DNA-Decorated PET Nanochannels for Sensitive Biosensing
Abstract
1. Introduction
2. Experimental Details
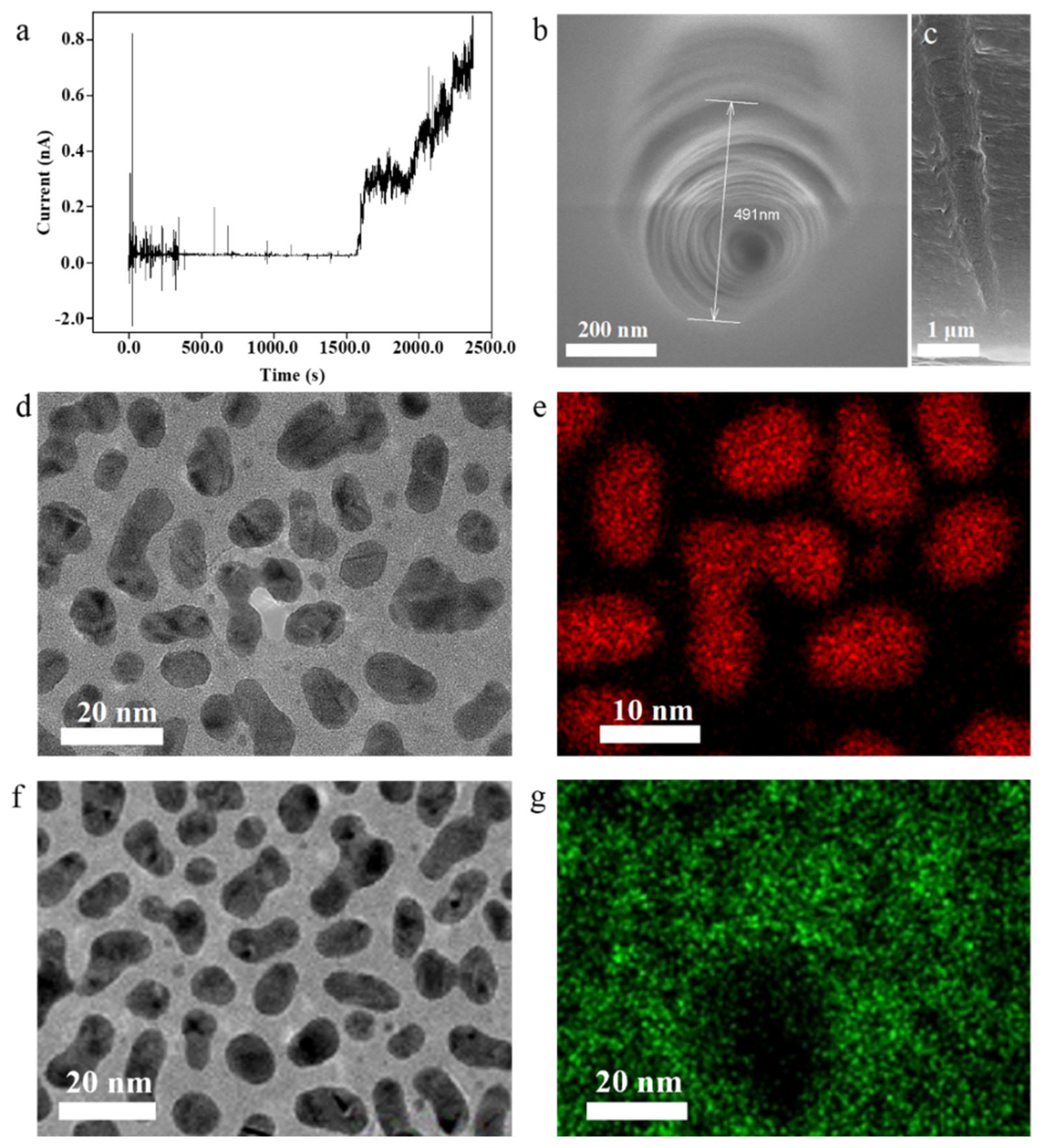
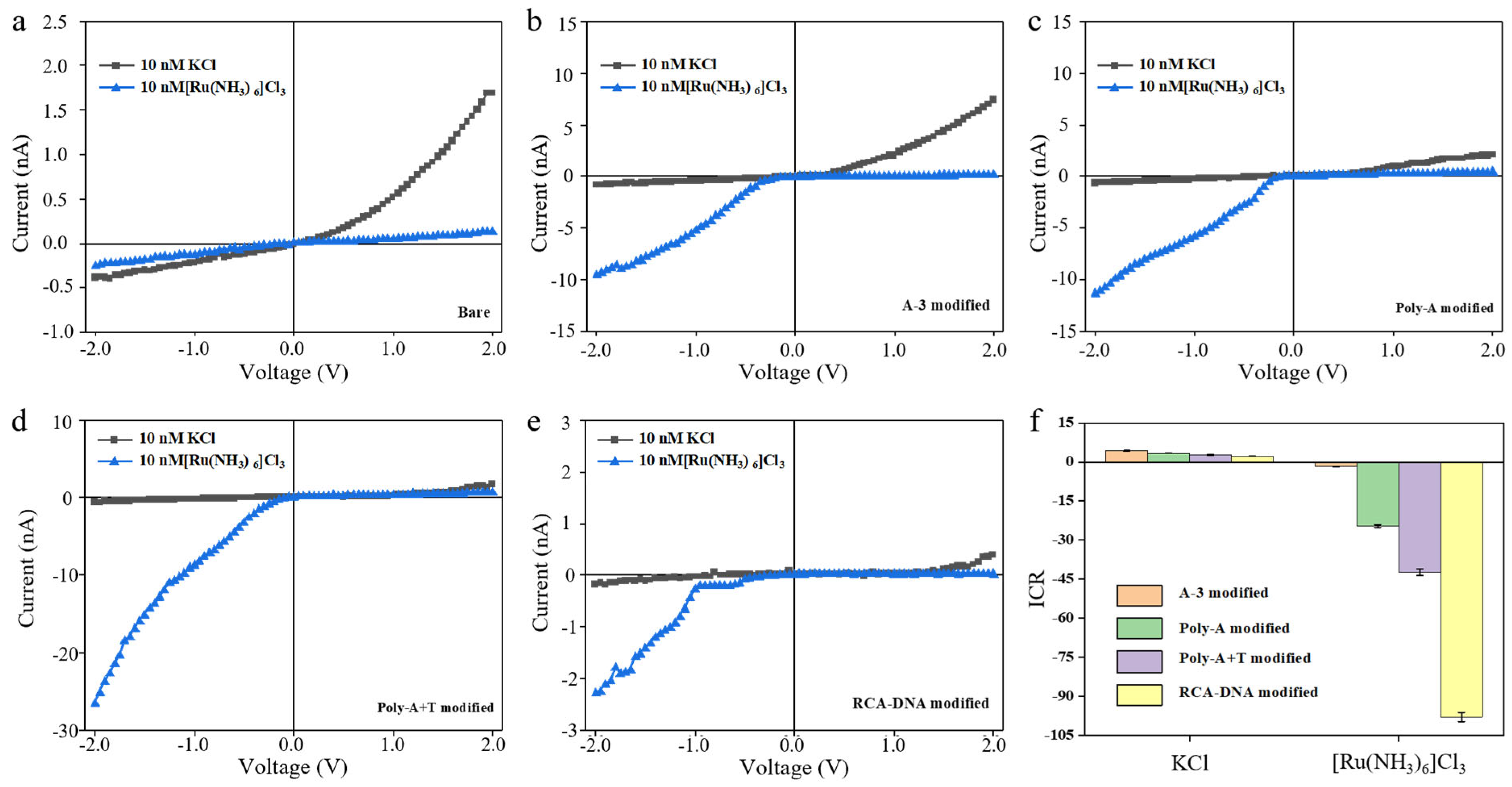
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, X.; Guo, W.; Jiang, L. Biomimetic smart nanopores and nanochannels. Chem. Soc. Rev. 2011, 40, 2385–2401. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, H.; Che, C.; Wu, X.; Feng, Y.; Gao, P.; Xia, F. Size and density adjustment of nanostructures in nanochannels for screening performance improvement. RSC Adv. 2021, 11, 2325–2328. [Google Scholar] [CrossRef]
- Doyle, D.A.; Morais-Cabral, J.; Pfuetzner, R.A.; Kuo, A.; Gulbis, J.M.; Cohen, S.L.; Chait, B.T.; MacKinnon, R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef]
- Zhou, Y.; Morais-Cabral, J.; Kaufman, A.; MacKinnon, R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 angstrom resolution. Nature 2001, 414, 43–48. [Google Scholar] [CrossRef]
- Duan, Z.; Ouyang, Y.; Fu, Y.; Huang, F.; Xia, F.; Willner, I. Optical and electrochemical probes for monitoring cytochrome c in subcellular compartments during apoptosis. Angew. Chem. Int. Ed. 2023, 62, e202301476. [Google Scholar] [CrossRef]
- Karmi, A.; Dachlika, H.; Sakala, G.P.; Rotem, D.; Reches, M.; Porath, D. Detection of Au nanoparticles using peptide-modified Si3N4 nanopores. ACS Appl. Nano Mater. 2021, 4, 1000–1008. [Google Scholar] [CrossRef]
- Lee, S.B.; Mitchell, D.T.; Trofin, L.; Nevanen, T.K.; Söderlund, H.; Martin, C.R. Antibody-based bio-nanotube membranes for enantiomeric drug separations. Science 2002, 296, 2198–2200. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Guo, W.; Mao, Y.; Hou, X.; Xue, J.; Xia, H.; Wang, L.; Song, Y.; Ji, H.; Ouyang, Q.; et al. Gating of single synthetic nanopores by proton-driven DNA molecular motors. J. Am. Chem. Soc. 2008, 130, 8345–8350. [Google Scholar] [CrossRef]
- Ali, M.; Schiedt, B.; Healy, K.; Neumann, R.; Ensinger, W. Modifying the surface charge of single tracketched conical nanopores in polyimide. Nanotechnology 2008, 19, 085713–085719. [Google Scholar] [CrossRef]
- Iqbal, S.M.; Akin, D.; Bashir, R. Solid-state nanopore channels with DNA selectivity. Nat. Nanotechnol. 2007, 2, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Yameen, B.; Neumann, R.; Ensinger, W.; Knoll, W.; Azzaroni, O. Biosensing and supramolecular bioconjugation in single conical polymer nanochannels. facile incorporation of biorecognition elements into nanoconfined geometries. J. Am. Chem. Soc. 2008, 130, 16351–16357. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Yameen, B.; Cervera, J.; Ramírez, P.; Neumann, R.; Ensinger, W.; Knoll, W.; Azzaroni, O. Layer-by-Layer assembly of polyelectrolytes into ionic current rectifying solid-state nanopores: Insights from theory and experiment. J. Am. Chem. Soc. 2010, 132, 8338–8348. [Google Scholar] [CrossRef]
- Xiao, K.; Xie, G.; Li, P.; Liu, Q.; Hou, G.; Zhang, Z.; Ma, J.; Tian, Y.; Wen, L.; Jiang, L. A biomimetic multi-stimuli-response ionic gate using a hydroxypyrene derivation-functionalized asymmetric single nanochannel. Adv. Mater. 2014, 26, 6560–6565. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.; Rao, K.V.; Sampath, S.; George, S.J.; Eswaramoorthy, M. Supramolecular gating of ion transport in nanochannels. Angew. Chem. Int. Ed. 2014, 53, 13073–13077. [Google Scholar] [CrossRef]
- Hou, X.; Dong, H.; Zhu, D.; Jiang, L. Fabrication of stable single nanochannels with controllable ionic rectification. Small 2010, 6, 361–365. [Google Scholar] [CrossRef]
- Tian, Y.; Hou, X.; Jiang, L. Biomimetic ionic rectifier systems: Asymmetric modification of single nanochannels by ion sputtering technology. J. Electroanal. Chem. 2011, 656, 231–236. [Google Scholar] [CrossRef]
- Wang, D.; Gao, P.; Zheng, M.; Duan, Z.; Wang, D.; Ding, D.; Xia, F. Mechanically durable plant-based composite surface towards enhanced antifouling properties. J. Colloid Interface Sci. 2025, 679, 457–466. [Google Scholar] [CrossRef]
- Vlassiouk, I.; Kozel, T.; Siwy, Z.S. Biosensing with nanofluidic diodes. J. Am. Chem. Soc. 2009, 131, 8211–8220. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, H.; Li, K.; Heng, L.; Wang, S.; Tian, Y.; Jiang, L. A bio-inspired potassium and pH responsive doublegated nanochannel. Adv. Funct. Mater. 2015, 25, 421–426. [Google Scholar] [CrossRef]
- Kalman, E.B.; Sudre, O.; Vlassiouk, I.; Siwy, Z.S. Control of ionic transport through gated single conical nanopores. Anal. Bioanal. Chem. 2009, 394, 413–419. [Google Scholar] [CrossRef]
- Pevarnik, M.; Healy, K.; Davenport, M.; Yen, J.; Siwy, Z.S. A hydrophobic entrance enhances ion current rectification and induces dewetting in asymmetric nanopores. Analyst 2012, 137, 2944–2950. [Google Scholar] [CrossRef]
- Hou, X.; Liu, Y.; Dong, H.; Yang, F.; Li, L.; Jiang, L. A pH-gating ionic transport nanodevice: Asymmetric chemical modification of single nanochannels. Adv. Mater. 2010, 22, 2440–2443. [Google Scholar] [CrossRef]
- Hou, X.; Yang, F.; Li, L.; Song, Y.; Jiang, L.; Zhu, D. A biomimetic asymmetric responsive single nanochannel. J. Am. Chem. Soc. 2010, 132, 11736–11742. [Google Scholar] [CrossRef]
- Zhang, H.C.; Hou, X.; Hou, J.; Zeng, L.; Tian, Y.; Li, L.; Jiang, L. Synthetic asymmetric-shaped nanodevices with symmetric pH-gating characteristics. Adv. Funct. Mater. 2015, 25, 1102–1110. [Google Scholar] [CrossRef]
- Nasir, S.; Ali, M.; Ramirez, P.; Gómez, V.; Oschmann, B.; Muench, F.; Tahir, M.N.; Zentel, R.; Mafe, S.; Ensinger, W. Fabrication of single cylindrical Au-coated nanopores with non-homogeneous fixed charge distribution exhibiting high current rectifications. ACS Appl. Mater. Interfaces 2014, 6, 12486–12494. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, Y.; Xie, Y.; Chen, L.; Xue, J.; Yan, S.; Wang, Y. A method to tune the ionic current rectification of track-etched nanopores by using surfactant. Phys. Chem. Chem. Phys. 2011, 13, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeon, J.; Wang, C.; Chang, G.T.; Park, J. Asymmetric nanochannel network-based bipolar ionic diode for enhanced heavy metal ion detection. ACS Nano 2022, 16, 8253–8263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Cai, H.W.; Hu, T.T.; Lin, M.H.; Dai, Y.; Xia, F. DNA-Functionalized Solid-State Nanochannels with Enhanced Sensing. Acc. Mater. Res. 2024, 6, 285–293. [Google Scholar] [CrossRef]
- Hu, J.; Lin, N.; Yuan, L.; Lou, X.; Xia, F. Detection of Analytes with the Outer Surface of Solid-State Nanochannels: From pm to μm. Acc. Chem. Res. 2025, 58, 834–846. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.S.; Zheng, S.C.; Li, S.Z.; Wang, D.; He, M.L.; Wang, L.; Wang, X.D. Ion transport behavior in a vertically-oriented asymmetric Ti3C2Tx nanochannel membrane. J. Membr. Sci. 2023, 668, 121232. [Google Scholar] [CrossRef]
- Harrell, C.C.; Kohli, P.; Siwy, Z.; Martin, C.R. DNA—Nanotube artificial ion channels. J. Am. Chem. Soc. 2004, 126, 15646–15647. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, Y.; Zhou, Y.; Xia, F.; Guo, W.; Jiang, L. Two-way nanopore sensing of sequence-specific oligonucleotides and small-molecule targets in complex matrices using integrated DNA supersandwich structures. Angew. Chem.-Int. Ed. 2013, 52, 2007–2011. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Q.; Wang, W.; Fang, F.; Zhang, J. Solid-state nanopore array: Manufacturing and applications. Small 2023, 19, 2205680. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Wang, D.; Che, C.; Ma, Q.; Wu, X.; Chen, Y.; Xu, H.; Li, X.; Lin, Y.; Ding, D.; et al. Regional and functional division of functional elements of solid-state nanochannels for enhanced sensitivity and specificity of biosensing in complex matrices. Nat. Protoc. 2021, 16, 4201–4226. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhai, T.; Gao, P.; Cheng, H.; Hou, R.; Lou, X.; Xia, F. Role of outer surface probes for regulating ion gating of nanochannels. Nat. Commun. 2018, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Qian, R.; Wu, M.; Yang, Z.; Wu, Y.; Guo, W.; Zhou, Z.; Wang, X.; Li, D.; Lu, Y. Rectifying artificial nanochannels with multiple interconvertible permeability states. Nat. Commun. 2024, 15, 2051–2064. [Google Scholar] [CrossRef]
- Li, Y.; Meng, S.C.; Wang, Y.; Platnich, C.M.; Earle, M.K.; Mylona, E.; Naydenova, P.; Baker, S.; Zhu, J.; Keyser, U.F. Nanopore detection of single-nucleotide RNA mutations and modifications with programmable nanolatches. Nat. Nanotechnol. 2025, 20, 1473–1481. [Google Scholar] [CrossRef]
- Cai, S.; Pataillot-Meakin, T.; Shibakawa, A.; Ren, R.; Bevan, C.L.; Ladame, S.; Ivanov, A.P.; Edel, J.B. Single-molecule amplification-free multiplexed detection of circulating microRNA cancer biomarkers from serum. Nat. Commun. 2021, 12, 3515–3527. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X. Bioinspired Solid-State Nanochannel Sensors: From Ionic Current Signals, Current, and Fluorescence Dual Signals to Faraday Current Signals. Small 2021, 17, 2100495. [Google Scholar] [CrossRef]
- Zhao, X.P.; Zhou, Y.; Zhang, Q.W.; Yang, D.R.; Wang, C.; Xia, X.H. Nanochannel–ion channel hybrid device for ultrasensitive monitoring of biomolecular recognition events. Anal. Chem. 2018, 91, 1185–1193. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Jiang, L. Fundamental studies and practical applications of bio-inspired smart solid-state nanopores and nanochannels. Nano Today 2016, 11, 61–81. [Google Scholar] [CrossRef]
- Bogatyrev, V.A.; Dykman, L.A.; Khlebtsov, B.N.; Plotnikov, V.K.; Khlebtsov, N.G. Optical properties of colloidal gold-oligothymidine conjugates and their variations on hybridization with polyadenylic acid. Colloid J. 2005, 67, 413–421. [Google Scholar] [CrossRef]
- Plekan, O.; Feyer, V.; Cassidy, A.; Lyamayev, V.; Tsud, N.; Ptasińska, S.; Reiff, S.; Acres, R.G.; Prince, K.C. Functionalisation and immobilisation of an Au (110) surface via uracil and 2-thiouracil anchored layer. Phys. Chem. Chem. Physics. 2015, 17, 15181–15192. [Google Scholar] [CrossRef] [PubMed]
- Apel, P.Y.; Korchev, Y.E.; Siwy, Z.; Spohr, R.; Yoshida, M. Diode-like single-ion track membrane prepared by electro-stop. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2001, 184, 337–346. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, X.; Xu, H.; Zhang, X.; Wang, D. DNA-Decorated PET Nanochannels for Sensitive Biosensing. Biosensors 2025, 15, 751. https://doi.org/10.3390/bios15110751
Gong X, Xu H, Zhang X, Wang D. DNA-Decorated PET Nanochannels for Sensitive Biosensing. Biosensors. 2025; 15(11):751. https://doi.org/10.3390/bios15110751
Chicago/Turabian StyleGong, Xianyan, Hongquan Xu, Xigui Zhang, and Dagui Wang. 2025. "DNA-Decorated PET Nanochannels for Sensitive Biosensing" Biosensors 15, no. 11: 751. https://doi.org/10.3390/bios15110751
APA StyleGong, X., Xu, H., Zhang, X., & Wang, D. (2025). DNA-Decorated PET Nanochannels for Sensitive Biosensing. Biosensors, 15(11), 751. https://doi.org/10.3390/bios15110751




