Recent Advances in Nanomaterials for Modulation of Stem Cell Differentiation and Its Therapeutic Applications
Abstract
1. Introduction
2. Metal-Based Stem Cell Differentiation Approaches and Therapeutics
2.1. Autophagy
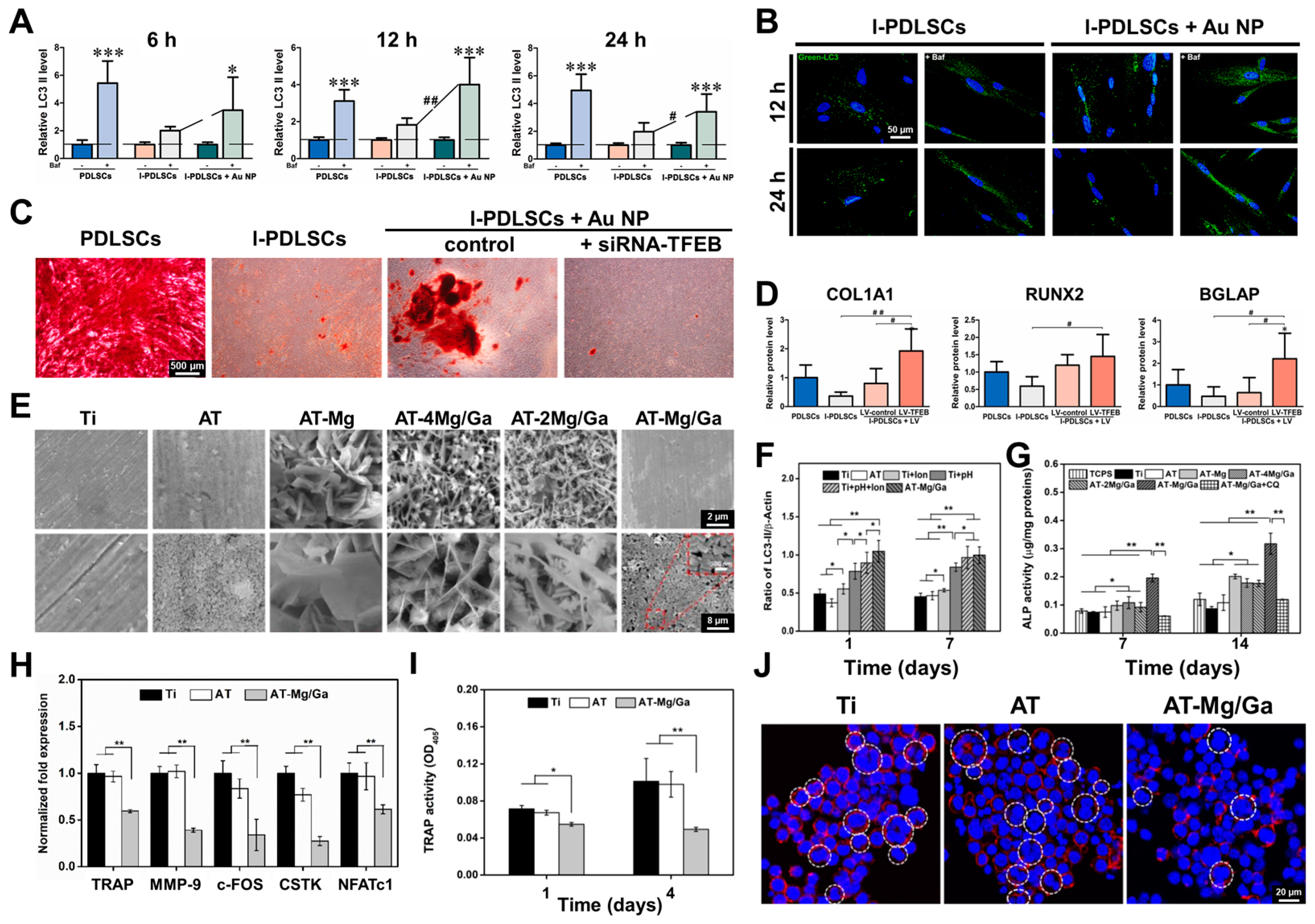
2.2. ROS Scavenger
3. Carbon-Based Stem Cell Differentiation Approaches and Therapeutics
3.1. Electrical Signals to Enhance Stem Cell Differentiation
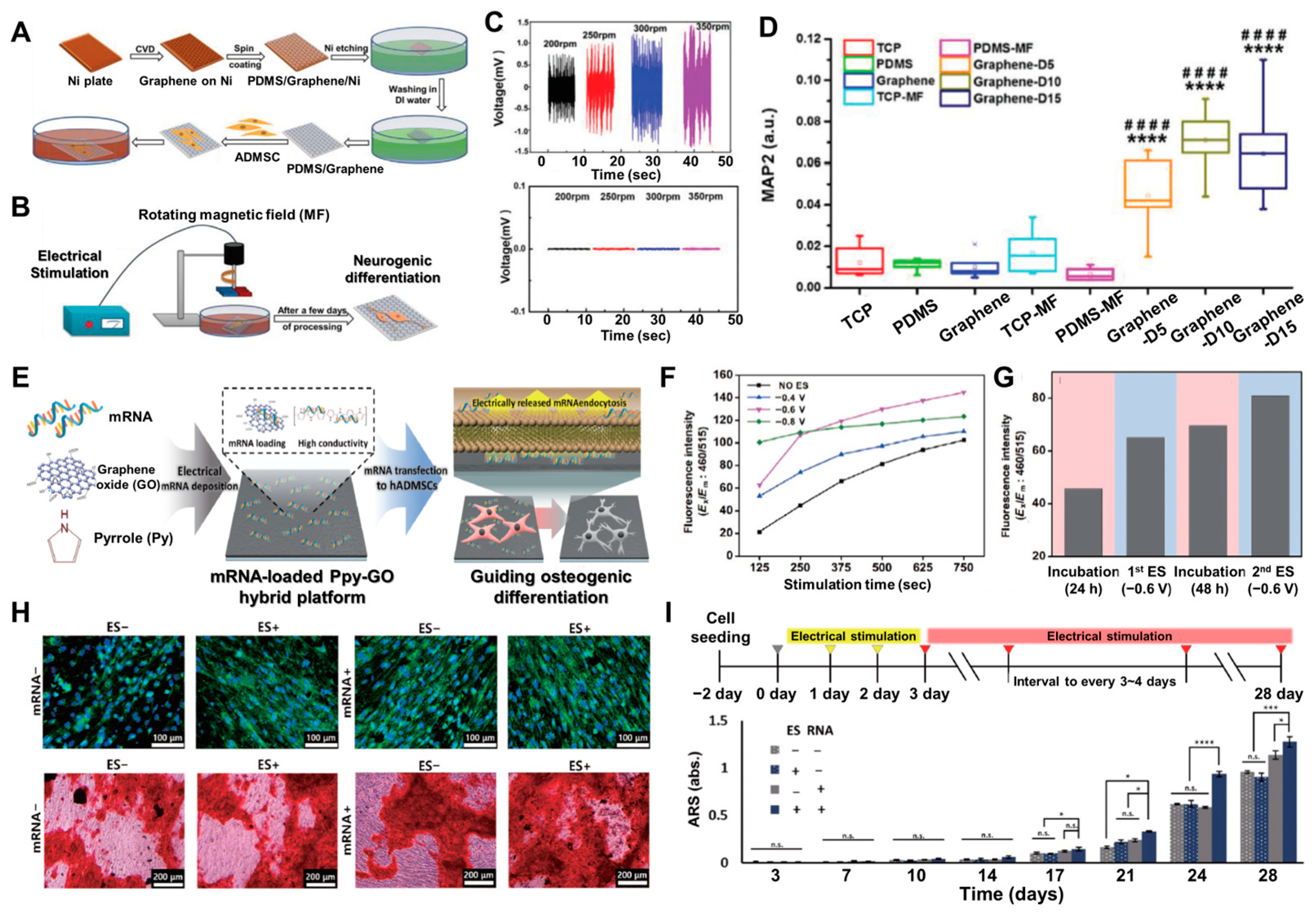
3.2. Hydrogel Formation
4. Other Nanomaterials for Stem Cell Differentiation and Therapeutics Applications
4.1. Metal–Organic Framework
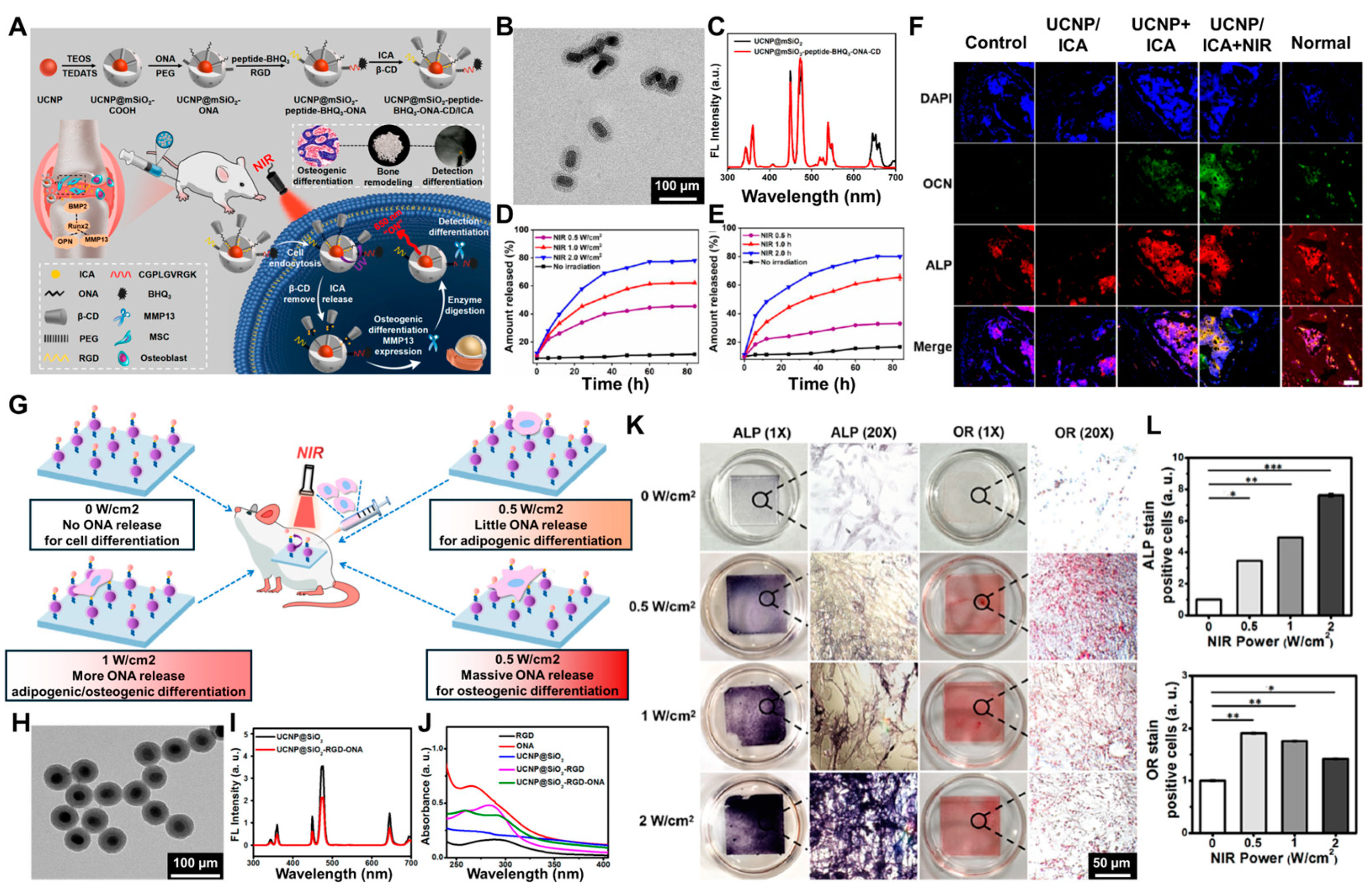
4.2. Upconversion Nanoparticles
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cahan, P.; Li, H.; Morris, S.A.; Lummertz da Rocha, E.L.; Daley, G.Q.; Collins, J.J. CellNet: Network biology applied to stem cell engineering. Cell 2014, 158, 903–915. [Google Scholar] [CrossRef]
- Hsu, M.-N.; Chang, Y.-H.; Truong, V.A.; Lai, P.-L.; Nguyen, T.K.N.; Hu, Y.-C. CRISPR technologies for stem cell engineering and regenerative medicine. Biotechnol. Adv. 2019, 37, 107447. [Google Scholar] [CrossRef] [PubMed]
- Kerativitayanan, P.; Carrow, J.K.; Gaharwar, A.K. Nanomaterials for engineering stem cell responses. Adv. Healthc. Mater. 2015, 4, 1600–1627. [Google Scholar] [CrossRef] [PubMed]
- Peerani, R.; Zandstra, P.W. Enabling stem cell therapies through synthetic stem cell–niche engineering. J. Clin. Investig. 2010, 120, 60–70. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.; Hellwarth, P.B.; Bao, X. Biomaterials for stem cell engineering and biomanufacturing. Bioact. Mater. 2019, 4, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.; Jang, Y.; Yoo, J.; Seong, H. Surface modification strategies for biomedical applications: Enhancing cell–biomaterial interfaces and biochip performances. BioChip J. 2023, 17, 174–191. [Google Scholar] [CrossRef]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal stem cell immunomodulation: Mechanisms and therapeutic potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef]
- Lee, B.-C.; Kang, K.-S. Functional enhancement strategies for immunomodulation of mesenchymal stem cells and their therapeutic application. Stem Cell Res. Ther. 2020, 11, 397. [Google Scholar] [CrossRef]
- Shin, M.; Kim, S.; Melvin, A.A.; Choi, J.-W. Towards nanomaterial-incorporated soft actuators: From inorganic/organic material-based soft robot to biomaterial-based biohybrid robot. BioChip J. 2024, 18, 68–84. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Yan, E.; Do, M.; Kim, Y.; Lee, Y.; Cho, S.-G.; Kim, D.-H. Engineering extracellular vesicles for ROS scavenging and tissue regeneration. Nano Converg. 2024, 11, 24. [Google Scholar] [CrossRef]
- Han, S.; Cruz, S.H.; Park, S.; Shin, S.R. Nano-biomaterials and advanced fabrication techniques for engineering skeletal muscle tissue constructs in regenerative medicine. Nano Converg. 2023, 10, 48. [Google Scholar] [CrossRef]
- Kharbikar, B.N.; Mohindra, P.; Desai, T.A. Biomaterials to enhance stem cell transplantation. Cell Stem Cell 2022, 29, 692–721. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, P.; Zhang, X.; Lv, L.; Zhou, Y. Advances in mesenchymal stem cell transplantation for the treatment of osteoporosis. Cell Prolif. 2021, 54, e12956. [Google Scholar] [CrossRef]
- Sehgal, A.; Hoda, D.; Riedell, P.A.; Ghosh, N.; Hamadani, M.; Hildebrandt, G.C.; Godwin, J.E.; Reagan, P.M.; Wagner-Johnston, N.; Essell, J.; et al. Lisocabtagene maraleucel as second-line therapy in adults with relapsed or refractory large B-cell lymphoma who were not intended for haematopoietic stem cell transplantation (PILOT): An open-label, phase 2 study. Lancet Oncol. 2022, 23, 1066–1077. [Google Scholar] [CrossRef]
- Alexander, T.; Greco, R.; Snowden, J.A. Hematopoietic stem cell transplantation for autoimmune disease. Annu. Rev. Med. 2021, 72, 215–228. [Google Scholar] [CrossRef]
- Abramson, M.H.; Gutgarts, V.; Zheng, J.; Maloy, M.A.; Ruiz, J.D.; Scordo, M.; Jaimes, E.A.; Sathick, I.J. Acute kidney injury in the modern era of allogeneic hematopoietic stem cell transplantation. Clin. J. Am. Soc. Nephrol. 2021, 16, 1318–1327. [Google Scholar] [CrossRef]
- Zipser, C.M.; Cragg, J.J.; Guest, J.D.; Fehlings, M.G.; Jutzeler, C.R.; Anderson, A.J.; Curt, A. Cell-based and stem-cell-based treatments for spinal cord injury: Evidence from clinical trials. Lancet Neurol. 2022, 21, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Yue, G.; Gao, S.; Ju, R.; Wang, Y. Human umbilical cord blood mononuclear cells transplantation for perinatal brain injury. Stem Cell Res. Ther. 2022, 13, 458. [Google Scholar] [CrossRef]
- Li, Y.; Hao, J.; Hu, Z.; Yang, Y.-G.; Zhou, Q.; Sun, L.; Wu, J. Current status of clinical trials assessing mesenchymal stem cell therapy for graft versus host disease: A systematic review. Stem Cell Res. Ther. 2022, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, H.; Cheng, L.; Wang, B. Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell Biosci. 2021, 11, 187. [Google Scholar] [CrossRef]
- Dholaria, B.; Savani, B.N.; Hamilton, B.K.; Oran, B.; Liu, H.D.; Tallman, M.S.; Ciurea, S.O.; Holtzman, N.G.; Ii, G.L.P.; Devine, S.M.; et al. Hematopoietic cell transplantation in the treatment of newly diagnosed adult acute myeloid leukemia: An evidence-based review from the American Society of Transplantation and cellular Therapy. Transplant. Cell. Ther. 2021, 27, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fang, S.; Ding, Q.; Jiang, R.; He, J.; Wang, Q.; Jin, Y.; Huang, X.; Liu, S.; Capitano, M.L.; et al. ADGRG1 enriches for functional human hematopoietic stem cells following ex vivo expansion–induced mitochondrial oxidative stress. J. Clin. Investig. 2021, 131, e148329. [Google Scholar] [CrossRef]
- Xiao, Y.; McGuinness, C.S.; Doherty-Boyd, W.S.; Salmeron-Sanchez, M.; Donnelly, H.; Dalby, M.J. Current insights into the bone marrow niche: From biology in vivo to bioengineering ex vivo. Biomaterials 2022, 286, 121568. [Google Scholar] [CrossRef]
- Hwang, N.S.; Varghese, S.; Elisseeff, J. Controlled differentiation of stem cells. Adv. Drug Deliv. Rev. 2008, 60, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Shichino, Y.; Abe, T.; Suetsugu, T.; Omori, A.; Kiyonari, H.; Iwasaki, S.; Matsuzaki, F. Selective translation of epigenetic modifiers affects the temporal pattern and differentiation of neural stem cells. Nat. Commun. 2022, 13, 470. [Google Scholar] [CrossRef]
- Lee, M.R.; Kwon, K.W.; Jung, H.; Kim, H.N.; Suh, K.Y.; Kim, K.; Kim, K.-S. Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. Biomaterials 2010, 31, 4360–4366. [Google Scholar] [CrossRef] [PubMed]
- Zouani, O.F.; Chanseau, C.; Brouillaud, B.; Bareille, R.; Deliane, F.; Foulc, M.-P.; Mehdi, A.; Durrieu, M.-C. Altered nanofeature size dictates stem cell differentiation. J. Cell Sci. 2012, 125, 1217–1224. [Google Scholar] [CrossRef]
- Higuchi, A.; Ling, Q.-D.; Chang, Y.; Hsu, S.-T.; Umezawa, A. Physical cues of biomaterials guide stem cell differentiation fate. Chem. Rev. 2013, 113, 3297–3328. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Liu, T.; Jin, Q.; Liu, H. Extracellular vesicles carrying RUNX3 promote differentiation of dental pulp stem cells. Tissue Eng. Regen. Med. 2024, 21, 111–122. [Google Scholar] [CrossRef]
- Ito, K.; Ito, K. Metabolism and the control of cell fate decisions and stem cell renewal. Annu. Rev. Cell Dev. Biol. 2016, 32, 399–409. [Google Scholar] [CrossRef]
- Prakash, N.; Kim, J.; Jeon, J.; Kim, S.; Arai, Y.; Bello, A.B.; Park, H.; Lee, S.-H. Progress and emerging techniques for biomaterial-based derivation of mesenchymal stem cells (MSCs) from pluripotent stem cells (PSCs). Biomater. Res. 2023, 27, 31. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.T.; Conklin, B.; Stein, J.B.; Pan, G.; Lee, K.-B. Nanotechnology-enabled immunoengineering approaches to advance therapeutic applications. Nano Converg. 2022, 9, 19. [Google Scholar] [CrossRef]
- Vallier, L.; Touboul, T.; Brown, S.; Cho, C.; Bilican, B.; Alexander, M.; Cedervall, J.; Chandran, S.; Ährlund-Richter, L.; Weber, A.; et al. Signaling pathways controlling pluripotency and early cell fate decisions of human induced pluripotent stem cells. Stem Cells 2009, 27, 2655–2666. [Google Scholar] [CrossRef]
- Byun, H.; Jang, G.N.; Hong, M.-H.; Yeo, J.; Shin, H.; Kim, W.J.; Shin, H. Biomimetic anti-inflammatory and osteogenic nanoparticles self-assembled with mineral ions and tannic acid for tissue engineering. Nano Converg. 2022, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Z.; Li, J.; Yang, S.; Zhang, Y.; Yao, B.; Song, W.; Fu, X.; Huang, S. Stiffness-mediated mesenchymal stem cell fate decision in 3D-bioprinted hydrogels. Burns Trauma 2020, 8, tkaa029. [Google Scholar] [CrossRef] [PubMed]
- Hayward, M.-K.; Muncie, J.M.; Weaver, V.M. Tissue mechanics in stem cell fate, development, and cancer. Dev. Cell 2021, 56, 1833–1847. [Google Scholar] [CrossRef]
- Simunovic, M.; Siggia, E.D.; Brivanlou, A.H. In vitro attachment and symmetry breaking of a human embryo model assembled from primed embryonic stem cells. Cell Stem Cell 2022, 29, 962–972.e4. [Google Scholar] [CrossRef]
- Northcote-Smith, J.; Suntharalingam, K. Targeting chemotherapy-resistant tumour sub-populations using inorganic chemistry: Anti-cancer stem cell metal complexes. Curr. Opin. Chem. Biol. 2023, 72, 102237. [Google Scholar] [CrossRef]
- Raghav, P.K.; Mann, Z.; Ahlawat, S.; Mohanty, S. Mesenchymal stem cell-based nanoparticles and scaffolds in regenerative medicine. Eur. J. Pharmacol. 2022, 918, 174657. [Google Scholar] [CrossRef]
- Asadniaye Fardjahromi, M.; Nazari, H.; Ahmadi Tafti, S.M.; Razmjou, A.; Mukhopadhyay, S.; Warkiani, M.E. Metal–organic framework-based nanomaterials for bone tissue engineering and wound healing. Mater. Today Chem. 2022, 23, 100670. [Google Scholar] [CrossRef]
- Ma, X.; Luan, Z.; Li, J. Inorganic nanoparticles-based systems in biomedical applications of stem cells: Opportunities and challenges. Int. J. Nanomed. 2023, 18, 143–182. [Google Scholar] [CrossRef]
- Bao, L.; Cui, X.; Mortimer, M.; Wang, X.; Wu, J.; Chen, C. The renaissance of one-dimensional carbon nanotubes in tissue engineering. Nano Today 2023, 49, 101784. [Google Scholar] [CrossRef]
- Koo, K.-M.; Go, Y.-H.; Kim, S.-M.; Kim, C.-D.; Do, J.T.; Kim, T.-H.; Cha, H.-J. Label-free and non-destructive identification of naïve and primed embryonic stem cells based on differences in cellular metabolism. Biomaterials 2023, 293, 121939. [Google Scholar] [CrossRef]
- Ju, F.N.; Kim, C.-H.; Lee, K.-H.; Kim, C.-D.; Lim, J.; Lee, T.; Park, C.G.; Kim, T.-H. Gold nanostructure-integrated conductive microwell arrays for uniform cancer spheroid formation and electrochemical drug screening. Biosens. Bioelectron. 2023, 222, 115003. [Google Scholar] [CrossRef]
- Cho, Y.-W.; Park, J.-H.; Kang, M.-J.; Lee, J.-H.; Kim, Y.K.; Luo, Z.; Kim, T.-H. Electrochemical detection of dopamine release from living neurons using graphene oxide-incorporated polypyrrole/gold nanocluster hybrid nanopattern arrays. Small 2023, 19, e2304271. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, M.; Sun, X.; Feliu, N.; Feng, L.; Parak, W.J.; Liu, S. Quantitative comparison of gold nanoparticle delivery via the enhanced permeation and retention (EPR) effect and mesenchymal stem cell (MSC)-based targeting. ACS Nano 2023, 17, 2039–2052. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lee, M.; Kwon, S.; Kim, J.; Kwon, Y. Systematic and mechanistic analysis of AuNP-induced nanotoxicity for risk assessment of nanomedicine. Nano Converg. 2022, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-D.; Koo, K.-M.; Kim, H.; Kim, H.-J.; Kim, T.-H. Thermally annealed large-scale gold nanostructure platform for long-term and label-free electrochemical monitoring of cellular metabolism. Chem. Eng. J. 2024, 485, 149864. [Google Scholar] [CrossRef]
- Liu, D.; Lu, G.; Shi, B.; Ni, H.; Wang, J.; Qiu, Y.; Yang, L.; Zhu, Z.; Yi, X.; Du, X. ROS-scavenging hydrogels synergize with neural stem cells to enhance spinal cord injury repair via regulating microenvironment and facilitating nerve regeneration. Adv. Healthc. Mater. 2023, 12, 2300123. [Google Scholar] [CrossRef]
- Shahabad, Z.A.; Avci, C.B.; Bani, F.; Zarebkohan, A.; Sadeghizadeh, M.; Salehi, R.; Ghafarkhani, M.; Rahbarghazi, R.; Bagca, B.G.; Ozates, N.P. Photothermal effect of albumin-modified gold nanorods diminished neuroblastoma cancer stem cells dynamic growth by modulating autophagy. Sci. Rep. 2022, 12, 11774. [Google Scholar] [CrossRef]
- Koo, K.-M.; Kim, C.-D.; Kim, H.; Cho, Y.-W.; Suhito, I.R.; Kim, T.-H. Extracellularly detectable electrochemical signals of living cells originate from metabolic reactions. Adv. Sci. 2023, 10, e2207084. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Wang, C.-P.J.; Lee, H.-J.; Hong, K.S.; Ahn, J.H.; Cho, Y.-W.; Lee, J.-H.; Seo, H.S.; Park, W.; Kim, S.-N.; et al. Uniform gold nanostructure formation via weakly adsorbed gold films and thermal annealing for reliable localized surface plasmon resonance-based detection of DNase-I. Small 2023, 19, 2302023. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-W.; Tsai, K.-C.; Shrestha, L.K.; Ariga, K.; Hsu, S.-H. Effects of hydrophilic fullerene nanoarchitectured structures on the behaviour of neural stem cells. Nanoscale 2022, 14, 11152–11161. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, P.; Gaur, S.; Yadav, H.; Urgunde, A.B.; Singh, V.; Patel, A.; Vishwakarma, K.; Kalirawana, D.; Gupta, R.; Kumar, P. 2D materials: Increscent quantum flatland with immense potential for applications. Nano Converg. 2022, 9, 26. [Google Scholar] [CrossRef]
- Rajendran, A.K.; Sankar, D.; Amirthalingam, S.; Kim, H.D.; Rangasamy, J.; Hwang, N.S. Trends in mechanobiology guided tissue engineering and tools to study cell-substrate interactions: A brief review. Biomater. Res. 2023, 27, 55. [Google Scholar] [CrossRef]
- Baheiraei, N.; Razavi, M.; Ghahremanzadeh, R. Reduced graphene oxide coated alginate scaffolds: Potential for cardiac patch application. Biomater. Res. 2023, 27, 109. [Google Scholar] [CrossRef]
- Kim, J.; Kang, M.S.; Jun, S.W.; Jo, H.J.; Han, D.-W.; Kim, C.-S. A systematic study on the use of multifunctional nanodiamonds for neuritogenesis and super-resolution imaging. Biomater. Res. 2023, 27, 37. [Google Scholar] [CrossRef]
- He, Q.; Wu, Z.; Zhang, L. Carbon dots as a new class of multifunctional nanomaterial in mesenchymal stem cells: Opportunities and challenges. J. Mater. Chem. B 2023, 11, 3511–3536. [Google Scholar] [CrossRef]
- Shin, J.; Kang, N.; Kim, B.; Hong, H.; Yu, L.; Kim, J.; Kang, H.; Kim, J.S. One-dimensional nanomaterials for cancer therapy and diagnosis. Chem. Soc. Rev. 2023, 52, 4488–4514. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, Z.; Wu, Q.; Gao, Z.; Mi, G.; Wang, R.; Sun, H.-B.; Zhao, Y.; Du, Y. Intelligent wearable devices based on nanomaterials and nanostructures for healthcare. Nanoscale 2023, 15, 405–433. [Google Scholar] [CrossRef]
- Meng, L.; Ren, N.; Dong, M.; Zhang, S.; Wang, A.; Zhuang, Z.; Wang, J.; Sun, C.; Liu, H. Metal–organic frameworks for nerve repair and neural stem cell therapy. Adv. Funct. Mater. 2024, 34, 2309974. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.; Luan, Z.; Zhao, Q.; Yang, A. NIR triggered release of NO by upconversion-based nanoplatforms to enhance osteogenic differentiation of mesenchymal stem cells for OP therapy. Biomater. Res. 2024, 28, 58. [Google Scholar]
- Xie, L.; Zhang, C.; Liu, M.; Huang, J.; Jin, X.; Zhu, C.; Lv, M.; Yang, N.; Chen, S.; Shao, M.; et al. Nucleus-targeting manganese dioxide nanoparticles coated with the human umbilical cord mesenchymal stem cell membrane for cancer cell therapy. ACS Appl. Mater. Interfaces 2023, 15, 10541–10553. [Google Scholar] [CrossRef]
- Kučuk, N.; Primožič, M.; Knez, Ž.; Leitgeb, M. Sustainable biodegradable biopolymer-based nanoparticles for healthcare applications. Int. J. Mol. Sci. 2023, 24, 3188. [Google Scholar] [CrossRef]
- Rahmani Del Bakhshayesh, A.; Saghebasl, S.; Asadi, N.; Kashani, E.; Mehdipour, A.; Nezami Asl, A.; Akbarzadeh, A. Recent advances in nano-scaffolds for tissue engineering applications: Toward natural therapeutics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1882. [Google Scholar] [CrossRef]
- Hu, J.; Li, C.; Yang, Z.; Wu, Q.; Wang, J.; Xu, Z.; Chen, Y.; Wan, Q.; Shuai, Y.; Yang, S.; et al. Hierarchically patterned protein scaffolds with nano-fibrillar and micro-lamellar structures modulate neural stem cell homing and promote neuronal differentiation. Biomater. Sci. 2023, 11, 7663–7677. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Ragab, M.H.; Galhom, R.A.; Hassan, H.Y. Evaluation of dental pulp stem cells behavior after odontogenic differentiation induction by three different bioactive materials on two different scaffolds. BMC Oral Health 2023, 23, 252. [Google Scholar] [CrossRef]
- Wang, M.; Yao, J.; Shen, S.; Heng, C.; Zhang, Y.; Yang, T.; Zheng, X. A scaffold with zinc–whitlockite nanoparticles accelerates bone reconstruction by promoting bone differentiation and angiogenesis. Nano Res. 2023, 16, 757–770. [Google Scholar] [CrossRef]
- Szwed-Georgiou, A.; Płociński, P.; Kupikowska-Stobba, B.; Urbaniak, M.M.; Rusek-Wala, P.; Szustakiewicz, K.; Piszko, P.; Krupa, A.; Biernat, M.; Gazińska, M.; et al. Bioactive materials for bone regeneration: Biomolecules and delivery systems. ACS Biomater. Sci. Eng. 2023, 9, 5222–5254. [Google Scholar] [CrossRef]
- Luan, X.; Kong, H.; He, P.; Yang, G.; Zhu, D.; Guo, L.; Wei, G. Self-assembled peptide-based nanodrugs: Molecular design, synthesis, functionalization, and targeted tumor bioimaging and biotherapy. Small 2023, 19, e2205787. [Google Scholar] [CrossRef]
- Duan, X.; Luo, Y.; Zhang, R.; Zhou, H.; Xiong, W.; Li, R.; Huang, Z.; Luo, L.; Rong, S.; Li, M.; et al. ZIF-8 as a protein delivery system enhances the application of dental pulp stem cell lysate in anti-photoaging therapy. Mater. Today Adv. 2023, 17, 100336. [Google Scholar] [CrossRef]
- Montazersaheb, P.; Pishgahzadeh, E.; Jahani, V.B.; Farahzadi, R.; Montazersaheb, S. Magnetic nanoparticle-based hyperthermia: A prospect in cancer stem cell tracking and therapy. Life Sci. 2023, 323, 121714. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Ma, M.; Pan, X.; Shafiq, M.; Yu, H.; Chen, H. Cobalt protoporphyrin-induced nano-self-assembly for CT imaging, magnetic-guidance, and antioxidative protection of stem cells in pulmonary fibrosis treatment. Bioact. Mater. 2023, 21, 129–141. [Google Scholar] [CrossRef]
- Dong, Z.; Lin, Y.; Xu, S.; Chang, L.; Zhao, X.; Mei, X.; Gao, X. NIR-triggered tea polyphenol-modified gold nanoparticles-loaded hydrogel treats periodontitis by inhibiting bacteria and inducing bone regeneration. Mater. Des. 2023, 225, 111487. [Google Scholar] [CrossRef]
- Shin, M.; Lim, J.; An, J.; Yoon, J.; Choi, J.-W. Nanomaterial-based biohybrid hydrogel in bioelectronics. Nano Converg. 2023, 10, 8. [Google Scholar] [CrossRef]
- Mei, H.; Liu, H.; Sha, C.; Lv, Q.; Song, Q.; Jiang, L.; Tian, E.; Gao, Z.; Li, J.; Zhou, J. Multifunctional metal–phenolic composites promote efficient periodontitis treatment via antibacterial and osteogenic properties. ACS Appl. Mater. Interfaces 2024, 16, 13573–13584. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, W.; Yang, H.; Liu, F.; Kong, Y.; Wang, W.; Zhao, H.; Ma, W.; Sang, Y.; Yi, F.; et al. Calcium folate nanoparticles as dual-functional neural inducing factors to promote the differentiation of neural stem cells into cholinergic neurons. Adv. Funct. Mater. 2023, 33, 2208835. [Google Scholar] [CrossRef]
- Georgas, E.; Yuan, M.; Chen, J.; Wang, Y.; Qin, Y.-X. Bioactive superparamagnetic iron oxide-gold nanoparticles regulated by a dynamic magnetic field induce neuronal Ca2+ influx and differentiation. Bioact. Mater. 2023, 26, 478–489. [Google Scholar] [CrossRef]
- Han, G.H.; Ko, W.-K.; Kim, S.J.; Lee, D.; Jeong, D.; Han, I.; Sheen, S.H.; Sohn, S. Neuron-inducing therapy using embryonic neural progenitor cells embedding positively charged gold nanoparticles in rats with complete spinal cord injury. Clin. Transl. Med. 2022, 12, e981. [Google Scholar] [CrossRef]
- Kang, X.; Wang, Y.; Cai, X.-L.; Hua, Y.; Shao, Z.-H.; Chen, X.; Zhao, X.; Zang, S.-Q. Chiral gold clusters functionalized two-dimensional nanoparticle films to regulate the adhesion and differentiation of stem cells. J. Colloid Interface Sci. 2022, 625, 831–838. [Google Scholar] [CrossRef]
- Hung, H.-S.; Yang, Y.-C.; Chang, C.-H.; Chang, K.-B.; Shen, C.-C.; Tang, C.-L.; Liu, S.-Y.; Lee, C.-H.; Yen, C.-M.; Yang, M.-Y. Neural differentiation potential of mesenchymal stem cells enhanced by biocompatible chitosan-gold nanocomposites. Cells 2022, 11, 1861. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Zhao, J.; Xu, Z.; Chen, C.; Xu, L.; Xu, C.; Sun, M.; Kuang, H. Chiral nanoparticles force neural stem cell differentiation to alleviate Alzheimer’s disease. Adv. Sci. 2022, 9, e2202475. [Google Scholar] [CrossRef]
- Luo, J.; Zhu, S.; Tong, Y.; Zhang, Y.; Li, Y.; Cao, L.; Kong, M.; Luo, M.; Bi, Q.; Zhang, Q. Cerium oxide nanoparticles promote osteoplastic precursor differentiation by activating the Wnt pathway. Biol. Trace Elem. Res. 2023, 201, 865–873. [Google Scholar] [CrossRef]
- Algazlan, A.S.; Almuraikhi, N.; Muthurangan, M.; Balto, H.; Alsalleeh, F. Silver nanoparticles alone or in combination with calcium hydroxide modulate the viability, attachment, migration, and osteogenic differentiation of human mesenchymal stem cells. Int. J. Mol. Sci. 2022, 24, 702. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Cao, J.; Wang, Y.; Anwar, N.; Zhang, Z.; Zhang, D.; Ma, Y.; Xiao, Y.; Xiao, L.; et al. The role of autophagy in bone metabolism and clinical significance. Autophagy 2023, 19, 2409–2427. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.; Ma, L.; Jing, J.; Feng, J.; Yuan, Y.; Guo, T.; Han, X.; Ho, T.-V.; Lei, J.; He, J.; et al. Sensory nerve niche regulates mesenchymal stem cell homeostasis via FGF/mTOR/autophagy axis. Nat. Commun. 2023, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, J.; Zhang, B.-W.; Yang, L.; Wan, Y.-C.; Chen, B.-B.; Xu, N.; Xu, Q.-R.; Fan, J.; Shang, J.-N.; et al. ATG5 attenuates inflammatory signaling in mouse embryonic stem cells to control differentiation. Dev. Cell 2024, 59, 882–897.e6. [Google Scholar] [CrossRef]
- Borsa, M.; Obba, S.; Richter, F.C.; Zhang, H.; Riffelmacher, T.; Carrelha, J.; Alsaleh, G.; Jacobsen, S.E.W.; Simon, A.K. Autophagy preserves hematopoietic stem cells by restraining MTORC1-mediated cellular anabolism. Autophagy 2024, 20, 45–57. [Google Scholar] [CrossRef]
- Li, X.; Guo, L.; Chen, J.; Liang, H.; Liu, Y.; Chen, W.; Zhou, L.; Shan, L.; Wang, H. Intravenous injection of human umbilical cord-derived mesenchymal stem cells ameliorates not only blood glucose but also nephrotic complication of diabetic rats through autophagy-mediated anti-senescent mechanism. Stem Cell Res. Ther. 2023, 14, 146. [Google Scholar] [CrossRef]
- Wu, Y.; Li, L.; Ning, Z.; Li, C.; Yin, Y.; Chen, K.; Li, L.; Xu, F.; Gao, J. Autophagy-modulating biomaterials: Multifunctional weapons to promote tissue regeneration. Cell Commun. Signal. 2024, 22, 124. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Wang, Y.; Liu, X.; Zhu, W.; Jiang, F.; Li, S.; Liu, L. AuNP-loaded electrospinning membrane cooperated with CDs for periodontal tissue engineering. Tissue Eng. Regen. Med. 2023, 20, 1091–1108. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M.; Jomaa, A.A.; Kamoun, M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Univ. Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Tian, B.-M.; Li, X.; Yu, Y.-C.; Deng, D.-K.; Sun, L.-J.; Qu, H.-L.; Wu, R.-X.; Xu, X.-Y.; Sun, H.-H.; et al. Gold nanoparticles targeting the autophagy–lysosome system to combat the inflammation-compromised osteogenic potential of periodontal ligament stem cells: From mechanism to therapy. Biomaterials 2022, 288, 121743. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, P.E.; Ransohoff, J.D.; Nahid, A.; Wu, J.C. Immunogenicity of pluripotent stem cells and their derivatives. Circ. Res. 2013, 112, 549–561. [Google Scholar] [CrossRef]
- Kurtuldu, F.; Mutlu, N.; Boccaccini, A.R.; Galusek, D. Gallium containing bioactive materials: A review of anticancer, antibacterial, and osteogenic properties. Bioact. Mater. 2022, 17, 125–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hu, Y.; Hou, Y.; Li, M.; Tan, L.; Chen, M.; Geng, W.; Tao, B.; Jiang, H.; Luo, Z.; et al. Magnesium/gallium-layered nanosheets on titanium implants mediate osteogenic differentiation of MSCs and osseointegration under osteoporotic condition. Chem. Eng. J. 2022, 427, 130982. [Google Scholar] [CrossRef]
- Paul, M.K.; Bisht, B.; Darmawan, D.O.; Chiou, R.; Ha, V.L.; Wallace, W.D.; Chon, A.T.; Hegab, A.E.; Grogan, T.; Elashoff, D.A.; et al. Dynamic changes in intracellular ROS Levels Regulate airway basal stem cell homeostasis through Nrf2-dependent Notch signaling. Cell Stem Cell 2014, 15, 199–214. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Salah, M.; Akasaka, H.; Shimizu, Y.; Morita, K.; Nishimura, Y.; Kubota, H.; Kawaguchi, H.; Sogawa, T.; Mukumoto, N.; Ogino, C.; et al. Reactive oxygen species-inducing titanium peroxide nanoparticles as promising radiosensitizers for eliminating pancreatic cancer stem cells. J. Exp. Clin. Cancer Res. 2022, 41, 146. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, W.; Cao, L.; Tian, X.; Tian, G.; Chen, M.; Ma, L.; Liu, X.; Yuan, Z.; Cheng, C.; et al. Multifaceted catalytic ROS-scavenging via electronic modulated metal oxides for regulating stem cell fate. Adv. Mater. 2022, 34, e2207275. [Google Scholar] [CrossRef]
- Atashi, F.; Modarressi, A.; Pepper, M.S. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells Dev. 2015, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Lv, Y.; Li, X.; Bao, H.; Cao, X.; Huang, J.; Zhang, Z. SOD-functionalized gold nanoparticles as ROS scavenger and CT contrast agent for protection and imaging tracking of mesenchymal stem cells in idiopathic pulmonary fibrosis treatment. Chem. Eng. J. 2023, 459, 141603. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, T.; Che, M.; Wang, Y.; He, C.; Liu, L.; Lv, Z.; Xiao, C.; Wang, H.; Zhang, S. Recent advances in nanomaterials for the treatment of spinal cord injury. Mater. Today Bio 2023, 18, 100524. [Google Scholar] [CrossRef]
- Hu, X.; Wang, T.; Li, F.; Mao, X. Surface modifications of biomaterials in different applied fields. RSC Adv. 2023, 13, 20495–20511. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Asil, S.M.; Guerrero, E.D.; Bugarini, G.; Cayme, J.; De Avila, N.; Garcia, J.; Hernandez, A.; Mecado, J.; Madero, Y.; Moncayo, F.; et al. Theranostic applications of multifunctional carbon nanomaterials. View 2023, 4, 20220056. [Google Scholar] [CrossRef]
- Conklin, B.; Conley, B.M.; Hou, Y.; Chen, M.; Lee, K.-B. Advanced theragnostics for the central nervous system (CNS) and neurological disorders using functional inorganic nanomaterials. Adv. Drug Deliv. Rev. 2023, 192, 114636. [Google Scholar] [CrossRef]
- Olate-Moya, F.; Rubí-Sans, G.; Engel, E.; Mateos-Timoneda, M.Á.; Palza, H. 3D bioprinting of biomimetic alginate/gelatin/chondroitin sulfate hydrogel nanocomposites for intrinsically chondrogenic differentiation of human mesenchymal stem cells. Biomacromolecules 2024, 25, 3312–3324. [Google Scholar] [CrossRef]
- An, N.; Yan, X.; Qiu, Q.; Zhang, Z.; Zhang, X.; Zheng, B.; Zhao, Z.; Guo, J.; Liu, Y. Human periodontal ligament stem cell sheets activated by graphene oxide quantum dots repair periodontal bone defects by promoting mitochondrial dynamics dependent osteogenic differentiation. J. Nanobiotechnol. 2024, 22, 133. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuang, J.; Wei, C.; Jin, C.; Zhu, M.; Zhao, S.; Xie, H. Enhancing osteogenic differentiation of dental pulp stem cells with covalently bonded all-carbon scaffolds. Adv. Funct. Mater. 2024, 34, 2400766. [Google Scholar] [CrossRef]
- Nascimento, L.; Fernandes, C.; Silva, R.M.; Semitela, Â.; de Sousa, B.M.; Marques, P.A.A.P.; Vieira, S.I.; Silva, R.F.; Barroca, N.; Gonçalves, G. Customizing 3D structures of vertically aligned carbon nanotubes to direct neural stem cell differentiation. Adv. Healthc. Mater. 2023, 12, e2300828. [Google Scholar] [CrossRef]
- Nekounam, H.; Samadian, H.; Golmohammadi, H.; Asghari, F.; Shokrgozar, M.A.; Ahadian, S.; Majidi, R.F. Carbon nanofibers fabrication, surface modifications, and application as the innovative substrate for electrical stimulation of neural cell differentiation. Surf. Interfaces 2023, 40, 102926. [Google Scholar] [CrossRef]
- Tufan, Y.; Öztatlı, H.; Doganay, D.; Buyuksungur, A.; Cicek, M.O.; Döş, İ.T.; Berberoğlu, Ç.; Unalan, H.E.; Garipcan, B.; Ercan, B. Multifunctional silk fibroin/carbon nanofiber scaffolds for in vitro cardiomyogenic differentiation of induced pluripotent stem cells and energy harvesting from simulated cardiac motion. ACS Appl. Mater. Interfaces 2023, 15, 42271–42283. [Google Scholar] [CrossRef]
- Li, Y.-M.; Patel, K.D.; Han, Y.-K.; Hong, S.-M.; Meng, Y.-X.; Lee, H.-H.; Park, J.H.; Knowles, J.C.; Hyun, J.K.; Lee, J.-H.; et al. Electroconductive and mechano-competent PUCL@CNT nanohybrid scaffolds guiding neuronal specification of neural stem/progenitor cells. Chem. Eng. J. 2023, 466, 143125. [Google Scholar] [CrossRef]
- Kozlowska, U.; Nichols, C.; Wiatr, K.; Figiel, M. From psychiatry to neurology: Psychedelics as prospective therapeutics for neurodegenerative disorders. J. Neurochem. 2022, 162, 89–108. [Google Scholar] [CrossRef]
- Putra, V.D.L.; Kilian, K.A.; Knothe Tate, M.L. Biomechanical, biophysical and biochemical modulators of cytoskeletal remodelling and emergent stem cell lineage commitment. Commun. Biol. 2023, 6, 75. [Google Scholar] [CrossRef]
- Afjeh-Dana, E.; Naserzadeh, P.; Moradi, E.; Hosseini, N.; Seifalian, A.M.; Ashtari, B. Stem cell differentiation into cardiomyocytes: Current methods and emerging approaches. Stem Cell Rev. Rep. 2022, 18, 2566–2592. [Google Scholar] [CrossRef] [PubMed]
- Guillot-Ferriols, M.; Lanceros-Méndez, S.; Gómez Ribelles, J.L.; Gallego Ferrer, G. Electrical stimulation: Effective cue to direct osteogenic differentiation of mesenchymal stem cells? Biomater. Adv. 2022, 138, 212918. [Google Scholar] [CrossRef]
- Liang, L.; Liu, C.; Cai, P.; Han, S.; Zhang, R.; Ren, N.; Wang, J.; Yu, J.; Shang, S.; Zhou, W.; et al. Highly specific differentiation of MSCs into neurons directed by local electrical stimuli triggered wirelessly by electromagnetic induction nanogenerator. Nano Energy 2022, 100, 107483. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Zhang, Z.; Liu, H.; Li, L.; Wang, A.; Ouyang, J.; Xie, T.; Zhang, L.; Xue, J.; et al. Electrical stimulation system based on electroactive biomaterials for bone tissue engineering. Mater. Today 2023, 68, 177–203. [Google Scholar] [CrossRef]
- Guo, Z.; Sun, C.; Yang, H.; Gao, H.; Liang, N.; Wang, J.; Hu, S.; Ren, N.; Pang, J.; Wang, J. Regulation of neural differentiation of ADMSCs using graphene-mediated wireless-localized electrical signals driven by electromagnetic induction. Adv. Sci. 2022, 9, 2104424. [Google Scholar] [CrossRef]
- Rich, J.; Tian, Z.; Huang, T.J. Sonoporation: Past, present, and future. Adv. Mater. Technol. 2022, 7, 2100885. [Google Scholar] [CrossRef] [PubMed]
- Fraire, J.C.; Shaabani, E.; Sharifiaghdam, M.; Rombaut, M.; Hinnekens, C.; Hua, D.; Ramon, J.; Raes, L.; Bolea-Fernandez, E.; Brans, T.; et al. Light triggered nanoscale biolistics for efficient intracellular delivery of functional macromolecules in mammalian cells. Nat. Commun. 2022, 13, 1996. [Google Scholar] [CrossRef]
- Van de Vyver, T.; De Smedt, S.C.; Raemdonck, K. Modulating intracellular pathways to improve non-viral delivery of RNA therapeutics. Adv. Drug Deliv. Rev. 2022, 181, 114041. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Solak, K.; Han, Y.; Cho, Y.-W.; Koo, K.-M.; Kim, C.-D.; Luo, Z.; Son, H.; Kim, H.-R.; Mavi, A.; et al. Electrically controlled mRNA delivery using a polypyrrole-graphene oxide hybrid film to promote osteogenic differentiation of human mesenchymal stem cells. Nano Res. 2022, 15, 9253–9263. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Saleemi, M.A.; Hosseini Fouladi, M.; Yong, P.V.C.; Chinna, K.; Palanisamy, N.K.; Wong, E.H. Toxicity of carbon nanotubes: Molecular mechanisms, signaling cascades, and remedies in biomedical applications. Chem. Res. Toxicol. 2021, 34, 24–46. [Google Scholar] [CrossRef]
- Sun, M.; Li, P.; Qin, H.; Liu, N.; Ma, H.; Zhang, Z.; Li, J.; Lu, B.; Pan, X.; Wu, L. Liquid metal/CNTs hydrogel-based transparent strain sensor for wireless health monitoring of aquatic animals. Chem. Eng. J. 2023, 454, 140459. [Google Scholar] [CrossRef]
- Kim, S.D.; Kim, K.; Shin, M. Recent advances in 3D printable conductive hydrogel inks for neural engineering. Nano Converg. 2023, 10, 41. [Google Scholar] [CrossRef]
- Tringides, C.M.; Boulingre, M.; Khalil, A.; Lungjangwa, T.; Jaenisch, R.; Mooney, D.J. Tunable conductive hydrogel scaffolds for neural cell differentiation. Adv. Healthc. Mater. 2023, 12, e2202221. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, W.; Yin, H.; Chen, X.; Cai, J.; Guo, J.; Zhou, S.; Chai, R.; Tang, M. Super-aligned carbon nanotubes and GelMA hydrogel composite scaffolds promote spiral ganglion neuron growth and orientation. Mater. Today Nano 2022, 18, 100181. [Google Scholar] [CrossRef]
- Ha, T.; Park, S.; Shin, M.; Lee, J.-Y.; Choi, J.-H.; Choi, J.-W. Biosensing system for drug evaluation of amyotrophic lateral sclerosis based on muscle bundle and nano-biohybrid hydrogel composed of multiple motor neuron spheroids and carbon nanotubes. Chem. Eng. J. 2023, 463, 142284. [Google Scholar] [CrossRef]
- Mohammapdour, R.; Ghandehari, H. Mechanisms of immune response to inorganic nanoparticles and their degradation products. Adv. Drug Deliv. Rev. 2022, 180, 114022. [Google Scholar] [CrossRef]
- Park, B.; Oh, D.; Kim, J.; Kim, C. Functional photoacoustic imaging: From nano-and micro-to macro-scale. Nano Converg. 2023, 10, 29. [Google Scholar] [CrossRef]
- Zhao, Y.; Das, S.; Sekine, T.; Mabuchi, H.; Irie, T.; Sakai, J.; Wen, D.; Zhu, W.; Ben, T.; Negishi, Y. Record ultralarge-pores, low density three-dimensional covalent organic framework for controlled drug delivery. Angew. Chem. Int. Ed. Engl. 2023, 62, e202300172. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Q.; Wu, Y.; Li, X.; Zhou, Y.; Wang, Z.; Liang, H.; Ding, F.; Hong, S.; Steinmetz, N.F. Molecularly stimuli-responsive self-assembled peptide nanoparticles for targeted imaging and therapy. ACS Nano 2023, 17, 8004–8025. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Kong, H.; Sun, Z.; Xu, Y.; Han, P.; Xi, Y.; Wei, G. Recent advance in tailoring the structure and functions of self-assembled peptide nanomaterials for biomedical applications. Coord. Chem. Rev. 2023, 494, 215374. [Google Scholar] [CrossRef]
- Ren, N.; Liang, N.; Dong, M.; Feng, Z.; Meng, L.; Sun, C.; Wang, A.; Yu, X.; Wang, W.; Xie, J.; et al. Stem cell membrane-encapsulated zeolitic imidazolate Framework-8: A targeted nano-platform for osteogenic differentiation. Small 2022, 18, e2202485. [Google Scholar] [CrossRef]
- Liang, N.; Ren, N.; Feng, Z.; Sun, Z.; Dong, M.; Wang, W.; Liu, F.; Sun, C.; Zhou, W.; Xing, Z.; et al. Biomimetic metal−organic frameworks as targeted vehicles to enhance osteogenesis. Adv. Healthc. Mater. 2022, 11, e2102821. [Google Scholar] [CrossRef]
- Zhou, H.; Jing, S.; Xiong, W.; Zhu, Y.; Duan, X.; Li, R.; Peng, Y.; Kumeria, T.; He, Y.; Ye, Q. Metal–organic framework materials promote neural differentiation of dental pulp stem cells in spinal cord injury. J. Nanobiotechnol. 2023, 21, 316. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, H.; Liu, Z.; Liu, C.; Du, X.; Ren, J.; Qu, X. Hydrogen-bonded organic framework (HOF)-based single-neural stem cell encapsulation and transplantation to remodel impaired neural networks. Angew. Chem. Int. Ed. Engl. 2022, 61, e202201485. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, D.; Li, M.; He, Y.; He, T.; Chen, M.; Hu, Y.; Luo, Z.; Cai, K. Nanocatalytic biofunctional MOF coating on titanium implants promotes osteoporotic bone regeneration through cooperative pro-osteoblastogenesis MSC reprogramming. ACS Nano 2022, 16, 15397–15412. [Google Scholar] [CrossRef]
- Shu, C.; Qin, C.; Chen, L.; Wang, Y.; Shi, Z.; Yu, J.; Huang, J.; Zhao, C.; Huan, Z.; Wu, C.; et al. Metal–organic framework functionalized bioceramic scaffolds with antioxidative activity for enhanced osteochondral regeneration. Adv. Sci. 2023, 10, e2206875. [Google Scholar] [CrossRef]
- Jing, G.; Li, Y.; Sun, F.; Liu, Q.; Du, A.; Wang, H.; Niu, J.; Lu, J.; Qian, Y.; Wang, S. Near-infrared light-activatable upconversion nanoparticle/curcumin hybrid nanodrug: A potent strategy to induce the differentiation and elimination of glioma stem cells. Adv. Compos. Hybrid Mater. 2024, 7, 82. [Google Scholar] [CrossRef]
- Xia, G.; Wang, G.; Yang, H.; Wang, W.; Fang, J. Piezoelectric charge induced hydrophilic poly(L-lactic acid) nanofiber for electro-topographical stimulation enabling stem cell differentiation and expansion. Nano Energy 2022, 102, 107690. [Google Scholar] [CrossRef]
- Kim, N.H.; Chae, S.; Yi, S.A.; Sa, D.H.; Oh, S.; Kang, E.S.; Kim, S.; Choi, K.H.; Lee, J.; Choi, J.-Y.; et al. Peptide-assembled single-chain atomic crystal enhances pluripotent stem cell differentiation to neurons. Nano Lett. 2023, 23, 6859–6867. [Google Scholar] [CrossRef] [PubMed]
- Polo, Y.; Luzuriaga, J.; de Langarica, S.G.; Pardo-Rodríguez, B.; Martínez-Tong, D.E.; Tapeinos, C.; Manero-Roig, I.; Marin, E.; Muñoz-Ugartemendia, J.; Ciofani, G. Self-assembled three-dimensional hydrogels based on graphene derivatives and cerium oxide nanoparticles: Scaffolds for co-culture of oligodendrocytes and neurons derived from neural stem cells. Nanoscale 2023, 15, 4488–4505. [Google Scholar] [CrossRef]
- Suresh, D.; Suresh, A.; Kannan, R. Engineering biomolecular systems: Controlling the self-assembly of gelatin to form ultra-small bioactive nanomaterials. Bioact. Mater. 2022, 18, 321–336. [Google Scholar] [CrossRef]
- Si, Y.; Liu, H.; Li, M.; Jiang, X.; Yu, H.; Sun, D. An efficient metal–organic framework-based drug delivery platform for synergistic antibacterial activity and osteogenesis. J. Colloid Interface Sci. 2023, 640, 521–539. [Google Scholar] [CrossRef]
- Matusiak, J.; Przekora, A.; Franus, W. Zeolites and zeolite imidazolate frameworks on a quest to obtain the ideal biomaterial for biomedical applications: A review. Mater. Today 2023, 67, 495–517. [Google Scholar] [CrossRef]
- Gu, X.; Xu, X.; Jia, C.; Wang, J.; Zhang, J.; Gao, Q.; Chen, J. Molecular mechanisms involved in the regulation of neurodevelopment by miR-124. Mol. Neurobiol. 2023, 60, 3569–3583. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zheng, Y.; Wang, L.; Liu, Y.; Wang, X.; Li, Y.; Chi, G. miR-124: A promising therapeutic target for central nervous system injuries and diseases. Cell. Mol. Neurobiol. 2022, 42, 2031–2053. [Google Scholar] [CrossRef]
- Yang, H.; Han, M.; Li, J.; Ke, H.; Kong, Y.; Wang, W.; Wang, L.; Ma, W.; Qiu, J.; Wang, X.; et al. Delivery of miRNAs through metal–organic framework nanoparticles for assisting neural stem cell therapy for ischemic stroke. ACS Nano 2022, 16, 14503–14516. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-W.; Jee, S.; Suhito, I.R.; Lee, J.-H.; Park, C.G.; Choi, K.M.; Kim, T.-H. Single metal–organic framework–embedded nanopit arrays: A new way to control neural stem cell differentiation. Sci. Adv. 2022, 8, eabj7736. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion nanoparticles: Design, nanochemistry, and applications in theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Haase, M.; Schäfer, H. Upconverting nanoparticles. Angew. Chem. Int. Ed. Engl. 2011, 50, 5808–5829. [Google Scholar] [CrossRef]
- Ren, N.; Liang, N.; Yu, X.; Wang, A.; Xie, J.; Sun, C. Ligand-free upconversion nanoparticles for cell labeling and their effects on stem cell differentiation. Nanotechnology 2020, 31, 145101. [Google Scholar] [CrossRef]
- Yan, R.; Guo, Y.; Wang, X.; Liang, G.; Yang, A.; Li, J. Near-infrared light-controlled and real-time detection of osteogenic differentiation in mesenchymal stem cells by upconversion nanoparticles for osteoporosis therapy. ACS Nano 2022, 16, 8399–8418. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, R.; Wang, X.; Liang, G.; Yang, A.; Li, J. Near-infrared light-controlled activation of adhesive peptides regulates cell adhesion and multidifferentiation in mesenchymal stem cells on an up-conversion substrate. Nano Lett. 2022, 22, 2293–2302. [Google Scholar] [CrossRef]




| Nanomaterials | Lineages | Cells | Mechanism of Actions | Therapeutic Applications | Ref. |
|---|---|---|---|---|---|
| AuNPs | Osteogenic differentiation | MSCs | ROS scavenging | Periodontitis | [76] |
| CaFO NPs (calcium folate) | Neuronal differentiation | Mouse embryonic-derived NSCs | Decomposition into Ca2+ and folic acid | Alzheimer’s disease | [77] |
| SPIO-AuNPs | Neuronal differentiation | PC-12 | Dynamic magnetic field to Ca2+ influx | Alzheimer’s disease | [78] |
| AuNPs | Neuronal differentiation | Embryonic-derived NPCs | GFAP barriers from activated astrocytes | Spinal cord injury | [79] |
| L/D-Au nanocluster films | Adipogenic/ Osteogenic differentiation | MSCs | Chirality at cluster scale to control cellular behaviors | Functional maintenance in organisms | [80] |
| Chitosan-AuNPs | Neuronal differentiation | MSCs | Strengthen MSC colony formation | Neurodegenerative diseases | [81] |
| Chiral AuNPs | Neuronal differentiation | Mouse neural NSCs | CPL illumination to direct differentiation | Alzheimer’s disease | [82] |
| CeO2 NPs | Osteogenic differentiation | MC3T3-E1 | Activating the Wnt pathway | Bone metabolic disease | [83] |
| AgNPs-Ca(OH)2 | Osteogenic differentiation | MSCs | Upregulation of TGF-β1 | Necrosis in immature teeth | [84] |
| Nanomaterials | Lineages | Cells | Mechanism of Actions | Therapeutic Applications | Ref. |
|---|---|---|---|---|---|
| Graphene oxide | Chondrogenic differentiation | MSCs | Activating osmosensitive receptor | Cartilage repair | [109] |
| Graphene oxide QDs | Osteogenic differentiation | MSCs | Promoting mitochondrial dynamics | Bone defects | [110] |
| 3DGp/CNT scaffolds | Osteogenic differentiation | MSCs | Upregulation of BMP pathway | Dental clinical engineering | [111] |
| CNTs | Neuronal differentiation | NSCs | Enhancing cellular attachment and communication | Neurological diseases and injuries | [112] |
| Carbon nanofibers | Neuronal differentiation | MSCs | Increasing cellular connection | Neural tissue regeneration | [113] |
| Silk fibroin/ carbon nanofiber scaffolds | Cardiomyogenic differentiation | iPSCs | Mimicking mechanical/physical properties of cardiac tissue | Heart failure | [114] |
| CNTs | Neuronal differentiation | NSCs | Regulating focal adhesion, calcium ion channels/ PI3K-AK pathways | Cortical injury | [115] |
| Nanomaterials | Lineages | Cells | Mechanisms | Therapeutic Applications | Ref. |
|---|---|---|---|---|---|
| Stem cell membrane/ZIF-8 | Osteogenic differentiation | MSCs | Improve the targeted internalization of nanoparticles | Bone regeneration | [139] |
| Dexamethasone/ZIF-8 | Osteogenic differentiation | MSCs | Activation of PI3K-Akt signaling pathways | Bone regeneration | [140] |
| ZIF-8/GelMA hydrogel | Neuronal differentiation | DPSCs | Activation of MAPK signaling pathway | Spinal cord injury | [141] |
| Porous carbon nanozyme/HOFs | Neuronal differentiation | NSCs | Oxidative stress resistance, drug carrier | Alzheimer’s disease | [142] |
| Ce/Sr dual-loaded bio-MOF | Osteogenic differentiation | MSCs | Restore mitochondrial dynamics and normalize senescent MSCs | Osteoporotic fracture | [143] |
| Zn/Co-MOF/β-TCP scaffolds | Osteogenic/ chondrogenic differentiation | MSCs | ROS scavenging | Osteoarthritis | [144] |
| UCNPs-F127@Cur | Glioma stem cells | NIH3T3 | Suppressing the Wnt-β-catenin and Jak-Stat pathways | Glioblastoma | [145] |
| PLLA nanofiber | Neural differentiation | NSCs | Piezoelectric charge to topographical stimulation | Neural tissue repair | [146] |
| Single-chain atomic crystal | Neural differentiation | ESCs | Supporting adhesion and growth | Neurodegenerative disease | [147] |
| Graphene derivatives/CeO2-nanoparticle-containing hydrogels | Neuronal differentiation | NSCs | Self-assembly of graphene oxide sheets incorporating a reducing agent and CeO2 nanoparticles | Neuroregenerative cell therapies | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.-D.; Koo, K.-M.; Kim, H.-J.; Kim, T.-H. Recent Advances in Nanomaterials for Modulation of Stem Cell Differentiation and Its Therapeutic Applications. Biosensors 2024, 14, 407. https://doi.org/10.3390/bios14080407
Kim C-D, Koo K-M, Kim H-J, Kim T-H. Recent Advances in Nanomaterials for Modulation of Stem Cell Differentiation and Its Therapeutic Applications. Biosensors. 2024; 14(8):407. https://doi.org/10.3390/bios14080407
Chicago/Turabian StyleKim, Chang-Dae, Kyeong-Mo Koo, Hyung-Joo Kim, and Tae-Hyung Kim. 2024. "Recent Advances in Nanomaterials for Modulation of Stem Cell Differentiation and Its Therapeutic Applications" Biosensors 14, no. 8: 407. https://doi.org/10.3390/bios14080407
APA StyleKim, C.-D., Koo, K.-M., Kim, H.-J., & Kim, T.-H. (2024). Recent Advances in Nanomaterials for Modulation of Stem Cell Differentiation and Its Therapeutic Applications. Biosensors, 14(8), 407. https://doi.org/10.3390/bios14080407





