil Fertilized with Liquid Manure by High-Performance Liquid Chromatography with Electrospray Ionization Tandem Mass Spectrometry. Anal. Chem. 2002, 74, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Poudel, A.; Hathcock, T.; Butaye, P.; Kang, Y.; Price, S.; Macklin, K.; Walz, P.; Cattley, R.; Kalalah, A.; Adekanmbi, F.; et al. Multidrug-Resistant Escherichia coli, Klebsiella pneumoniae and Staphylococcus spp. in Houseflies and Blowflies from Farms and Their Environmental Settings. Int. J. Environ. Res. Public Health 2019, 16, 3583. [Google Scholar] [CrossRef] [Green Version]
- Adenipekun, E.O.; Jackson, C.R.; Oluwadun, A.; Iwalokun, B.; Frye, J.; Barrett, J.B.; Hiott, L.M.; Woodley, T.A. Prevalence and Antimicrobial Resistance inEscherichia colifrom Food Animals in Lagos, Nigeria. Microb. Drug Resist. 2015, 21, 358–365. [Google Scholar] [CrossRef]
- Nishikawa, R.; Murase, T.; Ozaki, H. Plasmid-mediated quinolone resistance in Escherichia coli isolates from commercial broiler chickens and selection of fluoroquinolone-resistant mutants. Poult. Sci. 2019, 98, 5900–5907. [Google Scholar] [CrossRef]
- Han, T.; Zhang, Q.; Liu, N.; Wang, J.; Li, Y.; Huang, X.; Liu, J.; Wang, J.; Qu, Z.; Qi, K. Changes in antibiotic resistance of Escherichia coli during the broiler feeding cycle. Poult. Sci. 2020, 99, 6983–6989. [Google Scholar] [CrossRef]
- Koutsianos, D.; Gantelet, H.; Franzo, G.; Lecoupeur, M.; Thibault, E.; Cecchinato, M.; Koutoulis, K.C. An Assessment of the Level of Protection Against Colibacillosis Conferred by Several Autogenous and/or Commercial Vaccination Programs in Conventional Pullets upon Experimental Challenge. Vet. Sci. 2020, 7, 80. [Google Scholar] [CrossRef]
- Ostrolenk, M.; Welch, H. The House Fly as a Vector of Food Poisoning Organisms in Food Producing Establishments. Am. J. Public Health Nations Health 1942, 32, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Wales, A.D.; Davies, R.H. Co-Selection of Resistance to Antibiotics, Biocides and Heavy Metals, and Its Relevance to Foodborne Pathogens. Antibiotics 2015, 4, 567–604. [Google Scholar] [CrossRef] [Green Version]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [Green Version]
- Graham, J.P.; Eisenberg, J.N.; Trueba, G.; Zhang, L.; Johnson, T. Small-Scale Food Animal Production and Antimicrobial Resistance: Mountain, Molehill, or Something in-between? Environ. Health Perspect. 2017, 125, 104501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshar, D.; Wright, L.T.; McCullough, C.E.; KuKanich, B. Pharmacokinetics of enrofloxacin and its metabolite ciprofloxacin following single-dose subcutaneous injection in black-tailed prairie dogs (Cynomys ludovicianus). Am. J. Vet. Res. 2018, 79, 658–663. [Google Scholar] [CrossRef]
- Agius, J.E.; Kimble, B.; Govendir, M.; Rose, K.; Pollard, C.-L.; Phalen, D.N. Pharmacokinetic profile of enrofloxacin and its metabolite ciprofloxacin in Asian house geckos (Hemidactylus frenatus) after single-dose oral administration of enrofloxacin. Vet. Anim. Sci. 2020, 9, 100116. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Kehrenberg, C.; Doublet, B.; Cloeckaert, A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004, 28, 519–542. [Google Scholar] [CrossRef] [Green Version]
- Grossman, T.H. Tetracycline Antibiotics and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, K.; Tomita, H.; Fujimoto, S.; Kudo, M.; Kuwano, H.; Ike, Y. Drug resistance ofEnterococcus faeciumclinical isolates and the conjugative transfer of gentamicin and erythromycin resistance traits. FEMS Microbiol. Lett. 2005, 243, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantón, R.; Coque, T.M. The CTX-M β-lactamase pandemic. Curr. Opin. Microbiol. 2006, 9, 466–475. [Google Scholar] [CrossRef]
- Abdi-Hachesoo, B.; Asasi, K.; Sharifiyazdi, H. Rapid detection of Escherichia coli gyrA and parC mutants in one-day-old broiler chicks in Iran. Vet. Ital. 2013, 49, 291–297. [Google Scholar]
- Lee, Y.-J.; Cho, J.-K.; Kim, K.-S.; Tak, R.-B.; Kim, A.-R.; Kim, J.-W.; Im, S.-K.; Kim, B.-H. Fluoroquinolone resistance and gyrA and parC mutations of Escherichia coli isolated from chicken. J. Microbiol. 2005, 43, 391–397. [Google Scholar]
- Oikarainen, P.E.; Pohjola, L.K.; Pietola, E.S.; Heikinheimo, A. Direct vertical transmission of ESBL/pAmpC-producing Escherichia coli limited in poultry production pyramid. Vet. Microbiol. 2019, 231, 100–106. [Google Scholar] [CrossRef]
- Sabat, G.; Rose, P.; Hickey, W.J.; Harkin, J.M. Selective and Sensitive Method for PCR Amplification of Escherichia coli 16S rRNA Genes in Soil. Appl. Environ. Microbiol. 2000, 66, 844–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- M100-S11. Performance standards for antimicrobial susceptibility testing. Clin. Microbiol. Newsl. 2001, 23, 49. [Google Scholar] [CrossRef]
- Lorenzo-Seva, U.; Ferrando, P.J. POLYMAT-C: A comprehensive SPSS program for computing the polychoric correlation matrix. Behav. Res. Methods 2014, 47, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Petroczi, A.; Naughton, D. Mercury, cadmium and lead contamination in seafood: A comparative study to evaluate the usefulness of Target Hazard Quotients. Food Chem. Toxicol. 2009, 47, 298–302. [Google Scholar] [CrossRef]
- Park, C.H.; Robicsek, A.; Jacoby, G.A.; Sahm, D.; Hooper, D.C. Prevalence in the United States of aac(6 ′ )—Ib—cr Encoding a Ciprofloxacin-Modifying Enzyme. Antimicrob. Agents Chemother. 2006, 50, 3953–3955. [Google Scholar] [CrossRef] [Green Version]
- Ling-Chao, K.; Zhi-Guo, L.; Rui-Hua, Z.; Yu-Xiang, J.; Ming-Jie, M.; Cui-Ping, G.; Yan-Li, Z.; Zhi-Jing, X.; Shi-Jin, J. Detection of Plasmid-Mediated Quinolone Resistance Genes in Avian Escherichia coli Isolates from Shandong Province in 2012−2013. Chin. J. Anim. Infect. Dis. 2014, 22, 56–62. [Google Scholar]



| Sampling Time | Chicken | Environment | Human | Total |
|---|---|---|---|---|
| Cycle 1 1.12–1.26 | 72.78(131/180) | 65.00(117/180) | 80.00(8/10) | 66.49(246/370) |
| Cycle 2 4.02–4.16 | 58.33(105/180) | 45.56(82/180) | 50.00(5/10) | 51.89(192/370) |
| Cycle 3 7.14–7.28 | 81.67(147/180) | 57.14(120/210) | 80.00(8/10) | 68.75(275/400) |
| Cycle 4 9.12–9.26 | 86.11(155/180) | 50.00(105/210) | 100.00(10/10) | 67.50(270/400) |
| Total | 74.72(538/720) | 54.36(424/780) | 77.50(31/40) | 63.83(983/1540) |
| Cycle | Chicken | Environment | Human | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | |
| 1 | 80.00 | 86.67 | 66.67 | 70.00 | 53.33 | 80.00 | 50.00 | 63.33 | 83.33 | 33.33 | 80.00 | 80.00 | 80.00 | |
| 2 | 40.00 | 66.67 | 73.33 | 76.67 | 43.33 | 50.00 | 66.67 | 23.33 | 56.67 | 46.67 | 36.67 | 43.33 | 50.00 | |
| 3 | 96.67 | 86.67 | 80.00 | 90.00 | 50.00 | 86.67 | 90.00 | 16.67 | 33.33 | 63.33 | 36.67 | 86.67 | 73.33 | 80.00 |
| 4 | 93.33 | 60.00 | 96.67 | 93.33 | 86.67 | 86.67 | 53.33 | 6.67 | 80.00 | 93.33 | 40.00 | 43.33 | 33.33 | 100.00 |
| Four-cycle average | 77.50 | 75.00 | 79.17 | 82.50 | 58.33 | 75.84 | 65.00 | 27.50 | 63.33 | 59.17 | 48.34 | 63.33 | 53.33 | 77.50 |
| Cycle | Chicken Seedlings | The Day before Medication | First Day of Medication | Third Day of Medication | One Day after Medication | Three Days after Medication | Environment | Human | Overall Average |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 79.17 | 88.46 | 100.00 | 100.00 | 100.00 | 100.00 | 96.21 | 100.00 | 95.20 |
| 2 | 100.00 | 100.00 | 100.00 | 100.00 | 92.31 | 100.00 | 97.56 | 100.00 | 98.44 |
| 3 | 93.10 | 96.15 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 98.90 |
| 4 | 89.29 | 95.00 | 96.55 | 100.00 | 100.00 | 100.00 | 97.14 | 100.00 | 95.20 |
| ENR | CIP | AMC | AMP | DO | TE | FFC | CN | CHL | ERY | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kendall tau_b | ENR | Correlation coefficient | 1.000 | 0.190 * | 0.172 | −0.065 | 0.034 | −0.010 | 0.027 | 0.100 | −0.054 | 0.015 |
| Sig.(Double tail) | . | 0.021 | 0.071 | 0.498 | 0.693 | 0.913 | 0.762 | 0.235 | 0.550 | 0.862 | ||
| N | 97 | 97 | 91 | 90 | 97 | 91 | 97 | 97 | 96 | 92 | ||
| CIP | Correlation coefficient | 0.190 * | 1.000 | 0.079 | 0.008 | 0.034 | 0.104 | 0.284 ** | 0.318 ** | 0.174 * | 0.156 | |
| Sig.(Double tail) | 0.021 | . | 0.366 | 0.930 | 0.663 | 0.224 | 0.001 | 0.000 | 0.036 | 0.056 | ||
| N | 97 | 107 | 101 | 100 | 107 | 101 | 106 | 106 | 105 | 101 | ||
| AMC | Correlation coefficient | 0.172 | 0.079 | 1.000 | −0.058 | 0.131 | 0.155 | −0.126 | 0.028 | −0.133 | 0.034 | |
| Sig.(Double tail) | 0.071 | 0.366 | . | 0.567 | 0.152 | 0.117 | 0.189 | 0.755 | 0.166 | 0.721 | ||
| N | 91 | 101 | 101 | 95 | 101 | 97 | 100 | 100 | 99 | 96 | ||
| AMP | Correlation coefficient | −0.065 | 0.008 | −0.058 | 1.000 | 0.138 | 0.064 | 0.006 | 0.032 | 0.079 | 0.026 | |
| Sig.(Double tail) | 0.498 | 0.930 | 0.567 | . | 0.136 | 0.523 | 0.955 | 0.724 | 0.416 | 0.781 | ||
| N | 90 | 100 | 95 | 100 | 100 | 95 | 99 | 99 | 98 | 96 | ||
| DO | Correlation coefficient | 0.034 | 0.034 | 0.131 | 0.138 | 1.000 | 0.349 ** | 0.159 | 0.044 | 0.285 ** | 0.231 ** | |
| Sig.(Double tail) | 0.693 | 0.663 | 0.152 | 0.136 | . | 0.000 | 0.066 | 0.594 | 0.001 | 0.007 | ||
| N | 97 | 107 | 101 | 100 | 107 | 101 | 106 | 106 | 105 | 101 | ||
| TE | Correlation coefficient | −0.010 | 0.104 | 0.155 | 0.064 | 0.349 ** | 1.000 | 0.270 ** | 0.073 | 0.187 * | 0.197 * | |
| Sig.(Double tail) | 0.913 | 0.224 | 0.117 | 0.523 | 0.000 | . | 0.004 | 0.410 | 0.047 | 0.032 | ||
| N | 91 | 101 | 97 | 95 | 101 | 101 | 100 | 100 | 99 | 97 | ||
| FFC | Correlation coefficient | 0.027 | 0.284 ** | −0.126 | 0.006 | 0.159 | 0.270 ** | 1.000 | 0.224 ** | 0.352 ** | 0.312 ** | |
| Sig.(Double tail) | 0.762 | 0.001 | 0.189 | 0.955 | 0.066 | 0.004 | . | 0.009 | 0.000 | 0.001 | ||
| N | 97 | 106 | 100 | 99 | 106 | 100 | 106 | 106 | 104 | 100 | ||
| CN | Correlation coefficient | 0.100 | 0.318 ** | 0.028 | 0.032 | 0.044 | 0.073 | 0.224 ** | 1.000 | 0.255 ** | 0.385 ** | |
| Sig.(Double tail) | 0.235 | 0.000 | 0.755 | 0.724 | 0.594 | 0.410 | 0.009 | . | 0.003 | 0.000 | ||
| N | 97 | 106 | 100 | 99 | 106 | 100 | 106 | 106 | 104 | 100 | ||
| CHL | Correlation coefficient | −0.054 | 0.174 * | −0.133 | 0.079 | 0.285 ** | 0.187 * | 0.352 ** | 0.255 ** | 1.000 | 0.285 ** | |
| Sig.(Double tail) | 0.550 | 0.036 | 0.166 | 0.416 | 0.001 | 0.047 | 0.000 | 0.003 | . | 0.001 | ||
| N | 96 | 105 | 99 | 98 | 105 | 99 | 104 | 104 | 105 | 100 | ||
| ERY | Correlation coefficient | 0.015 | 0.156 | 0.034 | 0.026 | 0.231 ** | 0.197 * | 0.312 ** | 0.385 ** | 0.285 ** | 1.000 | |
| Sig.(Double tail) | 0.862 | 0.056 | 0.721 | 0.781 | 0.007 | 0.032 | 0.001 | 0.000 | 0.001 | . | ||
| N | 92 | 101 | 96 | 96 | 101 | 97 | 100 | 100 | 100 | 101 |
| Gene Name | Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 |
|---|---|---|---|---|
| qnrA | 0 | 31.25(60/192) | 0 | 0 |
| qnrB | 1.21(3/246) | 57.29(110/192) | 0 | 0 |
| qnrC | 0 | 0.05(1/192) | 0 | 0 |
| qnrD | 1.63(4/246) | 40.10(77/192) | 40.00(110/275) | 10.37(28/270) |
| qnrS | 71.54(176/246) | 45.31(87/192) | 82.91(228/275) | 60.00(162/270) |
| oqxA | 64.23(158/246) | 69.27(133/192) | 70.55(194/275) | 68.15(184/270) |
| oqxB | 45.12(111/246) | 27.08(52/192) | 45.45(125/275) | 22.96(62/270) |
| qepA | 0 | 0 | 15.64(43/275) | 15.19(41/270) |
| aac(6)-lb-cr | 50.41(124/246) | 84.38(162/192) | 76.36(210/275) | 47.04(127/270) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, K.; Zhao, Y.; Cui, L.; Cao, Z.; Zhang, F.; Wang, X.; Peng, Z.; Feng, J.; Hu, T.; Dai, M. Longitudinal Surveillance and Risk Assessment of Resistance in Escherichia coli to Enrofloxacin from A Large-Scale Chicken Farm in Hebei, China. Antibiotics 2021, 10, 1222. https://doi.org/10.3390/antibiotics10101222
Guo K, Zhao Y, Cui L, Cao Z, Zhang F, Wang X, Peng Z, Feng J, Hu T, Dai M. Longitudinal Surveillance and Risk Assessment of Resistance in Escherichia coli to Enrofloxacin from A Large-Scale Chicken Farm in Hebei, China. Antibiotics. 2021; 10(10):1222. https://doi.org/10.3390/antibiotics10101222
Chicago/Turabian StyleGuo, Kaixuan, Yue Zhao, Luqing Cui, Zhengzheng Cao, Fan Zhang, Xiangru Wang, Zhong Peng, Jiawei Feng, Tianyu Hu, and Menghong Dai. 2021. "Longitudinal Surveillance and Risk Assessment of Resistance in Escherichia coli to Enrofloxacin from A Large-Scale Chicken Farm in Hebei, China" Antibiotics 10, no. 10: 1222. https://doi.org/10.3390/antibiotics10101222






