Design and Evaluation of a Non-Contact Bed-Mounted Sensing Device for Automated In-Home Detection of Obstructive Sleep Apnea: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
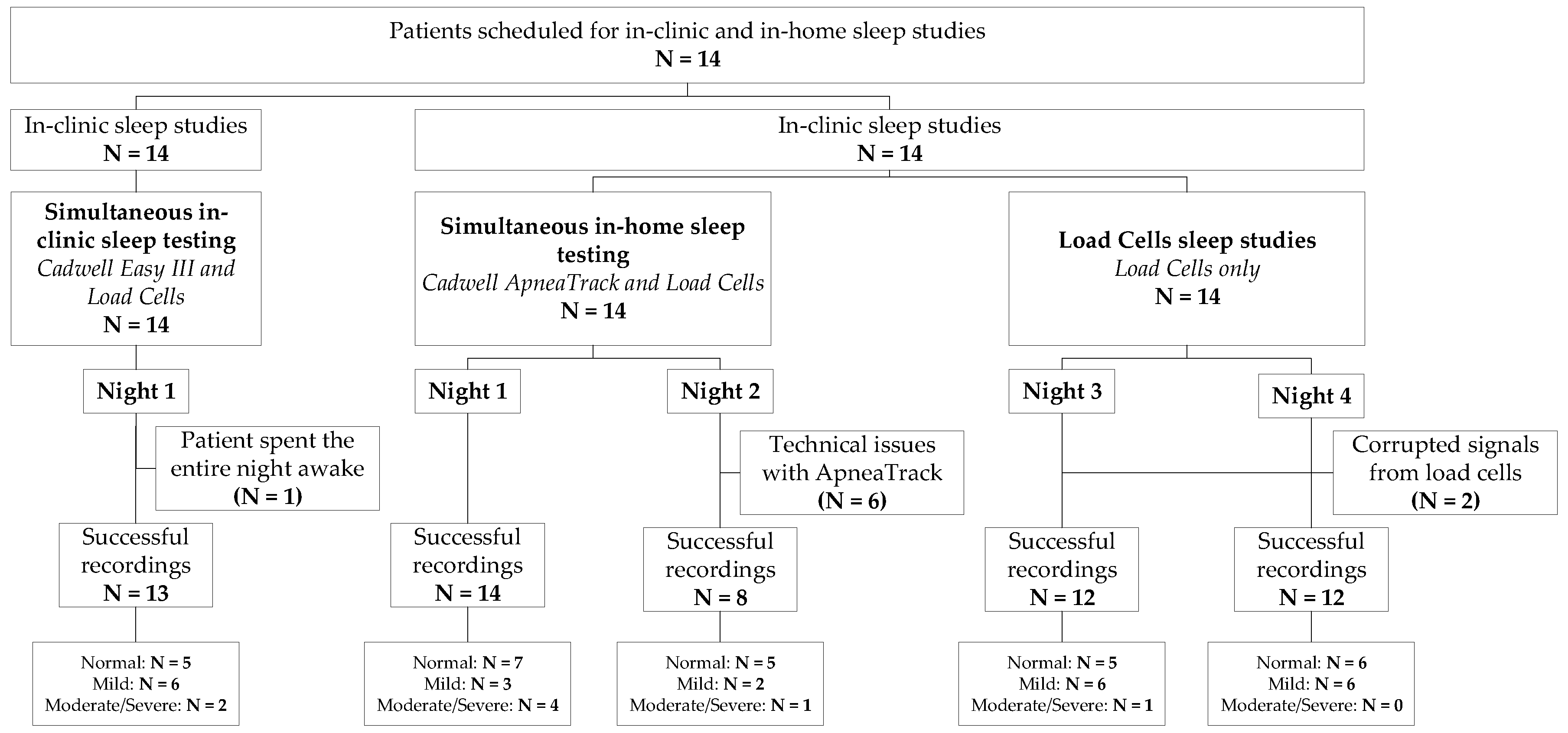
2.1. Cohort and Sleep Studies
2.2. Data Acquisition System
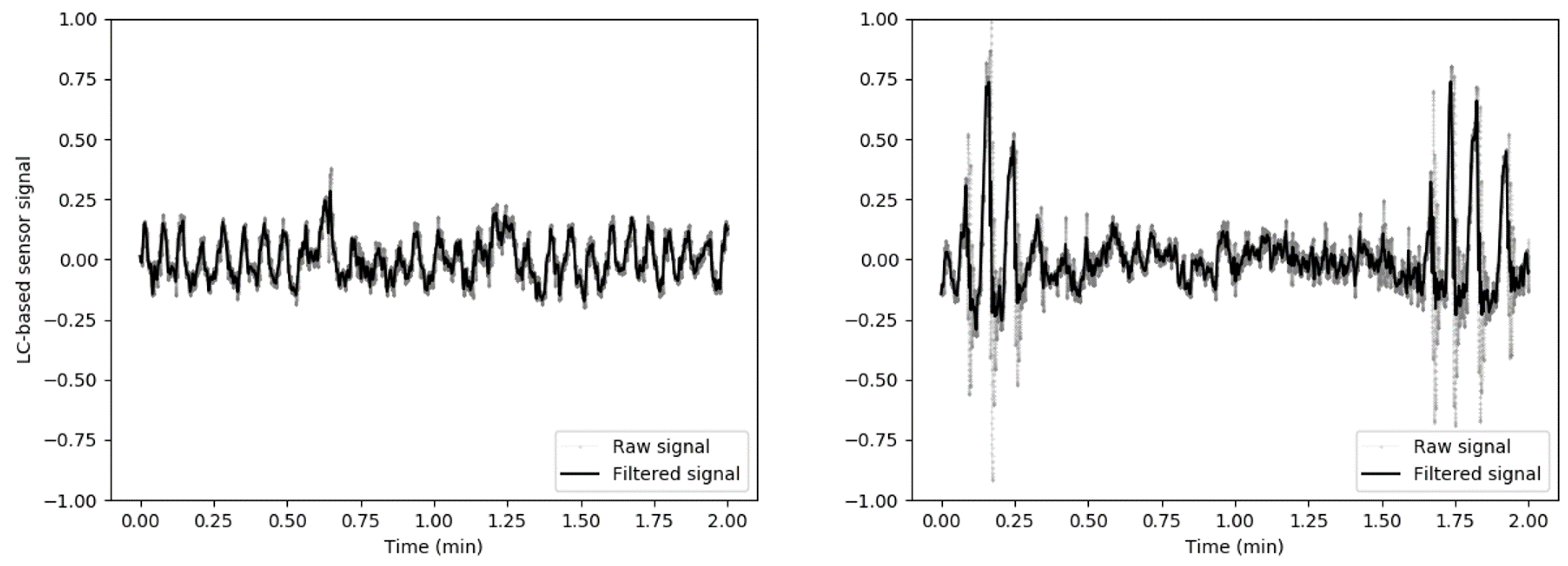
2.3. Load Cells Signal Processing and Feature Extraction
2.4. Machine Learning Models
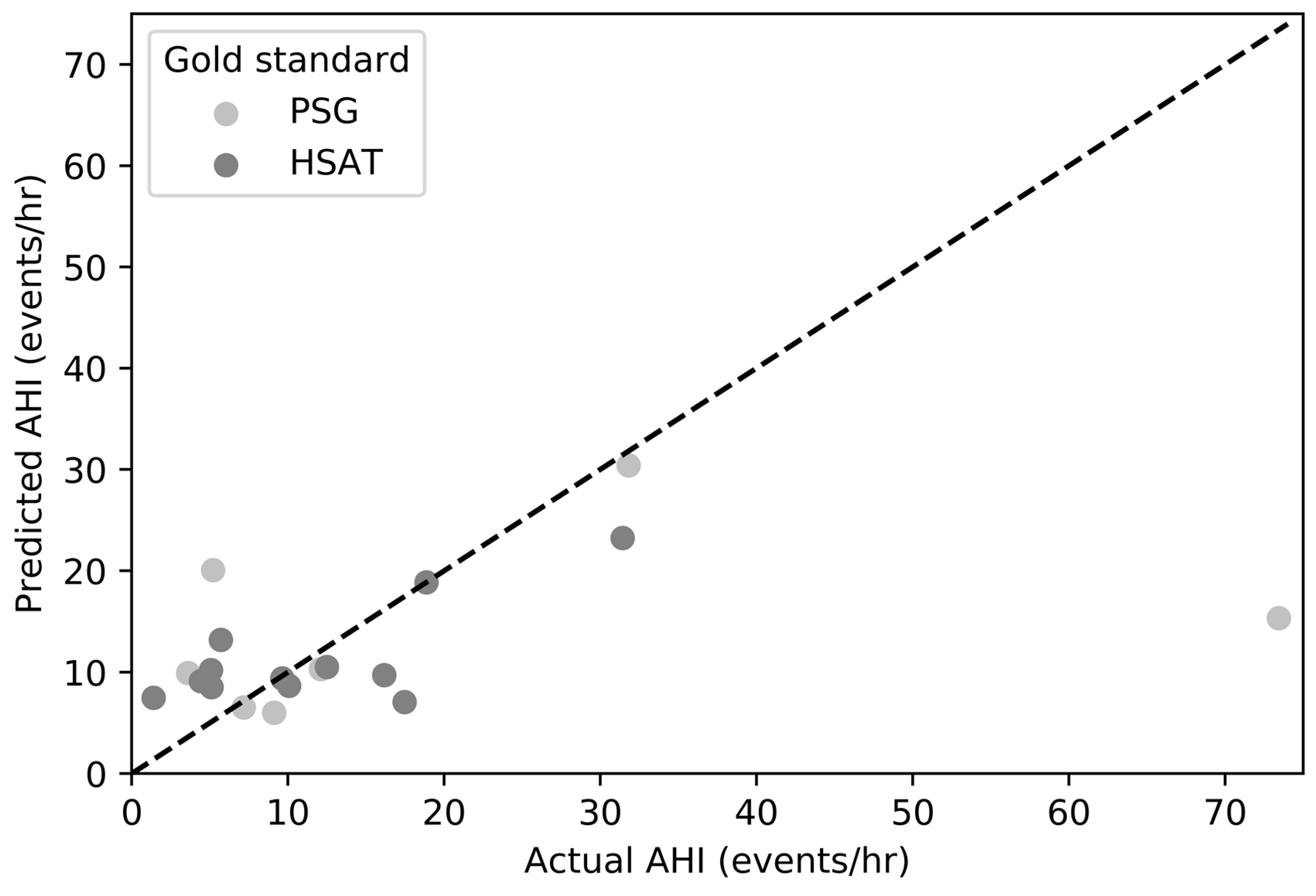
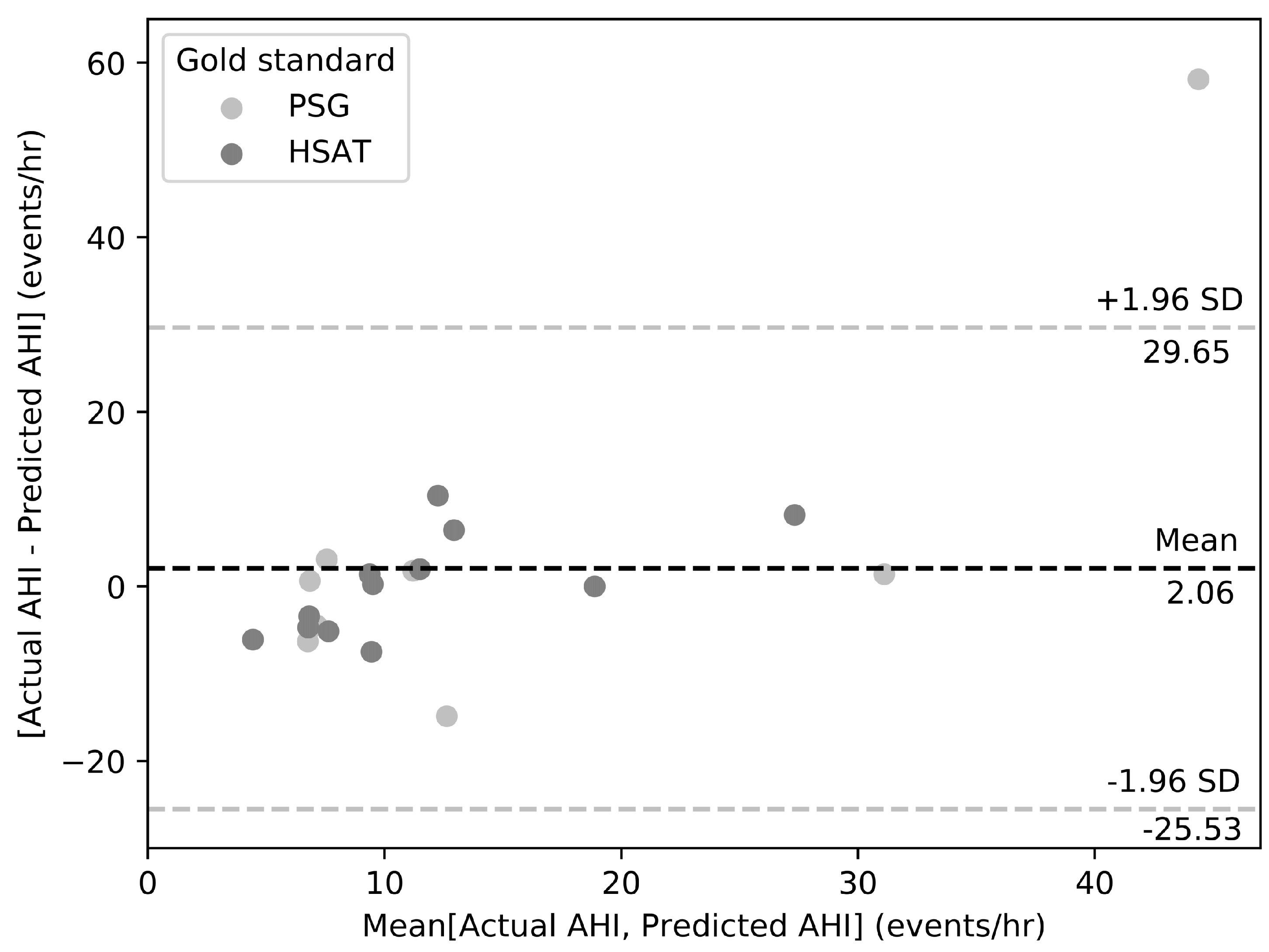
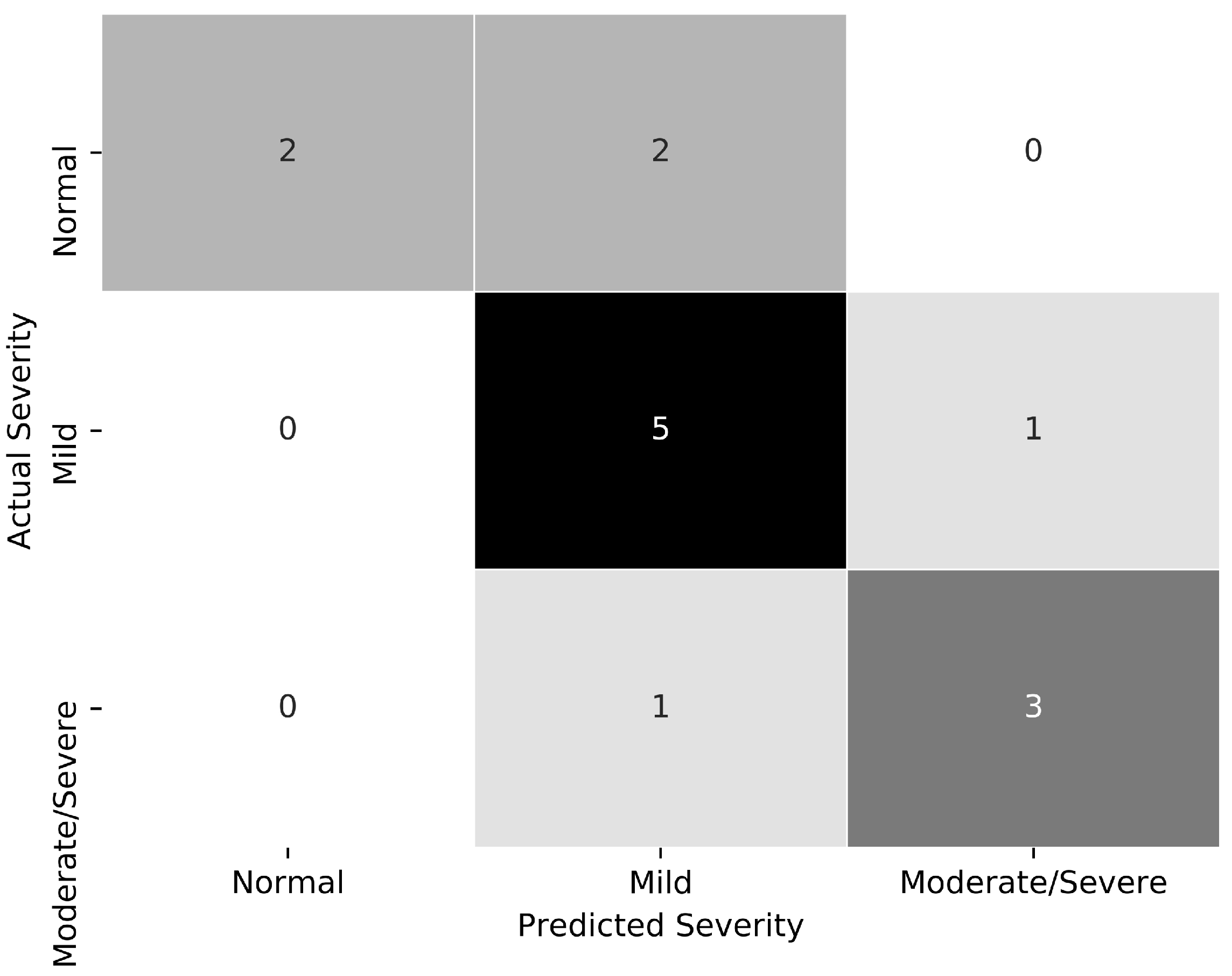
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Peppard, P.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 12, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Nagubadi, S.; Kryger, M.; Mokhlesi, B. Epidemiology of Obstructive Sleep Apnea: A Population-based Perspective. Expert Rev. Respir. Med. 2008, 2, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Neill, A. Home Setup Polysomnography in the Assessment of Suspected Obstructive Sleep Apnea. J. Sleep Res. 2011, 20, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G. Obstructive sleep apnoea syndrome: Underestimated and undertreated. Br. Med. Bull. 2004, 72, 49–64. [Google Scholar] [CrossRef] [PubMed]
- American Association of Sleep Medicine. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep 1999, 22, 667–689. [Google Scholar] [CrossRef]
- Agnew, H.; Webb, W.; Williams, R. The first night effect: An EEG study of sleep. Psychophysiology 1966, 2, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Aurora, R.; Chowdhuri, S.; Ramar, K.; Bista, S.R.; Casey, K.R.; Lamm, C.I.; Kristo, D.A.; Mallea, J.M.; Rowley, J.A.; Zak, R.S.; et al. The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses. Sleep 2012, 35, 17–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Medicare and Medicaid Services. National Coverage Determination for Continuous Positive Airway Pressure Therapy for Obstructive Sleep Apnea. 2018. Available online: https://www.cms.gov/medicare-coverage-database/ (accessed on 15 April 2018).
- Epstein, L.; Kristo, D.; Strollo, P.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar] [PubMed]
- Spicuzza, L.; Caruso, D.; Di Maria, D. Obstructive sleep apnoea syndrome and its management. Ther. Adv. Chronic Dis. 2015, 6, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Brink, M.; Muller, C.; Schierz, C. Contact-free measurement of heart rate, respiration rate, and body movements during sleep. Behav. Res. Methods 2006, 38, 511–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, D.; Beattie, Z.; Riley, T.; Adami, A.; Hagen, C.; Hayes, T. Unobtrusive classification of sleep and wakefulness using load cells under the bed. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 5254–5257. [Google Scholar]
- Beattie, Z.; Hagen, C.; Pavel, M.; Hayes, T. Classification of breathing events using load cells under the bed. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 3921–3924. [Google Scholar]
- Beattie, Z.; Jacobs, P.; Riley, T.; Hagen, C. A time-frequency respiration tracking system using non-contact bed sensors with harmonic artifact rejection. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 8111–8114. [Google Scholar]
- Beattie, Z.; Hayes, T.; Guilleminault, C.; Hagen, C. Accurate scoring of Apnea-Hypopnea Index using a simple non-contact breathing sensor. J. Sleep Res. 2013, 22, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Arlotto, P.; Grimaldi, M.; Naeck, R.; Ginoux, J.M. An ultrasonic contactless sensor for breathing monitoring. Sensors 2014, 14, 15371–15386. [Google Scholar] [CrossRef] [PubMed]
- Behar, J.; Roebuck, A.; Domingos, J.S.; Gederi, E.; Clifford, G.D. A review of current sleep screening applications for smartphones. Physiol. Meas. 2013, 34, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, R.; Gollakota, S.; Watson, N. Contactless sleep apnea detection on smartphones. In Proceedings of the 13th Annual International Conference on Mobile Systems, Applications, and Services, Florence, Italy, 18–22 May 2015. [Google Scholar]
- Mosquera-Lopez, C.; Leitschuh, J.; Condon, J.; Hagen, C.; Hanks, C.; Jacobs, P. In-Home Sleep Apnea Severity Classification Using Contact-Free Load Cells and an AdaBoosted Decision Tree Algorithm. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 6044–6047. [Google Scholar]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Sleep Medicine (AASM). The International Classification of Sleep Disorders (ICSD); AASM: Darien, IL, USA, 2004. [Google Scholar]
- White, L.; Lyons, O.; Yadollahi, A.; Ryan, C.M.; Bradley, T.D. Night-to-night variability in obstructive sleep apnea severity: Relationship to overnight rostral fluid shift. J. Clin. Sleep Med. 2015, 11, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Stoberl, A.; Schwarz, E.; Haile, S.; Turnbull, C.D.; Rossi, V.A.; Stradling, J.R.; Kohler, M. Night-to-night variability of obstructive sleep apnea. J. Sleep Res. 2017, 26, 782–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aurora, R.; Swartz, R.; Punjabi, N. Misclassification of OSA severity with automated scoring of home sleep recordings. Chest 2015, 147, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Goparaju, B. Potential underestimation of sleep apnea severity by at-home kits: Rescoring in-laboratory polysomnography without sleep staging. J. Clin. Sleep Med. 2017, 13, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.; McArdle, N.; James, A. A Comprehensive Evaluation of a Two-Channel Portable Monitor to “Rule in” Obstructive Sleep Apnea. J. Clin. Sleep Med. 2015, 11, 433–444. [Google Scholar] [CrossRef]

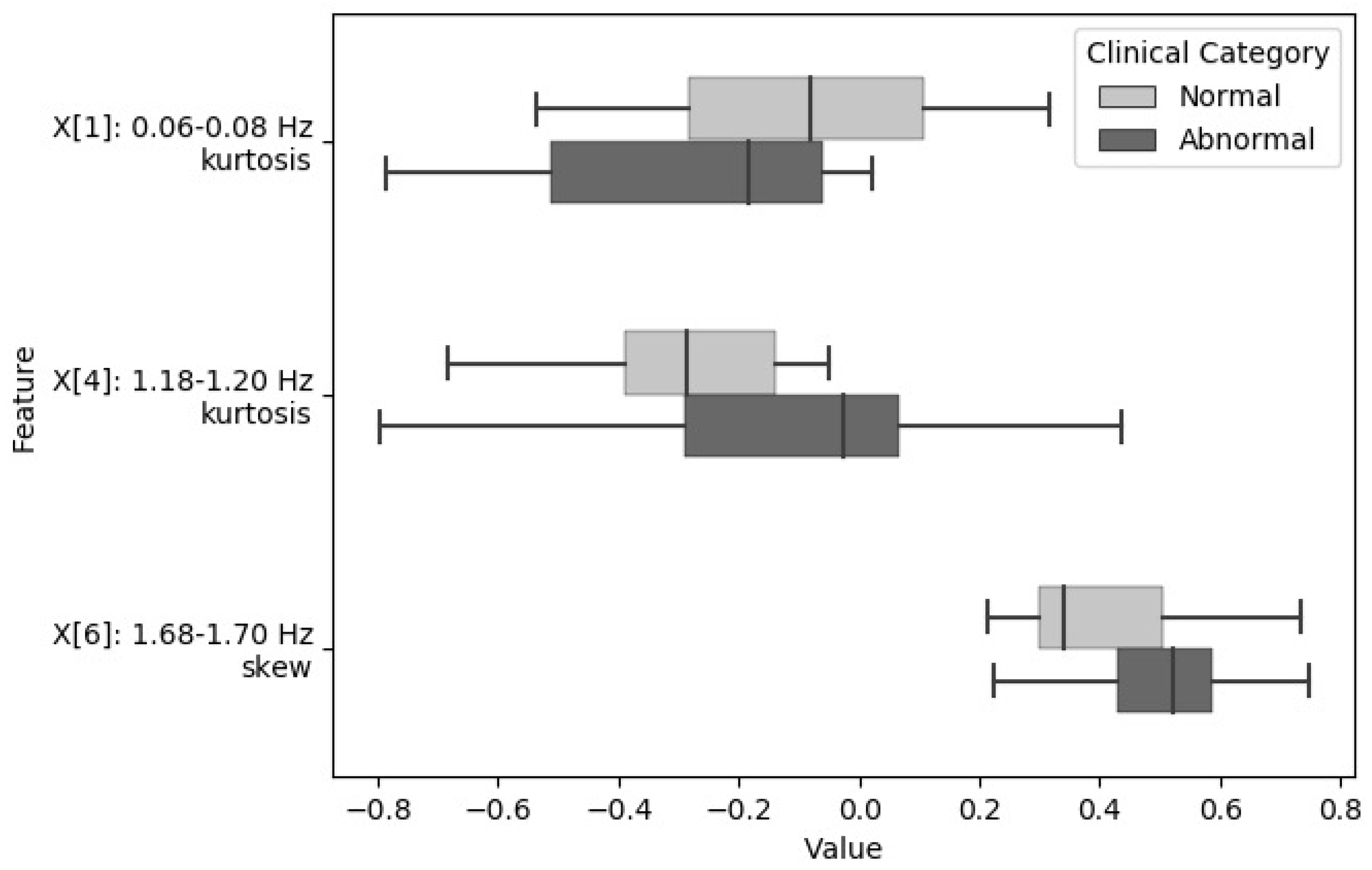
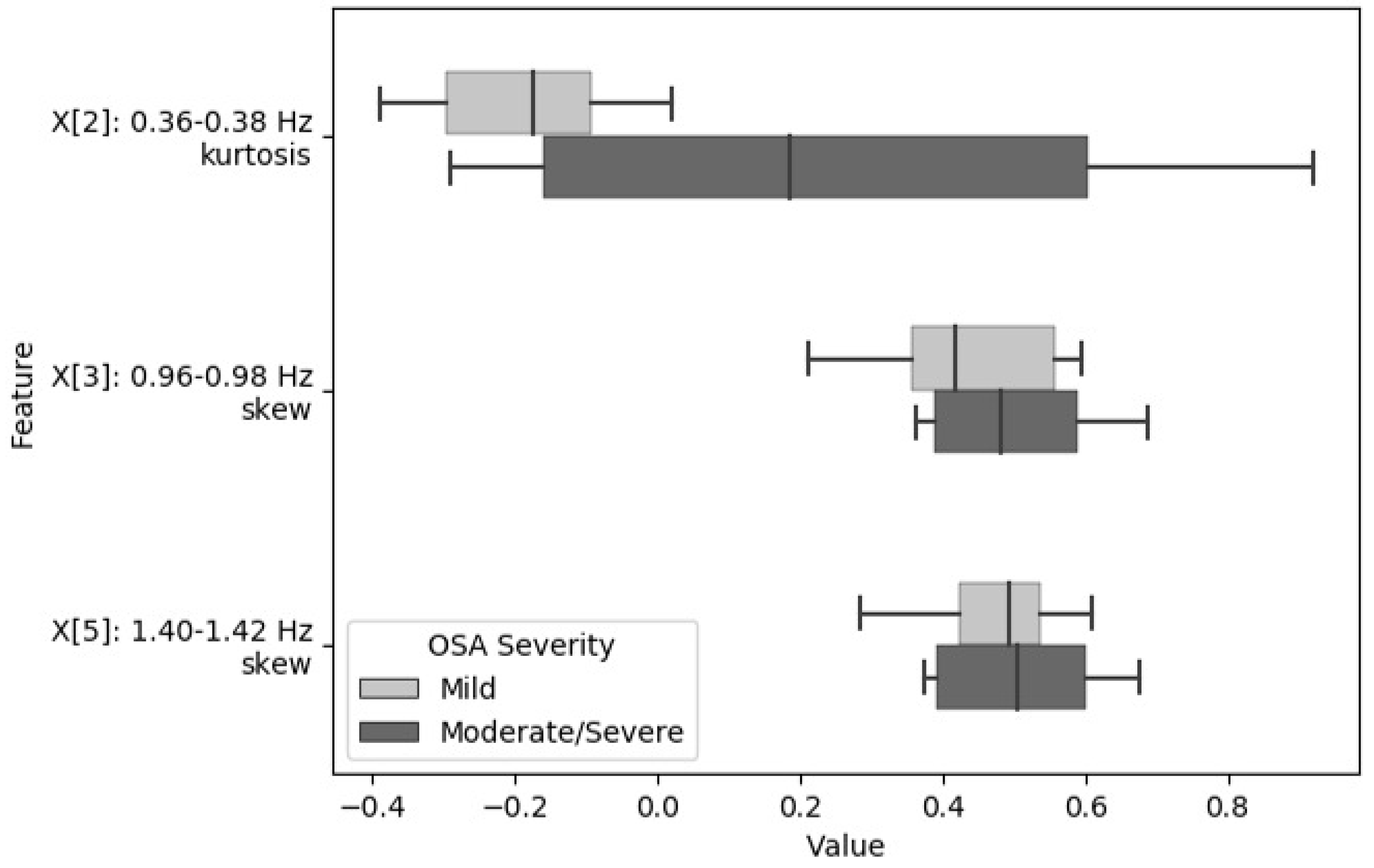
| Feature | Frequency Sub-Band | Statistic Calculated from Fast Fourier Transform (FFT) Coefficients | Classification Stage |
|---|---|---|---|
| X[1] | 0.06–0.08 Hz | kurtosis | S1DT |
| X[2] | 0.36–0.38 Hz | skewness | S2LR |
| X[3] | 0.96–0.98 Hz | skewness | S2LR |
| X[4] | 1.18–1.20 Hz | kurtosis | S1DT |
| X[5] | 1.40–1.42 Hz | kurtosis | S2LR |
| X[6] | 1.68–1.70 Hz | skewness | S1DT |
| Patient ID | Study Duration | Clinical Category | AHI | Severity | |||
|---|---|---|---|---|---|---|---|
| (hours) | Actual | Predicted | Actual | Predicted | Actual | Predicted | |
| 1 | 7.10 | Abnormal | Abnormal | 7.16 | 6.53 | Mild | Mild |
| 2 | 0.00 | - | - | - | - | - | - |
| 3 | 1.15 | Abnormal | Abnormal | 31.81 | 30.40 | Severe | Moderate/Severe |
| 4 | 6.34 | Abnormal | Abnormal | 9.12 | 6.01 | Mild | Mild |
| 5 | 1.12 | Abnormal | Abnormal | 73.43 | 15.32 | Severe | Moderate/Severe |
| 6 | 7.33 | Normal | Abnormal | 3.60 | 9.92 | Normal | Mild |
| 7 | 5.16 | Abnormal | Normal | 5.34 | <5 | Mild | Normal |
| 8 | 7.04 | Normal | Abnormal | 4.89 | 9.37 | Normal | Mild |
| 9 | 5.65 | Normal | Normal | 3.06 | <5 | Normal | Normal |
| 10 | 6.93 | Normal | Normal | 2.20 | <5 | Normal | Normal |
| 11 | 6.21 | Abnormal | Abnormal | 12.11 | 10.30 | Mild | Mild |
| 12 | 7.07 | Abnormal | Abnormal | 5.20 | 20.06 | Mild | Moderate/Severe |
| 13 | 6.01 | Abnormal | Normal | 7.06 | <5 | Mild | Normal |
| 14 | 7.39 | Normal | Normal | 0.83 | <5 | Normal | Normal |
| Patient ID | Night | Study Duration | Clinical Category | AHI | Severity | |||
|---|---|---|---|---|---|---|---|---|
| (hours) | Actual | Predicted | Actual | Predicted | Actual | Predicted | ||
| 1 | 1 | 7.76 | Abnormal | Abnormal | 9.63 | 9.38 | Mild | Mild |
| 2 | 9.08 | Abnormal | Abnormal | 10.07 | 8.66 | |||
| 2 | 1 | 6.93 | Abnormal | Abnormal | 17.46 | 7.05 | Moderate | Mild |
| 2 | 5.35 | Abnormal | Abnormal | 16.16 | 9.71 | |||
| 3 | 1 | 3.56 | Abnormal | Abnormal | 12.49 | 10.51 | Moderate | Moderate/Severe |
| 4 | 1 | 8.15 | Normal | Normal | 3.61 | <5 | Normal | Normal |
| 2 | 5.37 | Normal | Normal | 1.51 | <5 | |||
| 5 | 1 | 4.83 | Abnormal | Abnormal | 31.42 | 23.23 | Severe | Moderate/Severe |
| 6 | 1 | 5.07 | Normal | Abnormal | 4.42 | 9.12 | Normal | Mild |
| 2 | 0.77 | Normal | Normal | 1.34 | <5 | |||
| 7 | 1 | 5.00 | Abnormal | Abnormal | 5.70 | 13.19 | Mild | Mild |
| 2 | 1.40 | Abnormal | Abnormal | 5.07 | 10.20 | |||
| 8 | 1 | 8.57 | Abnormal | Abnormal | 5.11 | 8.53 | Mild | Mild |
| 9 | 1 | 1.54 | Normal | Normal | 4.02 | <5 | Normal | Mild |
| 2 | 1.45 | Normal | Abnormal | 1.40 | 7.48 | |||
| 10 | 1 | 9.59 | Normal | Normal | 1.49 | <5 | Normal | Normal |
| 2 | 9.05 | Normal | Normal | 2.14 | <5 | |||
| 11 | 1 | 3.03 | Normal | Normal | 1.67 | <5 | Normal | Normal |
| 12 | 1 | 2.82 | Normal | Normal | 2.17 | <5 | Normal | Normal |
| 13 | 1 | 4.26 | Abnormal | Abnormal | 18.87 | 18.87 | Moderate | Moderate/Severe |
| 14 | 1 | 8.70 | Normal | Normal | 0.71 | <5 | Normal | Normal |
| 2 | 5.88 | Normal | Normal | 0.18 | <5 | |||
| Patient ID | Night | Clinical Category | AHI | Severity |
|---|---|---|---|---|
| Predicted | Predicted | Predicted | ||
| 1 | 1 | Abnormal | 14.08 | Mild |
| 2 | Abnormal | 9.47 | ||
| 2 | 1 | Abnormal | 10.38 | Mild |
| 2 | Abnormal | 11.52 | ||
| 3 | 1 | Abnormal | 6.94 | Mild |
| 2 | Normal | <5 | ||
| 4 | 1 | Abnormal | 5.61 | Mild |
| 2 | Normal | <5 | ||
| 5 | 1 | Abnormal | 8.55 | Mild |
| 2 | Abnormal | 14.72 | ||
| 6 | 1 | Normal | <5 | Mild |
| 2 | Abnormal | 6.63 | ||
| 8 | 1 | Abnormal | 13.07 | Mild |
| 2 | Normal | <5 | ||
| 9 | 1 | Normal | <5 | Normal |
| 2 | Normal | <5 | ||
| 10 | 1 | Normal | <5 | Mild |
| 2 | Abnormal | 5.27 | ||
| 12 | 1 | Abnormal | 24.84 | Severe |
| 2 | Normal | <5 | ||
| 13 | 1 | Normal | <5 | Mild |
| 2 | Abnormal | 9.00 | ||
| 14 | 1 | Normal | <5 | Normal |
| 2 | Normal | <5 |
| Rating * | Ease of installation | Stability | Comfort |
|---|---|---|---|
| 1 | 0.00% | 0.00% | 0.00% |
| 2 | 7.14% | 0.00% | 0.00% |
| 3 | 7.14% | 0.00% | 0.00% |
| 4 | 28.57% | 14.29% | 14.29% |
| 5 | 57.14% | 85.71% | 85.71% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosquera-Lopez, C.; Leitschuh, J.; Condon, J.; Hagen, C.C.; Rajhbeharrysingh, U.; Hanks, C.; Jacobs, P.G. Design and Evaluation of a Non-Contact Bed-Mounted Sensing Device for Automated In-Home Detection of Obstructive Sleep Apnea: A Pilot Study. Biosensors 2019, 9, 90. https://doi.org/10.3390/bios9030090
Mosquera-Lopez C, Leitschuh J, Condon J, Hagen CC, Rajhbeharrysingh U, Hanks C, Jacobs PG. Design and Evaluation of a Non-Contact Bed-Mounted Sensing Device for Automated In-Home Detection of Obstructive Sleep Apnea: A Pilot Study. Biosensors. 2019; 9(3):90. https://doi.org/10.3390/bios9030090
Chicago/Turabian StyleMosquera-Lopez, Clara, Joseph Leitschuh, John Condon, Chad C. Hagen, Uma Rajhbeharrysingh, Cody Hanks, and Peter G. Jacobs. 2019. "Design and Evaluation of a Non-Contact Bed-Mounted Sensing Device for Automated In-Home Detection of Obstructive Sleep Apnea: A Pilot Study" Biosensors 9, no. 3: 90. https://doi.org/10.3390/bios9030090
APA StyleMosquera-Lopez, C., Leitschuh, J., Condon, J., Hagen, C. C., Rajhbeharrysingh, U., Hanks, C., & Jacobs, P. G. (2019). Design and Evaluation of a Non-Contact Bed-Mounted Sensing Device for Automated In-Home Detection of Obstructive Sleep Apnea: A Pilot Study. Biosensors, 9(3), 90. https://doi.org/10.3390/bios9030090





