Characterization of Fitness Cost Caused by Tigecycline-Resistance Gene tet(X6) in Different Host Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Plasmids
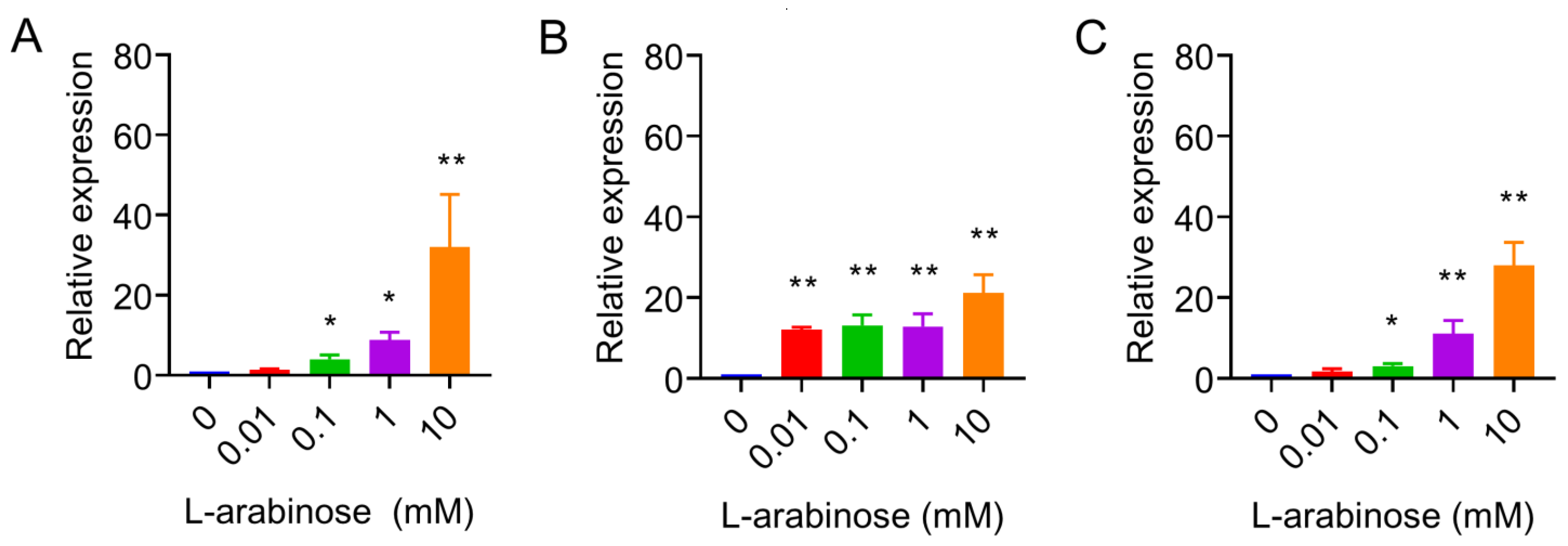
2.2. Quantitative Real-Time PCR
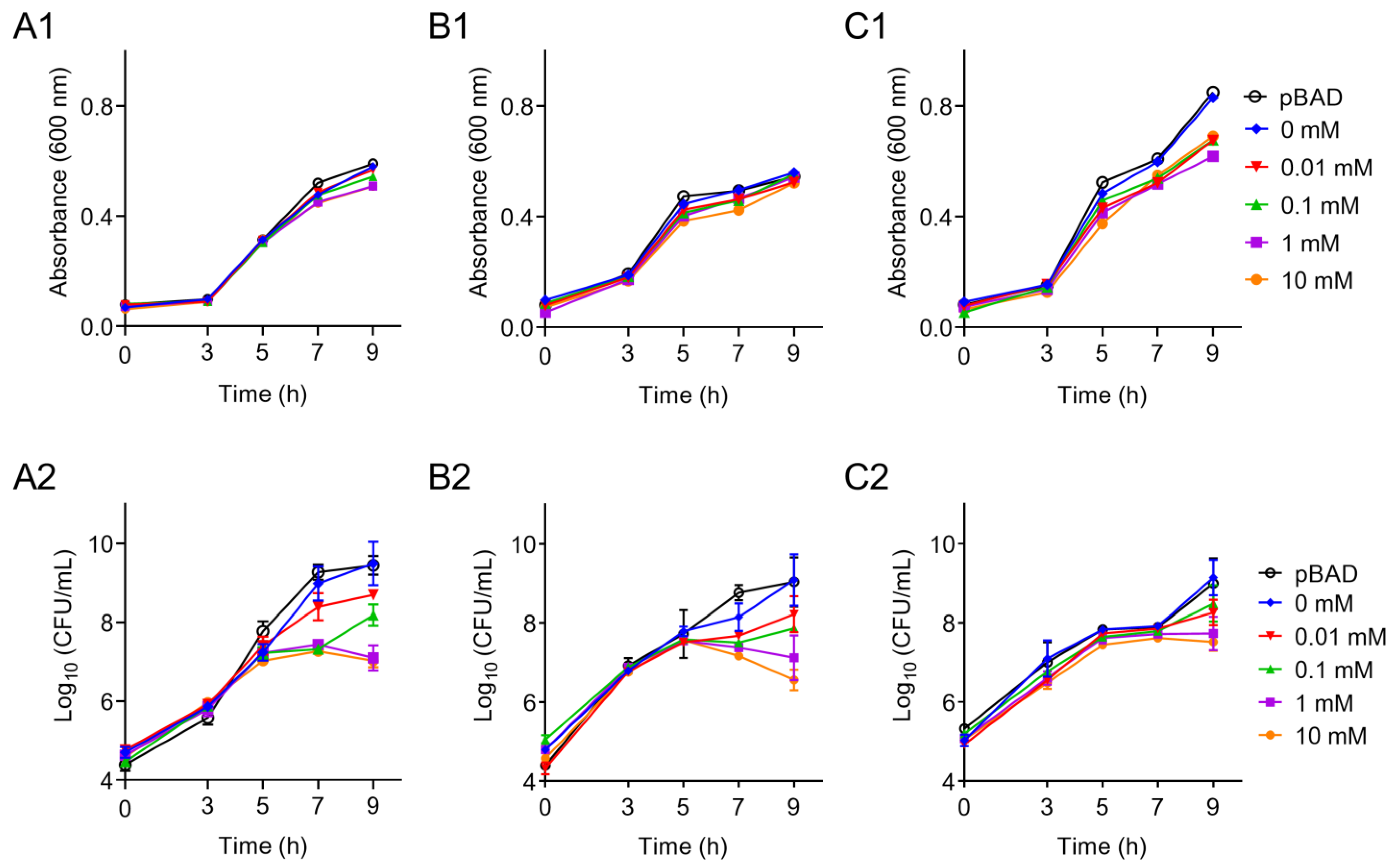
2.3. Effects of tet(X6) Overexpression on Bacterial Growth
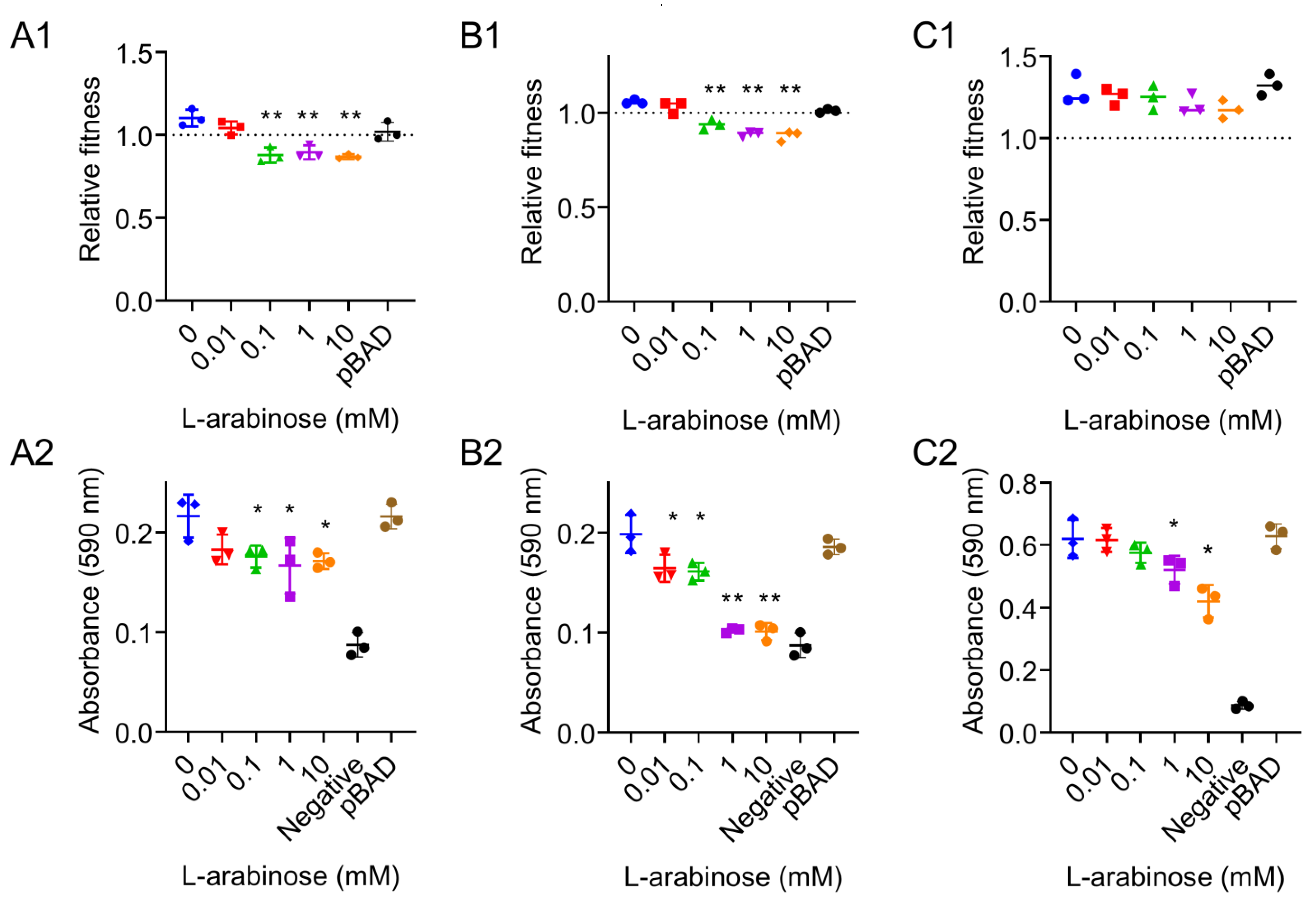
2.4. In Vitro Competition and Biofilm Formation
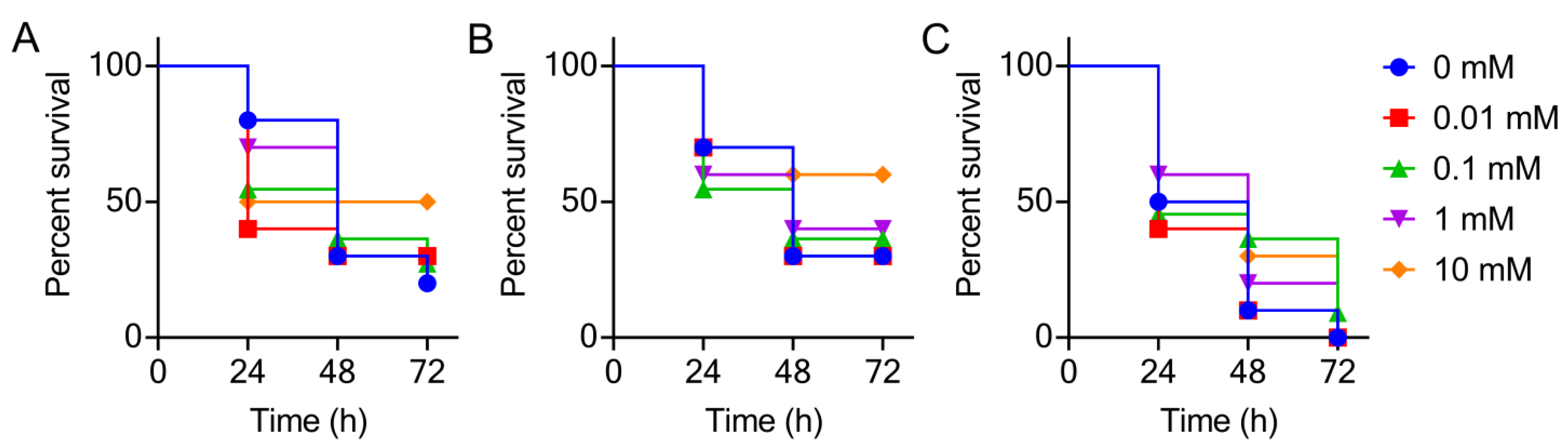
2.5. Galleria mellonella Infection Model
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yelin, I.; Kishony, R. Antibiotic resistance. Cell 2018, 172, 1136. [Google Scholar] [CrossRef] [PubMed]
- Niebel, M.; Quick, J.; Prieto, A.M.; Hill, R.L.; Pike, R.; Huber, D.; David, M.; Hornsey, M.; Wareham, D.; Oppenheim, B.; et al. Deletions in a ribosomal protein-coding gene are associated with tigecycline resistance in Enterococcus faecium. Int. J. Antimicrob. Agents 2015, 46, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cai, Y.; Liu, X.; Bai, N.; Liang, B.; Wang, R. The emergence of clinical resistance to tigecycline. Int. J. Antimicrob. Agents 2013, 41, 110–116. [Google Scholar] [CrossRef]
- Zeng, Y.; Dong, N.; Zhang, R.; Liu, C.; Sun, Q.; Lu, J.; Shu, L.; Cheng, Q.; Chan, E.W.; Chen, S. Emergence of an Empedobacter falsenii strain harbouring a tet(X)-variant-bearing novel plasmid conferring resistance to tigecycline. J. Antimicrob. Chemother. 2020, 75, 531–536. [Google Scholar] [CrossRef]
- Fang, L.X.; Chen, C.; Cui, C.Y.; Li, X.P.; Zhang, Y.; Liao, X.P.; Sun, J.; Liu, Y.H. Emerging high-level tigecycline resistance: Novel tetracycline destructases spread via the mobile Tet(X). BioEssays 2020, 42, e2000014. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Saw, W.-Y.; Tan, L.W.L.; Moong, D.K.N.; Nagarajan, N.; Teo, Y.Y.; Seedorf, H. Emergence of tigecycline- and eravacycline-resistant Tet(X4)-producing Enterobacteriaceae in the gut microbiota of healthy Singaporeans. J. Antimicrob. Chemother. 2020, 75, 3480–3484. [Google Scholar] [CrossRef]
- Sun, C.; Cui, M.; Zhang, S.; Wang, H.; Song, L.; Zhang, C.; Zhao, Q.; Liu, D.; Wang, Y.; Shen, J.; et al. Plasmid-mediated tigecycline-resistant gene tet(X4) in from food-producing animals, China, 2008–2018. Emerg. Microbes Infect. 2019, 8, 1524–1527. [Google Scholar] [CrossRef] [Green Version]
- He, D.; Wang, L.; Zhao, S.; Liu, L.; Liu, J.; Hu, G.; Pan, Y. A novel tigecycline resistance gene, tet(X6), on an SXT/R391 integrative and conjugative element in a Proteus genomospecies 6 isolate of retail meat origin. J. Antimicrob. Chemother. 2020, 75, 1159–1164. [Google Scholar] [CrossRef]
- Zheng, X.R.; Zhu, J.H.; Zhang, J.; Cai, P.; Sun, Y.H.; Chang, M.X.; Fang, L.X.; Sun, J.; Jiang, H.X. A novel plasmid-borne tet(X6) variant co-existing with blaNDM-1 and blaOXA-58 in a chicken Acinetobacter baumannii isolate. J. Antimicrob. Chemother. 2020, 75, 3397–3399. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I. The biological cost of mutational antibiotic resistance: Any practical conclusions? Curr. Opin. Microbiol. 2006, 9, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Levin, B.R. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 1999, 2, 489–493. [Google Scholar] [CrossRef]
- Yang, Q.; Li, M.; Spiller, O.B.; Andrey, D.O.; Hinchliffe, P.; Li, H.; MacLean, C.; Niumsup, P.; Powell, L.; Pritchard, M. Balancing mcr-1 expression and bacterial survival is a delicate equilibrium between essential cellular defence mechanisms. Nat. Commun. 2017, 8, 2054. [Google Scholar] [CrossRef] [Green Version]
- San Millan, A.; MacLean, R.C. Fitness costs of plasmids: A limit to plasmid transmission. Microbiol. Spectr. 2017, 5, MTBP-0016-2017. [Google Scholar] [CrossRef] [Green Version]
- San Millan, A. Evolution of plasmid-mediated antibiotic resistance in the clinical context. Trends Microbiol. 2018, 26, 978–985. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Wang, H.-H.; Lu, Y.; Yi, L.-X.; Deng, Y.; Lv, L.; Burrus, V.; Liu, J.-H. A ProQ/FinO family protein involved in plasmid copy number control favours fitness of bacteria carrying mcr-1-bearing IncI2 plasmids. Nucleic Acids Res. 2021, 49, 3981–3996. [Google Scholar] [CrossRef]
- Durante-Mangoni, E.; Del Franco, M.; Andini, R.; Bernardo, M.; Giannouli, M.; Zarrilli, R. Emergence of colistin resistance without loss of fitness and virulence after prolonged colistin administration in a patient with extensively drug-resistant Acinetobacter baumannii. Diagn. Microbiol. Infect. Dis. 2015, 82, 222–226. [Google Scholar] [CrossRef]
- Ommen, P.; Zobek, N.; Meyer, R.L. Quantification of biofilm biomass by staining: Non-toxic safranin can replace the popular crystal violet. J. Microbiol. Methods 2017, 141, 87–89. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, Y.; Yang, K.; Tong, Z.; Shi, J.; Li, R.; Xiao, X.; Ren, W.; Hardeland, R.; Reiter, R.J.; et al. Melatonin overcomes MCR-mediated colistin resistance in Gram-negative pathogens. Theranostics 2020, 10, 10697–10711. [Google Scholar] [CrossRef]
- Jousset, A.B.; Rosinski-Chupin, I.; Takissian, J.; Glaser, P.; Bonnin, R.A.; Naas, T. Transcriptional landscape of a plasmid and response to imipenem exposure in TOP10. Front. Microbiol. 2018, 9, 2929. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Zhang, P.; Zhang, H.; Zhou, Y.; Zhang, L.; Wang, R. Bio-control of Salmonella Enteritidis in foods using bacteriophages. Viruses 2015, 7, 4836–4853. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Peng, K.; Li, Y.; Liu, Y.; Wang, Z. Exploring tet(X)-bearing tigecycline-resistant bacteria of swine farming environments. Sci. Total Environ. 2020, 733, 139306. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 11th ed.; CLSI Standard M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Olaitan, A.O.; Morand, S.; Rolain, J.M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, Q.; Peng, K.; Liu, Y.; Li, R.; Wang, Z. Emergence of carbapenem- and tigecycline-resistant Proteus cibarius of animal origin. Front. Microbiol. 2020, 11, 1940. [Google Scholar] [CrossRef]
- Hall, J.P.J.; Brockhurst, M.A.; Dytham, C.; Harrison, E. The evolution of plasmid stability: Are infectious transmission and compensatory evolution competing evolutionary trajectories? Plasmid 2017, 91, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Deschaine, B.M.; Heysel, A.R.; Lenhart, B.A.; Murphy, H.A. Biofilm formation and toxin production provide a fitness advantage in mixed colonies of environmental yeast isolates. Ecol. Evol. 2018, 8, 5541–5550. [Google Scholar] [CrossRef]
- Kowalski, C.H.; Kerkaert, J.D.; Liu, K.W.; Bond, M.C.; Hartmann, R.; Nadell, C.D.; Stajich, J.E.; Cramer, R.A. Fungal biofilm morphology impacts hypoxia fitness and disease progression. Nat. Microbiol. 2019, 4, 2430–2441. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, D.; Xu, J.; Jia, R.; Chen, S.; Liu, M.; Zhao, X.; Yang, Q.; Wu, Y.; Zhang, S.; et al. Rifampin resistance and its fitness cost in Riemerella anatipestifer. BMC Microbiol. 2019, 19, 107. [Google Scholar] [CrossRef]
- Abdelraouf, K.; Kabbara, S.; Ledesma, K.R.; Poole, K.; Tam, V.H. Effect of multidrug resistance-conferring mutations on the fitness and virulence of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2011, 66, 1311–1317. [Google Scholar] [CrossRef]
| Genes | Sequences (5′ to 3′) | Amplicon Size (bp) |
|---|---|---|
| tet(X6) | F: CGAGCTCATGACTTTACTAAAACATAAAAAAATTAC R: CCCAAGCTTTTATAGATTCATTAGTTTTTGGAAAGAA | 1149 |
| qPCR-tet(X6) | F: TGTCGTTGATTTTCTCCTG R: TTGATTCTGCCTGTGCTT | 332 |
| 16S rRNA | F: TTCGGGAACCGTGAGA R: CTGGCAACAAAGGATAAGG | 103 |
| Strains | MIC (μg/mL) | MIC (μg/mL) with l-Arabinose (10 mM) |
|---|---|---|
| E. coli TOP10 (tet(X6)/pBAD) | 0.125 | 1 |
| S. Enteritidis ATCC13076 (tet(X6)/pBAD) | 1 | 8 |
| P. mirabilis HS1-T (tet(X6)/pBAD) | 4 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Cai, W.; Tang, F.; Wang, Z.; Liu, Y. Characterization of Fitness Cost Caused by Tigecycline-Resistance Gene tet(X6) in Different Host Bacteria. Antibiotics 2021, 10, 1172. https://doi.org/10.3390/antibiotics10101172
Jiang L, Cai W, Tang F, Wang Z, Liu Y. Characterization of Fitness Cost Caused by Tigecycline-Resistance Gene tet(X6) in Different Host Bacteria. Antibiotics. 2021; 10(10):1172. https://doi.org/10.3390/antibiotics10101172
Chicago/Turabian StyleJiang, Lijie, Wenhui Cai, Feifei Tang, Zhiqiang Wang, and Yuan Liu. 2021. "Characterization of Fitness Cost Caused by Tigecycline-Resistance Gene tet(X6) in Different Host Bacteria" Antibiotics 10, no. 10: 1172. https://doi.org/10.3390/antibiotics10101172
APA StyleJiang, L., Cai, W., Tang, F., Wang, Z., & Liu, Y. (2021). Characterization of Fitness Cost Caused by Tigecycline-Resistance Gene tet(X6) in Different Host Bacteria. Antibiotics, 10(10), 1172. https://doi.org/10.3390/antibiotics10101172






