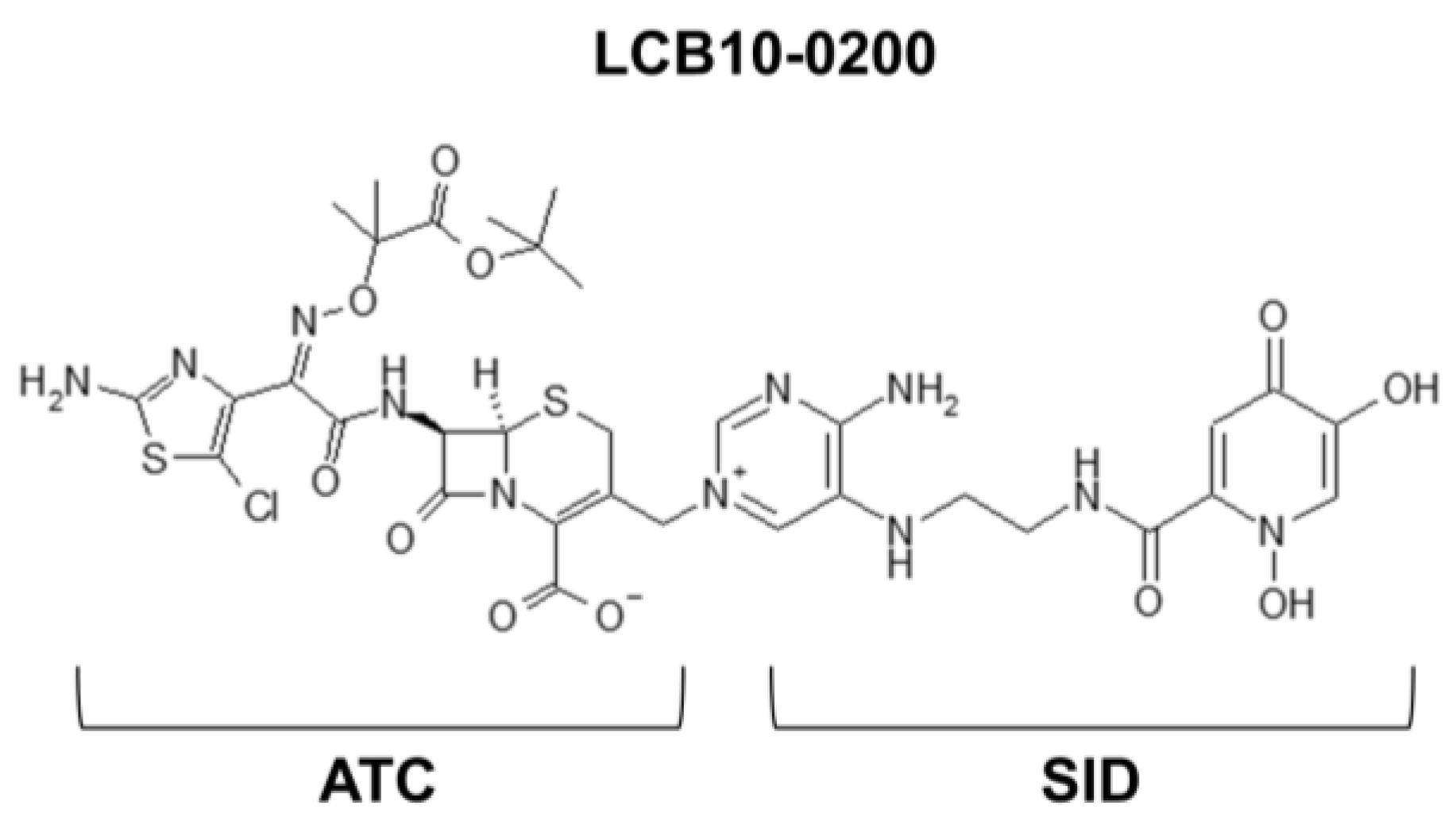
Antibacterial Activity of LCB10-0200 against Klebsiella pneumoniae
Abstract
:1. Introduction
2. Results
2.1. In Vitro Activity of LCB10-0200 against K. pneumoniae
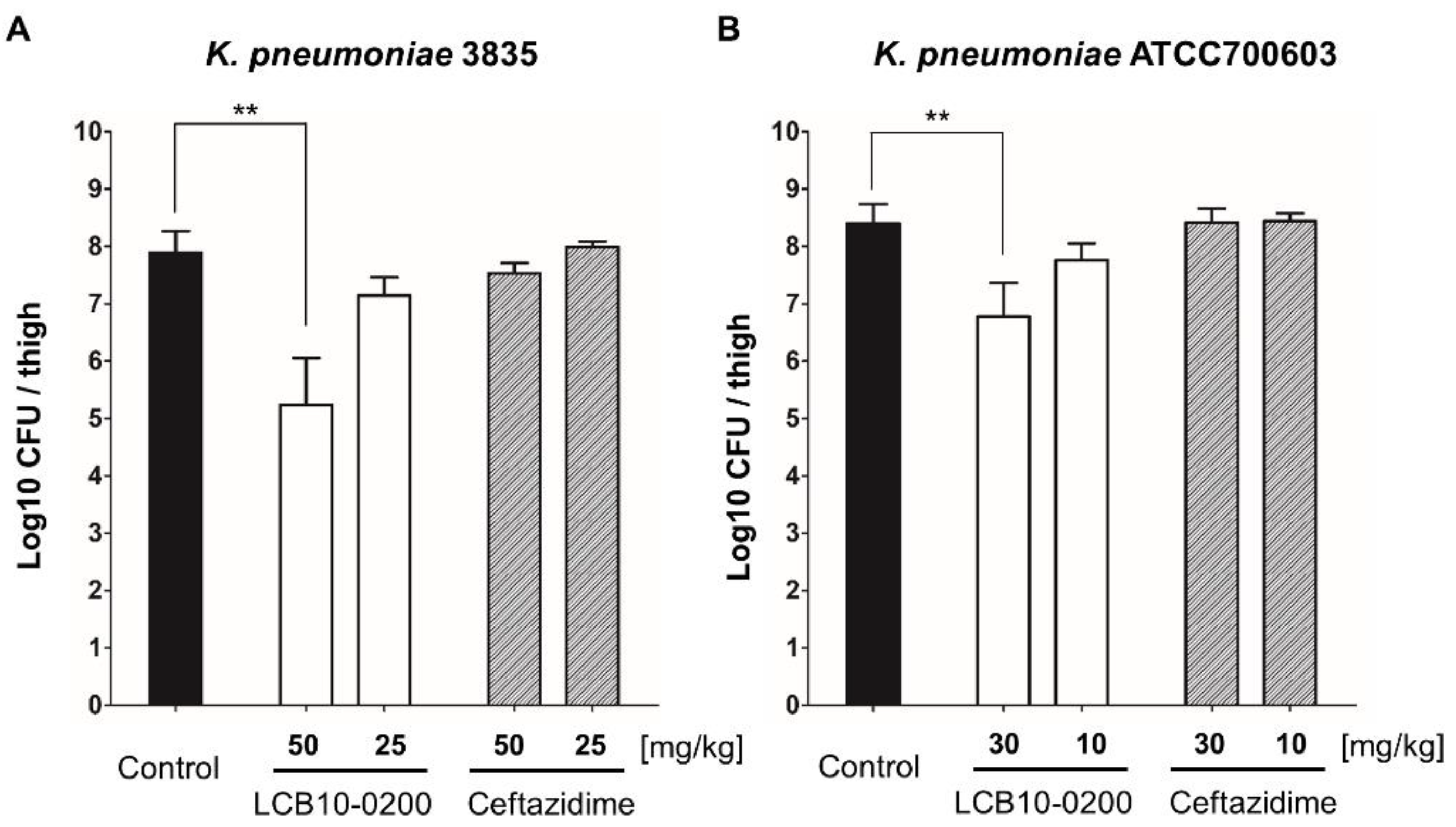
2.2. In Vivo Activity of LCB10-0200 against K. pneumoniae
3. Discussion
4. Materials and Methods
4.1. Antimicrobial Agents and Bacterial Strains
4.2. Minimum Inhibition Concentration (MIC) Determination
4.3. Systemic Infection Mouse Model
4.4. Thigh Infection Mouse Model
4.5. Urinary Tract Infection Mouse Model
4.6. Animal Ethical Approval
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gorrie, C.L.; Mirceta, M.; Wick, R.R.; Edwards, D.J.; Thomson, N.R.; Strugnell, R.A.; Pratt, N.F.; Garlick, J.S.; Watson, K.M.; Pilcher, D.V.; et al. Gastrointestinal Carriage Is a Major Reservoir of Klebsiella pneumoniae Infection in Intensive Care Patients. Clin. Infect. Dis. 2017, 65, 208–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Effah, C.Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella pneumoniae: An increasing threat to public health. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Starzyk-Luszcz, K.; Zielonka, T.M.; Jakubik, J.; Zycinska, K. Mortality Due to Nosocomial Infection with Klebsiella pneumoniae ESBL. Adv. Exp. Med. Biol. 2017, 1022, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.L.; da Silva, B.C.M.; Rezende, G.S.; Nakamura-Silva, R.; Pitondo-Silva, A.; Campanini, E.B.; Brito, M.C.A.; da Silva, E.M.L.; Freire, C.C.M.; da Cunha, A.F.; et al. High Prevalence of Multidrug-Resistant Klebsiella pneumoniae Harboring Several Virulence and beta-Lactamase Encoding Genes in a Brazilian Intensive Care Unit. Front. Microbiol. 2018, 9, 3198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Yang, W.; Zhao, X.; Wang, N.; Ren, H. Klebsiella pneumoniae presents antimicrobial drug resistance for beta-lactam through the ESBL/PBP signaling pathway. Exp. Ther. Med. 2020, 19, 2449–2456. [Google Scholar] [CrossRef] [Green Version]
- Garbati, M.A.; Al Godhair, A.I. The growing resistance of Klebsiella pneumoniae; the need to expand our antibiogram: Case report and review of the literature. Afr. J. Infect. Dis. 2013, 7, 8–10. [Google Scholar] [CrossRef] [Green Version]
- Oliva, A.; Mascellino, M.T.; Cipolla, A.; D’Abramo, A.; De Rosa, A.; Savinelli, S.; Ciardi, M.R.; Mastroianni, C.M.; Vullo, V. Therapeutic strategy for pandrug-resistant Klebsiella pneumoniae severe infections: Short-course treatment with colistin increases the in vivo and in vitro activity of double carbapenem regimen. Int. J. Infect. Dis. 2015, 33, 132–134. [Google Scholar] [CrossRef] [Green Version]
- Melano, R.; Corso, A.; Petroni, A.; Centron, D.; Orman, B.; Pereyra, A.; Moreno, N.; Galas, M. Multiple antibiotic-resistance mechanisms including a novel combination of extended-spectrum beta-lactamases in a Klebsiella pneumoniae clinical strain isolated in Argentina. J. Antimicrob. Chemother. 2003, 52, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Kidd, T.J.; Mills, G.; Sa-Pessoa, J.; Dumigan, A.; Frank, C.G.; Insua, J.L.; Ingram, R.; Hobley, L.; Bengoechea, J.A. A Klebsiella pneumoniae antibiotic resistance mechanism that subdues host defences and promotes virulence. EMBO Mol. Med. 2017, 9, 430–447. [Google Scholar] [CrossRef]
- Galani, I.; Karaiskos, I.; Giamarellou, H. Multidrug-resistant Klebsiella pneumoniae: Mechanisms of resistance including updated data for novel beta-lactam-beta-lactamase inhibitor combinations. Expert Rev. Anti. Infect. Ther. 2021, 1–12. [Google Scholar] [CrossRef]
- Bogdanovich, T.; Adams-Haduch, J.M.; Tian, G.B.; Nguyen, M.H.; Kwak, E.J.; Muto, C.A.; Doi, Y. Colistin-resistant, Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae belonging to the international epidemic clone ST258. Clin. Infect. Dis. 2011, 53, 373–376. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, S.; Fatima, J.; Shakil, S.; Rizvi, S.M.; Kamal, M.A. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi. J. Biol. Sci. 2015, 22, 90–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Angelis, G.; Del Giacomo, P.; Posteraro, B.; Sanguinetti, M.; Tumbarello, M. Molecular Mechanisms, Epidemiology, and Clinical Importance of beta-Lactam Resistance in Enterobacteriaceae. Int. J. Mol. Sci. 2020, 21, 5090. [Google Scholar] [CrossRef]
- Krewulak, K.D.; Vogel, H.J. Structural biology of bacterial iron uptake. Biochim. Biophys. Acta. 2008, 1778, 1781–1804. [Google Scholar] [CrossRef] [Green Version]
- Schalk, I.J.; Mislin, G.L.A. Bacterial Iron Uptake Pathways: Gates for the Import of Bactericide Compounds. J. Med. Chem. 2017, 60, 4573–4576. [Google Scholar] [CrossRef]
- Schalk, I.J. Siderophore-antibiotic conjugates: Exploiting iron uptake to deliver drugs into bacteria. Clin. Microbiol. Infect. 2018, 24, 801–802. [Google Scholar] [CrossRef] [Green Version]
- Tomaras, A.P.; Crandon, J.L.; McPherson, C.J.; Banevicius, M.A.; Finegan, S.M.; Irvine, R.L.; Brown, M.F.; O’Donnell, J.P.; Nicolau, D.P. Adaptation-based resistance to siderophore-conjugated antibacterial agents by Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 4197–4207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, A.; Sato, T.; Ota, M.; Takemura, M.; Nishikawa, T.; Toba, S.; Kohira, N.; Miyagawa, S.; Ishibashi, N.; Matsumoto, S.; et al. In Vitro Antibacterial Properties of Cefiderocol, a Novel Siderophore Cephalosporin, against Gram—Negative Bacteria. Antimicrob. Agents Chemother. 2018, 62, e01454-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPherson, C.J.; Aschenbrenner, L.M.; Lacey, B.M.; Fahnoe, K.C.; Lemmon, M.M.; Finegan, S.M.; Tadakamalla, B.; O’Donnell, J.P.; Mueller, J.P.; Tomaras, A.P. Clinically relevant Gram—Negative resistance mechanisms have no effect on the efficacy of MC-1, a novel siderophore-conjugated monocarbam. Antimicrob. Agents Chemother. 2012, 56, 6334–6342. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.H.; Park, H.S.; Kim, H.S.; Yun, J.Y.; Oh, K.; Cho, Y.L.; Kwak, J.H. Antimicrobial activities of LCB10-0200, a novel siderophore cephalosporin, against the clinical isolates of Pseudomonas aeruginosa and other pathogens. Int. J. Antimicrob. Agents 2017, 50, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.P.; Park, C.S.; Pinto, N.A.; Lee, H.; Seo, H.S.; Vu, T.N.; Mai, H.; Pham, A.H.T.; Jang, E.; Cho, Y.L.; et al. In Vitro Activity of a Novel Siderophore-Cephalosporin LCB10-0200 (GT-1), and LCB10-0200/Avibactam, against Carbapenem-Resistant Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa Strains at a Tertiary Hospital in Korea. Pharmaceuticals 2021, 14, 370. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, M.R.; Simor, A.E. Antimicrobial resistance in hospitals: How concerned should we be? CMAJ 2009, 180, 408–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.K.; Lee, J.Y.; Ko, K.S. Colistin resistance in Enterobacter spp. isolates in Korea. J. Microbiol. 2018, 56, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [Green Version]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Medicin. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [Green Version]
- Karaiskos, I.; Lagou, S.; Pontikis, K.; Rapti, V.; Poulakou, G. The “Old” and the “New” Antibiotics for MDR Gram—Negative Pathogens: For Whom, When, and How. Front. Public Health 2019, 7, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, T.; Yoshizawa, H.; Yamawaki, K.; Yokoo, K.; Sato, J.; Hisakawa, S.; Hasegawa, Y.; Kusano, H.; Sano, M.; Sugimoto, H.; et al. Cefiderocol (S-649266), A new siderophore cephalosporin exhibiting potent activities against Pseudomonas aeruginosa and other Gram—Negative pathogens including multi-drug resistant bacteria: Structure activity relationship. Eur. J. Med. Chem. 2018, 155, 847–868. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; M100; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 11th ed.; M07; CLSI: Wayne, PA, USA, 2018. [Google Scholar]

| Bacterial Strains | Compound MIC (mg/L) | ||
|---|---|---|---|
| LCB10-0200 | SID | ATC | |
| K. pneumoniae ATCC 13883 | 0.125 | >64 | 16 |
| K. pneumoniae ATCC700603 | 2 | >64 | 64 |
| K. pneumoniae 3835 | 1 | >64 | 64 |
| Strain | Beta-Lactamase | MIC (mg/L) | ||
|---|---|---|---|---|
| LCB10-0200 | Ceftazidime | Ciprofloxacin | ||
| K. pneumoniae 49 | KPC-2 | 8 | >32 | >32 |
| K. pneumoniae 50 | KPC-2 | 8 | >32 | >32 |
| K. pneumoniae 51 | KPC-2 | 4 | >32 | >32 |
| K. pneumoniae 52 | GES-5 | 1 | >32 | 2 |
| K. pneumoniae 53 | NDM-1 | >32 | >32 | >32 |
| K. pneumoniae 54 | OXA-232 | 2 | >32 | >32 |
| K. pneumoniae 55 | OXA-232 | 32 | >32 | >32 |
| Microorganism Inoculum (CFU/mouse) a | Antimicrobial Agents b | MIC (mg/L) | ED50 (mg/kg) (95% Confidence Limits) |
|---|---|---|---|
| K. pneumoniae ATCC13883 | LCB10-0200 | 0.125 | <0.8 |
| (5 × 107) | Ceftazidime | 0.125 | <0.8 |
| K. pneumoniae ATCC700603 | LCB10-0200 | 0.5 | <2.5 |
| (5 × 107) | Ceftazidime | 8 | >20 |
| K. pneumoniae 3835 | LCB10-0200 | 1 | 25.01 (11.37–50.01) |
| (2 × 108) | Ceftazidime | 64 | >40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.-H.; Kim, Y.-R.; Park, H.-S.; Oh, K.-M.; Cho, Y.-L.; Kwak, J.-H. Antibacterial Activity of LCB10-0200 against Klebsiella pneumoniae. Antibiotics 2021, 10, 1185. https://doi.org/10.3390/antibiotics10101185
Oh S-H, Kim Y-R, Park H-S, Oh K-M, Cho Y-L, Kwak J-H. Antibacterial Activity of LCB10-0200 against Klebsiella pneumoniae. Antibiotics. 2021; 10(10):1185. https://doi.org/10.3390/antibiotics10101185
Chicago/Turabian StyleOh, Sang-Hun, Young-Rok Kim, Hee-Soo Park, Kyu-Man Oh, Young-Lag Cho, and Jin-Hwan Kwak. 2021. "Antibacterial Activity of LCB10-0200 against Klebsiella pneumoniae" Antibiotics 10, no. 10: 1185. https://doi.org/10.3390/antibiotics10101185






