Double-Layered Microphysiological System Made of Polyethylene Terephthalate with Trans-Epithelial Electrical Resistance Measurement Function for Uniform Detection Sensitivity
Abstract
1. Introduction
2. Materials and Methods
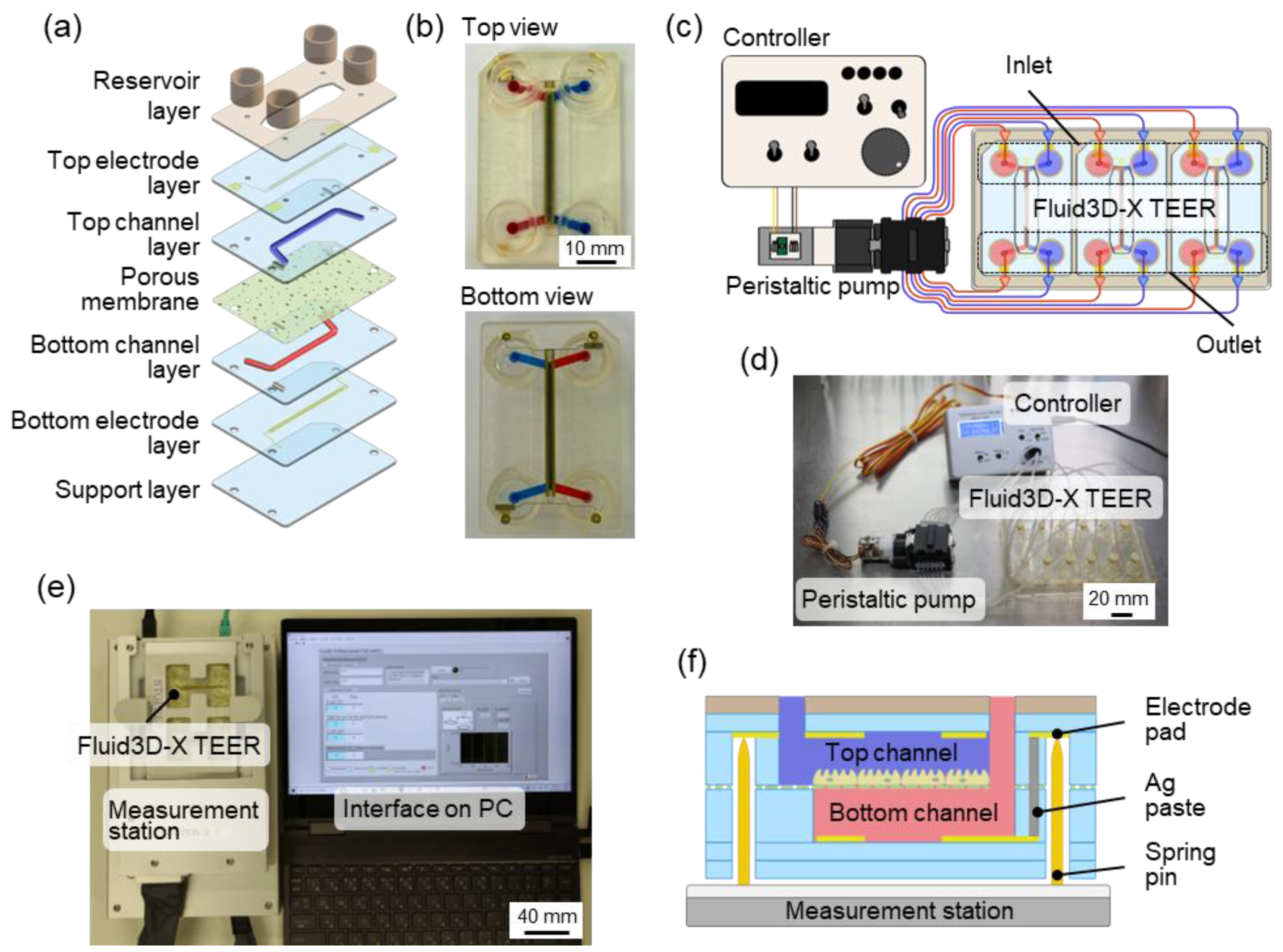
2.1. Double-Layered TEER Measurement Microfluidic Chip
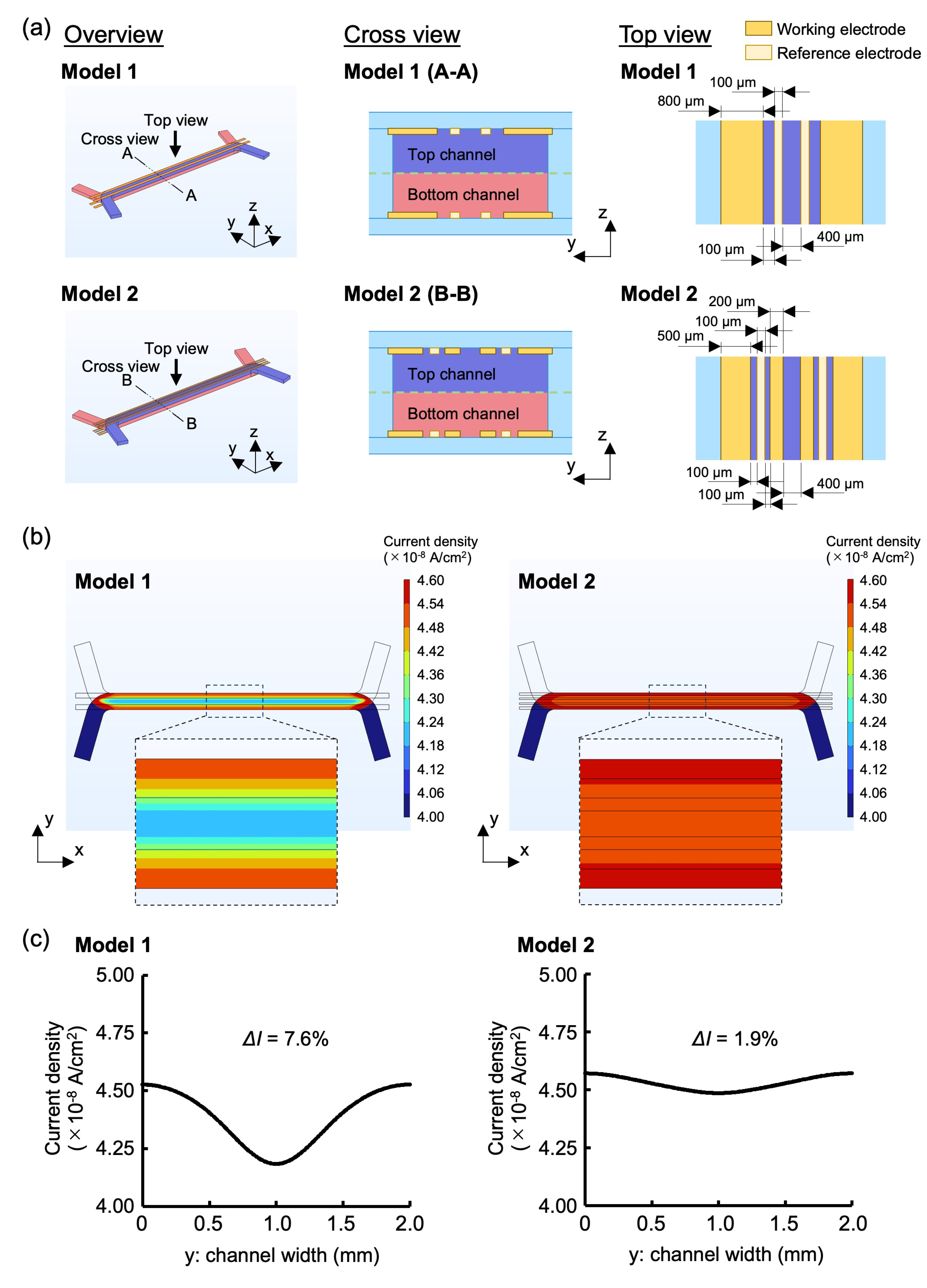
2.2. Electrode Design Through FEM Simulations
2.3. TEER Measurement Station
2.4. Cell Culture Using Fluid3D-X TEER
2.5. Online TEER Measurement with Staurosporine Exposure
2.6. Lucifer Yellow Permeation Assay
2.7. Immunocytochemistry
2.8. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Electrode Design Through FEM Simulations
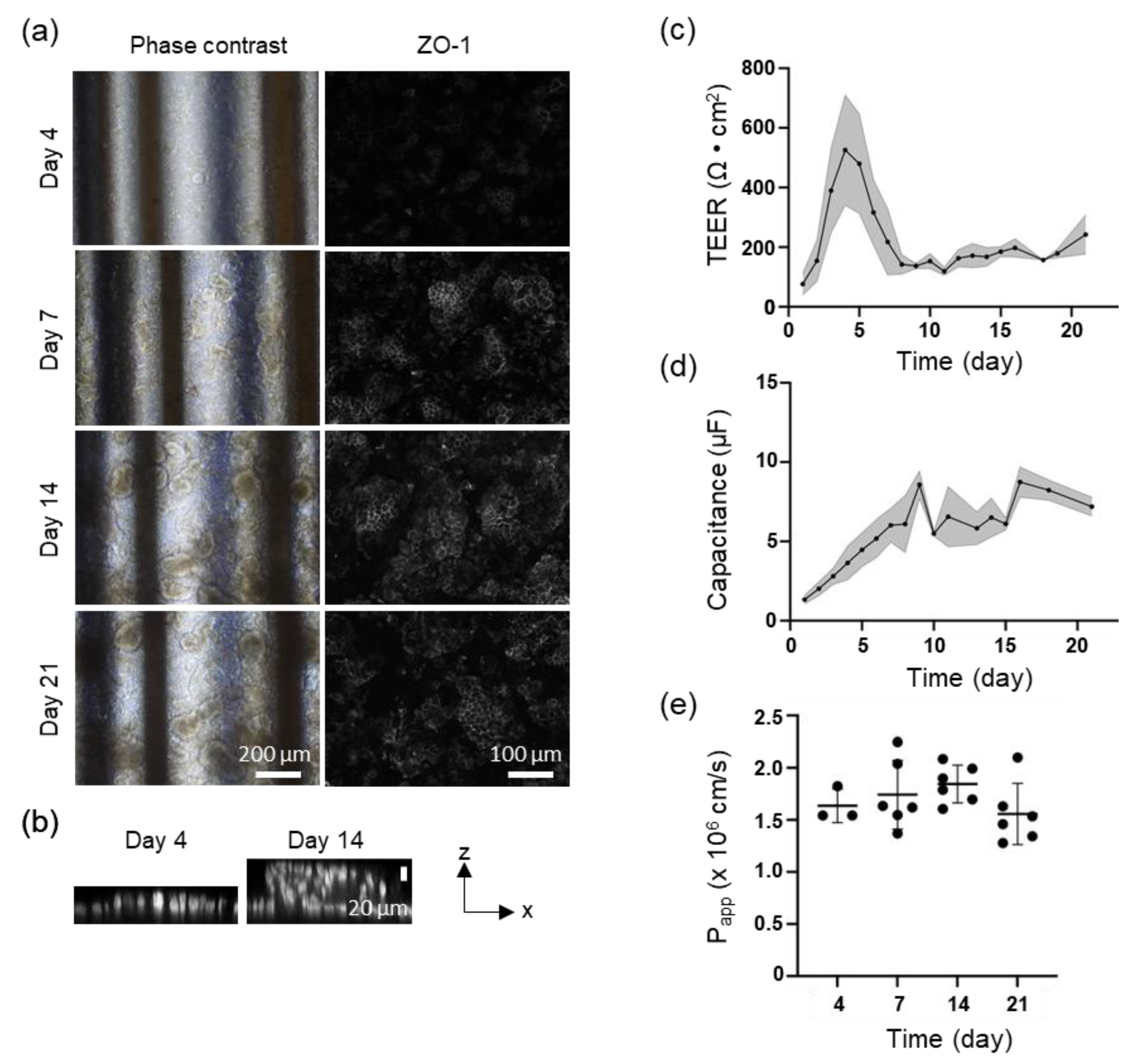
3.2. TEER Measurement for Caco-2 Perfusion Culture
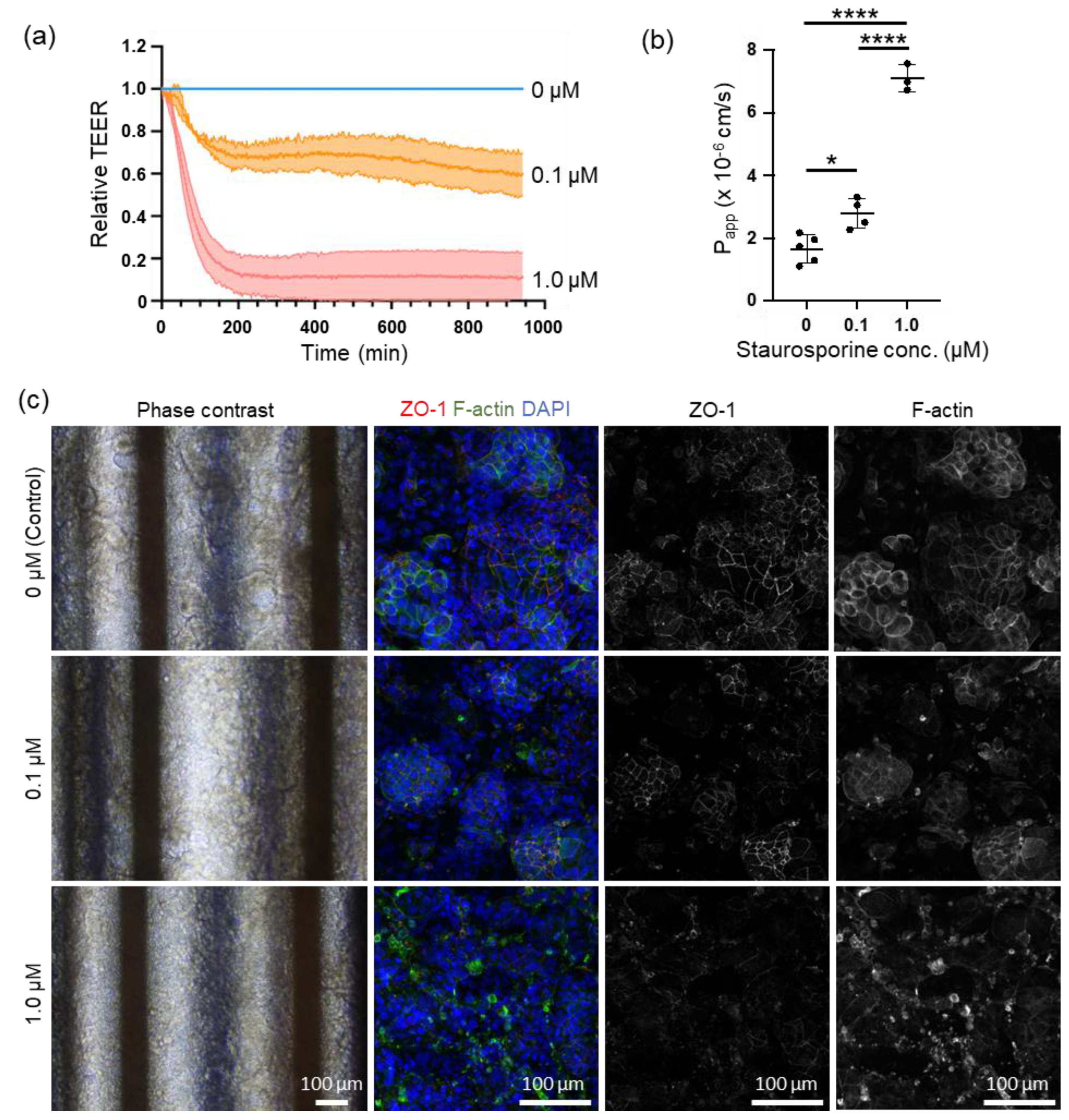
3.3. Online TEER Measurement with Drug Exposure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marx, U.; Akabane, T.; Andersson, T.B.; Baker, E.; Beilmann, M.; Beken, S.; Brendler-Schwaab, S.; Cirit, M.; David, R.; Dehne, E.M.; et al. Biology-inspired microphysiological systems to advance patient benefit and animal welfare in drug development. Altex 2020, 37, 365–394. [Google Scholar] [CrossRef] [PubMed]
- Han, J.J. FDA Modernization Act 2.0 allows for alternatives to animal testing. Artif. Organs 2023, 47, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Lehman-McKeeman, L.; Davis, M. Toxicology Modernization: Misnomer or Momentum. Med. Chem. Res. 2023, 32, 1235–1238. [Google Scholar] [CrossRef]
- Park, S.; Laskow, T.C.; Chen, J.; Guha, P.; Dawn, B.; Kim, D.H. Microphysiological systems for human aging research. Aging Cell 2024, 23, e14070. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Nishikawa, M.; Kutsuzawa, N.; Tokito, F.; Kobayashi, T.; Kurniawan, D.A.; Shioda, H.; Cao, W.; Shinha, K.; Nakamura, H.; et al. Advancements in Microphysiological systems: Exploring organoids and organ-on-a-chip technologies in drug development -focus on pharmacokinetics related organs. Drug Metab. Pharmacokinet. 2025, 60, 101046. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Kutsuzawa, N.; Ito, Y.; Kagawa, S.; Kohno, C.; Takiguchi, H.; Asano, K. Dexamethasone restores TNFα-induced epithelial barrier dysfunction in primary rat alveolar epithelial cells. PLoS ONE 2023, 18, e0295684. [Google Scholar] [CrossRef]
- Vigh, J.P.; Kincses, A.; Ozgür, B.; Walter, F.R.; Santa-Maria, A.R.; Valkai, S.; Vastag, M.; Neuhaus, W.; Brodin, B.; Dér, A.; et al. Transendothelial Electrical Resistance Measurement across the Blood–brain barrier: A Critical Review of Methods. Micromachines 2021, 12. [Google Scholar] [CrossRef]
- Takata, Y.; Banan Sadeghian, R.; Fujimoto, K.; Yokokawa, R. Online monitoring of epithelial barrier kinetics and cell detachment during cisplatin-induced toxicity of renal proximal tubule cells. Analyst 2024, 149, 3596–3606. [Google Scholar] [CrossRef]
- Ferrell, N.; Desai, R.R.; Fleischman, A.J.; Roy, S.; Humes, H.D.; Fissell, W.H. A microfluidic bioreactor with integrated transepithelial electrical resistance (TEER) measurement electrodes for evaluation of renal epithelial cells. Biotechnol. Bioeng. 2010, 107, 707–716. [Google Scholar] [CrossRef]
- Henry, O.Y.F.; Villenave, R.; Cronce, M.J.; Leineweber, W.D.; Benz, M.A.; Ingber, D.E. Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (TEER) measurements of human epithelial barrier function. Lab Chip 2017, 17, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Booth, R.; Kim, H. Characterization of a microfluidic in vitro model of the blood–brain barrier (μBBB). Lab Chip 2012, 12, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, G.S.; Occhetta, P.; Saccani, A.; Re, F.; Krol, S.; Rasponi, M.; Redaelli, A. Design and validation of a microfluidic device for blood–brain barrier monitoring and transport studies. J. Micromech. Microeng. 2018, 28, 044001. [Google Scholar] [CrossRef]
- Marrero, D.; Guimera, A.; Maes, L.; Villa, R.; Alvarez, M.; Illa, X. Organ-on-a-chip with integrated semitransparent organic electrodes for barrier function monitoring. Lab Chip 2023, 23, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Grimnes, S.; Martinsen, Ø.G. Sources of error in tetrapolar impedance measurements on biomaterials and other ionic conductors. J. Phys. D Appl. Phys. 2007, 40, 9. [Google Scholar] [CrossRef]
- Holzreuter, M.A.; Segerink, L.I. Innovative electrode and chip designs for transendothelial electrical resistance measurements in organs-on-chips. Lab Chip 2024, 24, 1121–1134. [Google Scholar] [CrossRef]
- Miyazaki, T.; Hirai, Y.; Kamei, K.-I.; Tsuchiya, T.; Tabata, O. Design strategy of electrode patterns based on finite element analysis in microfluidic device for Trans-Epithelial Electrical Resistance (TEER) measurement. Electron. Commun. Jpn. 2021, 104, e12296. [Google Scholar] [CrossRef]
- Kavand, H.; Nasiri, R.; Herland, A. Advanced Materials and Sensors for Microphysiological Systems: Focus on Electronic and Electrooptical Interfaces. Adv. Mater. 2022, 34, 2107876. [Google Scholar] [CrossRef]
- Sano, E.; Deguchi, S.; Matsuoka, N.; Tsuda, M.; Wang, M.; Kosugi, K.; Mori, C.; Yagi, K.; Wada, A.; Yamasaki, S.; et al. Generation of Tetrafluoroethylene–Propylene Elastomer-Based Microfluidic Devices for Drug Toxicity and Metabolism Studies. ACS Omega 2021, 6, 24859–24865. [Google Scholar] [CrossRef]
- Imaoka, T.; Onuki-Nagasaki, R.; Kimura, H.; Tai, K.; Ishii, M.; Nozue, A.; Kaisaki, I.; Hoshi, M.; Watanabe, K.; Maeda, K.; et al. Development of a novel gut microphysiological system that facilitates assessment of drug absorption kinetics in gut. Sci. Rep. 2024, 14, 29921. [Google Scholar] [CrossRef]
- Kutsuzawa, N.; Goto, T.; Nakamura, H.; Maeda, M.; Kinehara, M.; Sakagami, J.; Kimura, H. Evaluation of Perfusion Cell Culture Conditions in a Double-Layered Microphysiological System Using AI-Assisted Morphological Analysis. Micromachines 2025, 16, 327. [Google Scholar] [CrossRef]
- Kimura, H.; Nakamura, H.; Goto, T.; Uchida, W.; Uozumi, T.; Nishizawa, D.; Shinha, K.; Sakagami, J.; Doi, K. Standalone cell culture microfluidic device-based microphysiological system for automated cell observation and application in nephrotoxicity tests. Lab Chip 2024, 24, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.W. Barrier function of epithelia. Am. J. Physiol. 1981, 241, G275–G288. [Google Scholar] [CrossRef] [PubMed]
- Solsona, C.; Innocenti, B.; Fernández, J.M. Regulation of exocytotic fusion by cell inflation. Biophys. J. 1998, 74, 1061–1073. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Satoh, H.; Delbridge, L.M.; Blatter, L.A.; Bers, D.M. Surface:volume relationship in cardiac myocytes studied with confocal microscopy and membrane capacitance measurements: Species-dependence and developmental effects. Biophys. J. 1996, 70, 1494–1504. [Google Scholar] [CrossRef]
- Wang, X.B.; Huang, Y.; Gascoyne, P.R.; Becker, F.F.; Hölzel, R.; Pethig, R. Changes in Friend murine erythroleukaemia cell membranes during induced differentiation determined by electrorotation. Biochim. Biophys. Acta 1994, 1193, 330–344. [Google Scholar] [CrossRef]
- Nikulin, S.V.; Knyazev, E.N.; Poloznikov, A.A.; Shilin, S.A.; Gazizov, I.N.; Zakharova, G.S.; Gerasimenko, T.N. Expression of SLC30A10 and SLC23A3 Transporter mRNAs in Caco-2 Cells Correlates with an Increase in the Area of the Apical Membrane. Mol. Biol. 2018, 52, 577–582. [Google Scholar] [CrossRef]
- van der Helm, M.W.; Henry, O.Y.F.; Bein, A.; Hamkins-Indik, T.; Cronce, M.J.; Leineweber, W.D.; Odijk, M.; van der Meer, A.D.; Eijkel, J.C.T.; Ingber, D.E.; et al. Non-invasive sensing of transepithelial barrier function and tissue differentiation in organs-on-chips using impedance spectroscopy. Lab Chip 2019, 19, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Czupalla, C.J.; Liebner, S.; Devraj, K. In vitro models of the blood–brain barrier. Methods Mol. Biol. 2014, 1135, 415–437. [Google Scholar] [CrossRef] [PubMed]
- Ōmura, S.; Asami, Y.; Crump, A. Staurosporine: New lease of life for parent compound of today’s novel and highly successful anti-cancer drugs. J. Antibiot. 2018, 71, 688–701. [Google Scholar] [CrossRef]
- Funato, N.; Takayanagi, H.; Konda, Y.; Toda, Y.; Harigaya, Y.; Iwai, Y.; Ōmura, S. Absolute Configuration of Staurosporine By X-Ray Analysis. Tetrahedron Lett. 1994, 35, 1251–1254. [Google Scholar] [CrossRef]
- Schächtele, C.; Seifert, R.; Osswald, H. Stimulus-dependent inhibition of platelet aggregation by the protein kinase C inhibitors polymyxin B, H-7 and staurosporine. Biochem. Biophys. Res. Commun. 1988, 151, 542–547. [Google Scholar] [CrossRef]
- Rüegg, U.T.; Burgess, G.M. Staurosporine, K-252 and UCN-01: Potent but nonspecific inhibitors of protein kinases. Trends Pharmacol. Sci. 1989, 10, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Antonsson, A.; Persson, J.L. Induction of apoptosis by staurosporine involves the inhibition of expression of the major cell cycle proteins at the G(2)/m checkpoint accompanied by alterations in Erk and Akt kinase activities. Anticancer. Res. 2009, 29, 2893–2898. [Google Scholar] [PubMed]
- Chae, H.J.; Kang, J.S.; Byun, J.O.; Han, K.S.; Kim, D.U.; Oh, S.M.; Kim, H.M.; Chae, S.W.; Kim, H.R. Molecular mechanism of staurosporine-induced apoptosis in osteoblasts. Pharmacol. Res. 2000, 42, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Lee, E.Y.; Chung, D.; Cho, K.; Yu, W.J.; Nam, S.J.; Park, S.K.; Choi, I.W. The Inhibition Effect and Mechanism of Staurosporine Isolated from Streptomyces sp. SNC087 Strain on Nasal Polyp. Mar. Drugs 2024, 22. [Google Scholar] [CrossRef]
- Trietsch, S.J.; Naumovska, E.; Kurek, D.; Setyawati, M.C.; Vormann, M.K.; Wilschut, K.J.; Lanz, H.L.; Nicolas, A.; Ng, C.P.; Joore, J.; et al. Membrane-free culture and real-time barrier integrity assessment of perfused intestinal epithelium tubes. Nat. Commun. 2017, 8, 262. [Google Scholar] [CrossRef]
- Nicolas, A.; Schavemaker, F.; Kosim, K.; Kurek, D.; Haarmans, M.; Bulst, M.; Lee, K.; Wegner, S.; Hankemeier, T.; Joore, J.; et al. High throughput transepithelial electrical resistance (TEER) measurements on perfused membrane-free epithelia. Lab Chip 2021, 21, 1676–1685. [Google Scholar] [CrossRef]
- Lee, S.M.; Han, N.; Lee, R.; Choi, I.H.; Park, Y.B.; Shin, J.S.; Yoo, K.H. Real-time monitoring of 3D cell culture using a 3D capacitance biosensor. Biosens. Bioelectron. 2016, 77, 56–61. [Google Scholar] [CrossRef]
- Park, G.; Kang, S.; Kwon, Y.; An, J.; Park, H.; Lee, M.-H.; Lee, T. Electrical capacitance-based cancer cell viability monitoring device for accelerated drug development. Sens. Actuators B Chem. 2024, 409, 135566. [Google Scholar] [CrossRef]
- Patel, P.; Markx, G.H. Dielectric measurement of cell death. Enzym. Microb. Technol. 2008, 43, 463–470. [Google Scholar] [CrossRef]
- Wu, S.; Ketcham, S.A.; Corredor, C.C.; Both, D.; Drennen, J.K.; Anderson, C.A. Rapid at-line early cell death quantification using capacitance spectroscopy. Biotechnol. Bioeng. 2022, 119, 857–867. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutsuzawa, N.; Nakamura, H.; Chen, L.; Fujioka, R.; Mori, S.; Nakatani, N.; Yoshioka, T.; Kimura, H. Double-Layered Microphysiological System Made of Polyethylene Terephthalate with Trans-Epithelial Electrical Resistance Measurement Function for Uniform Detection Sensitivity. Biosensors 2025, 15, 663. https://doi.org/10.3390/bios15100663
Kutsuzawa N, Nakamura H, Chen L, Fujioka R, Mori S, Nakatani N, Yoshioka T, Kimura H. Double-Layered Microphysiological System Made of Polyethylene Terephthalate with Trans-Epithelial Electrical Resistance Measurement Function for Uniform Detection Sensitivity. Biosensors. 2025; 15(10):663. https://doi.org/10.3390/bios15100663
Chicago/Turabian StyleKutsuzawa, Naokata, Hiroko Nakamura, Laner Chen, Ryota Fujioka, Shuntaro Mori, Noriyuki Nakatani, Takahiro Yoshioka, and Hiroshi Kimura. 2025. "Double-Layered Microphysiological System Made of Polyethylene Terephthalate with Trans-Epithelial Electrical Resistance Measurement Function for Uniform Detection Sensitivity" Biosensors 15, no. 10: 663. https://doi.org/10.3390/bios15100663
APA StyleKutsuzawa, N., Nakamura, H., Chen, L., Fujioka, R., Mori, S., Nakatani, N., Yoshioka, T., & Kimura, H. (2025). Double-Layered Microphysiological System Made of Polyethylene Terephthalate with Trans-Epithelial Electrical Resistance Measurement Function for Uniform Detection Sensitivity. Biosensors, 15(10), 663. https://doi.org/10.3390/bios15100663





