Dynamic Monitoring of Time-Dependent Evolution of Biomolecules Using Quantum Dots-Based Biosensors Assemblies
Abstract
1. Introduction
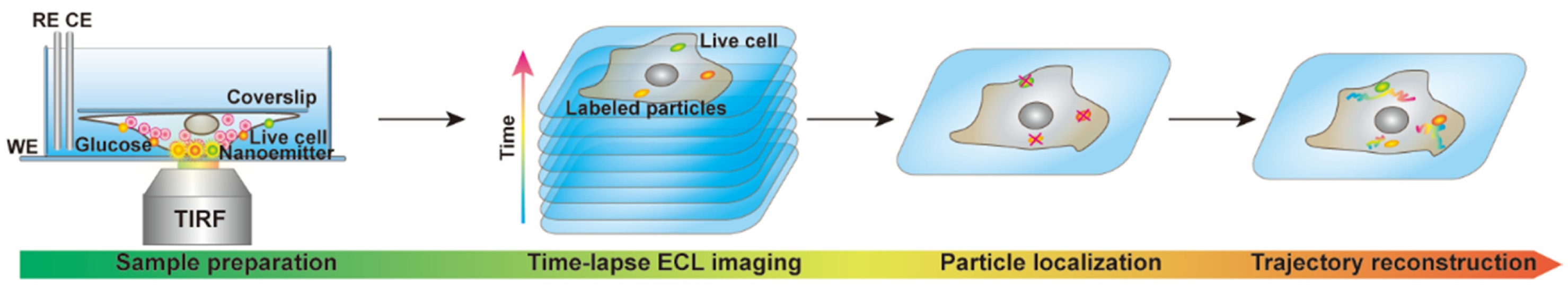
2. Materials and Methods
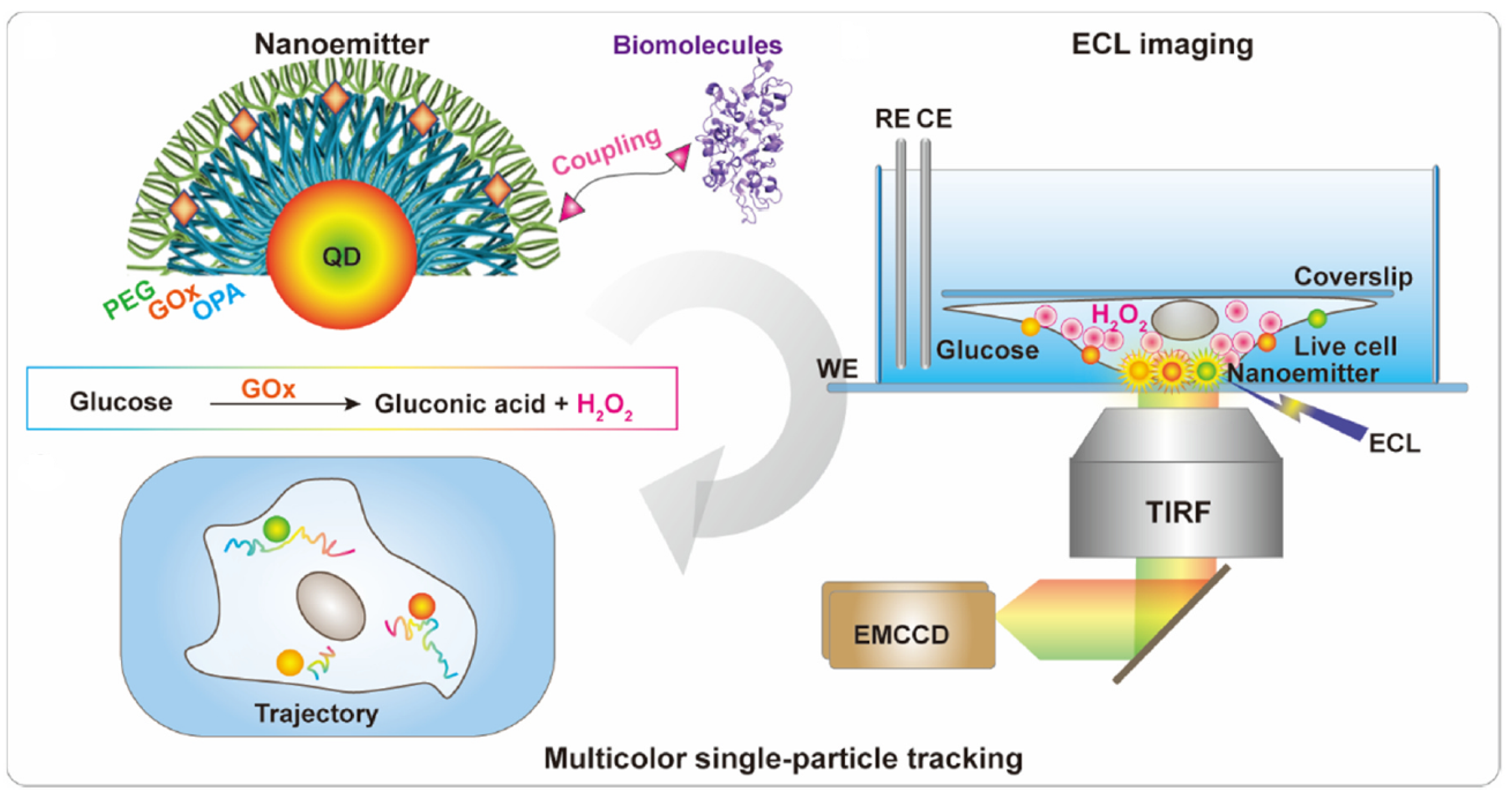
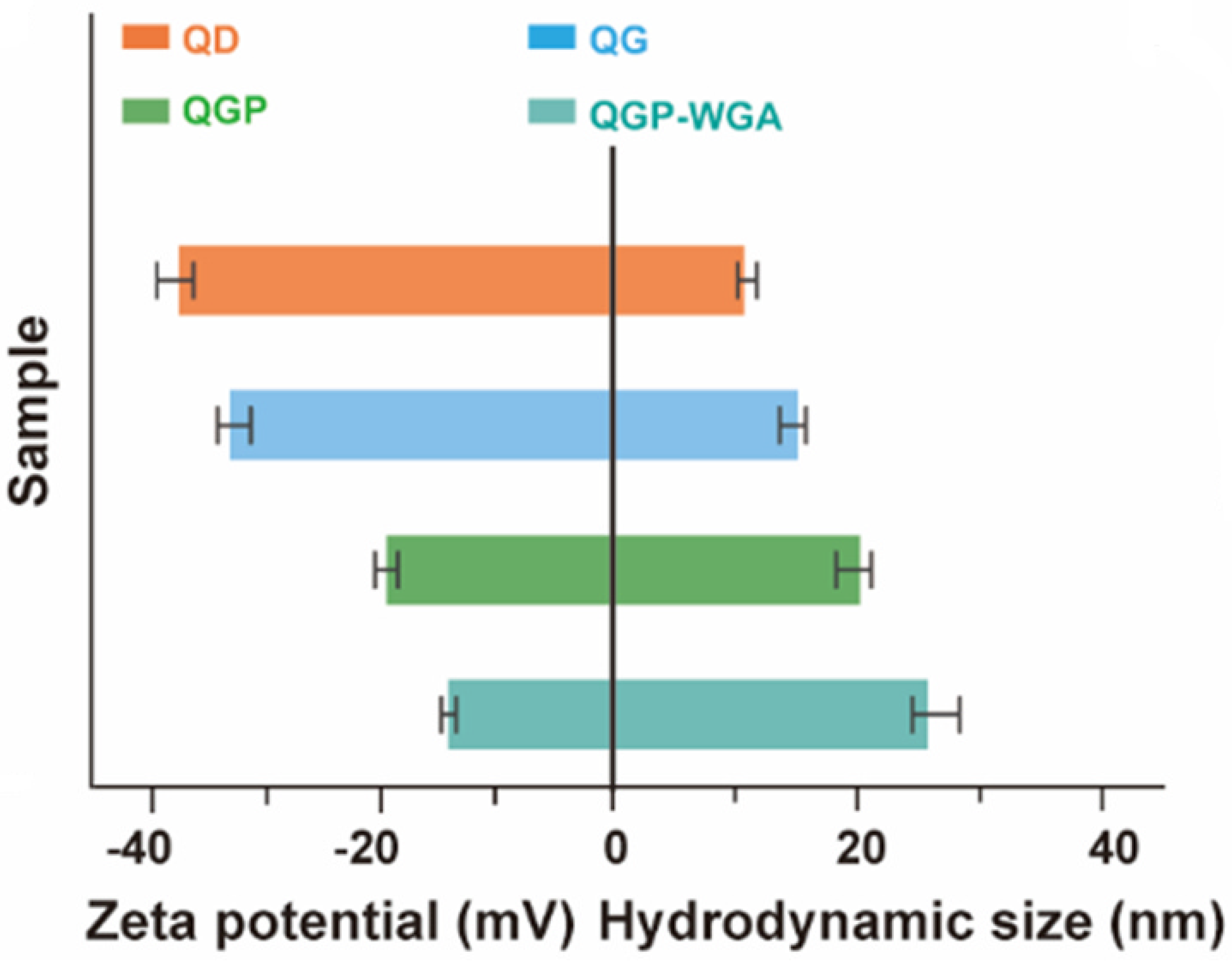
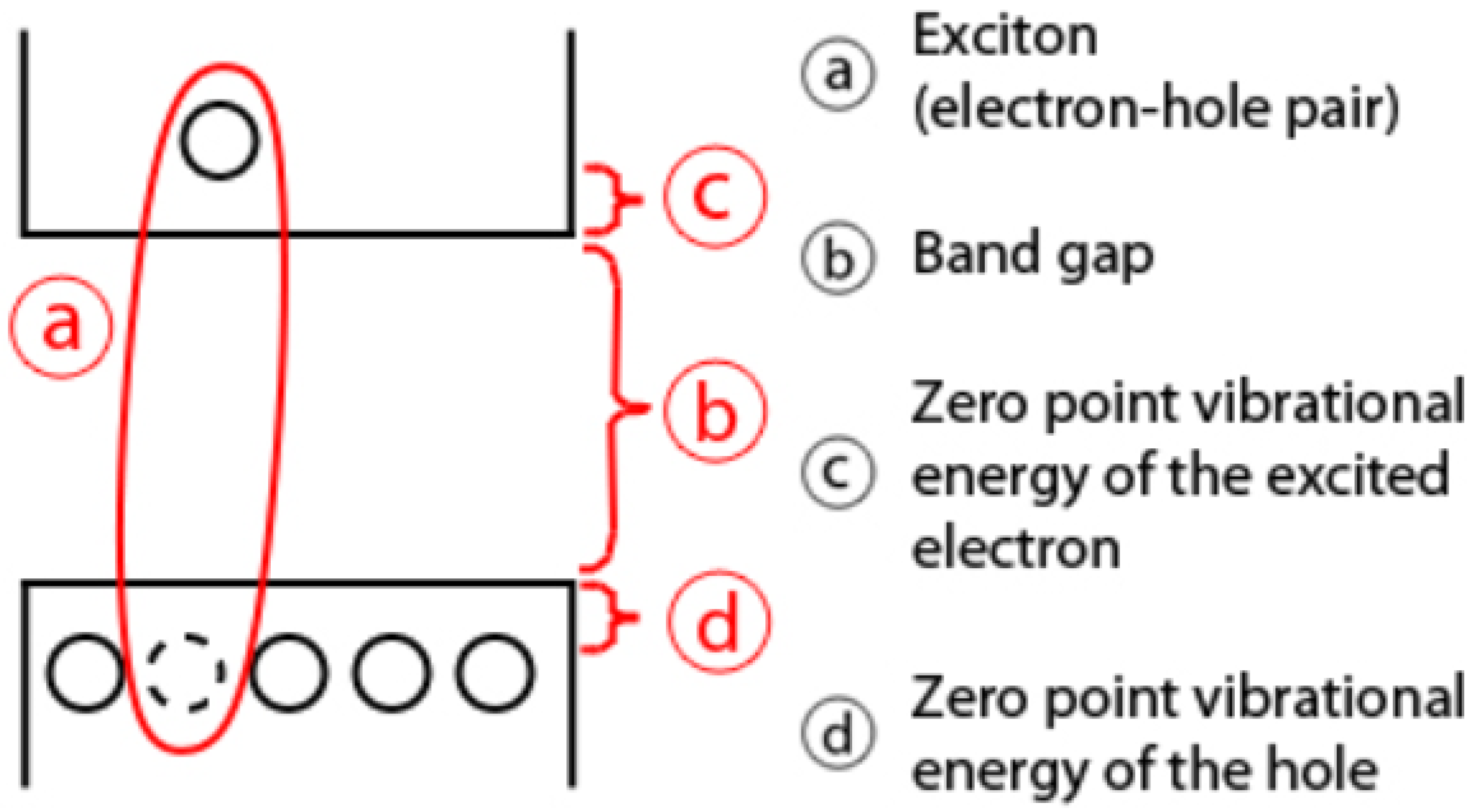
2.1. Design of the ECL Emitters
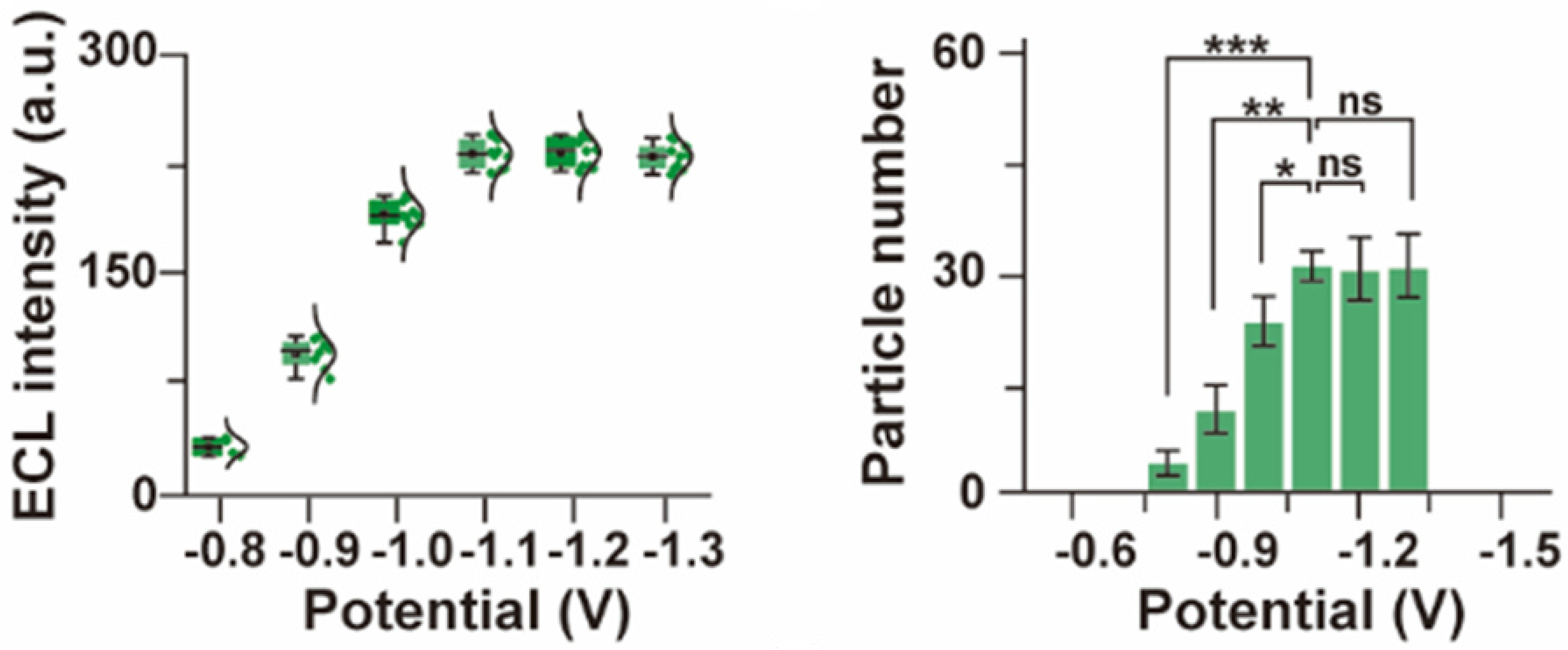
2.2. Evaluation of the Color ECL Emitters
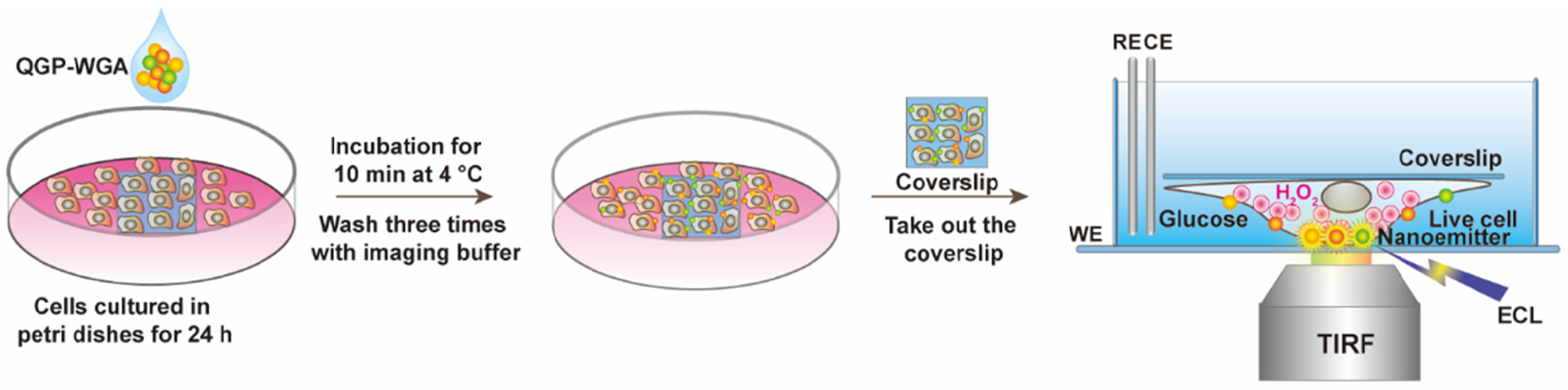
2.3. Practical Relevance of Quantum Dots
3. Results and Discussion
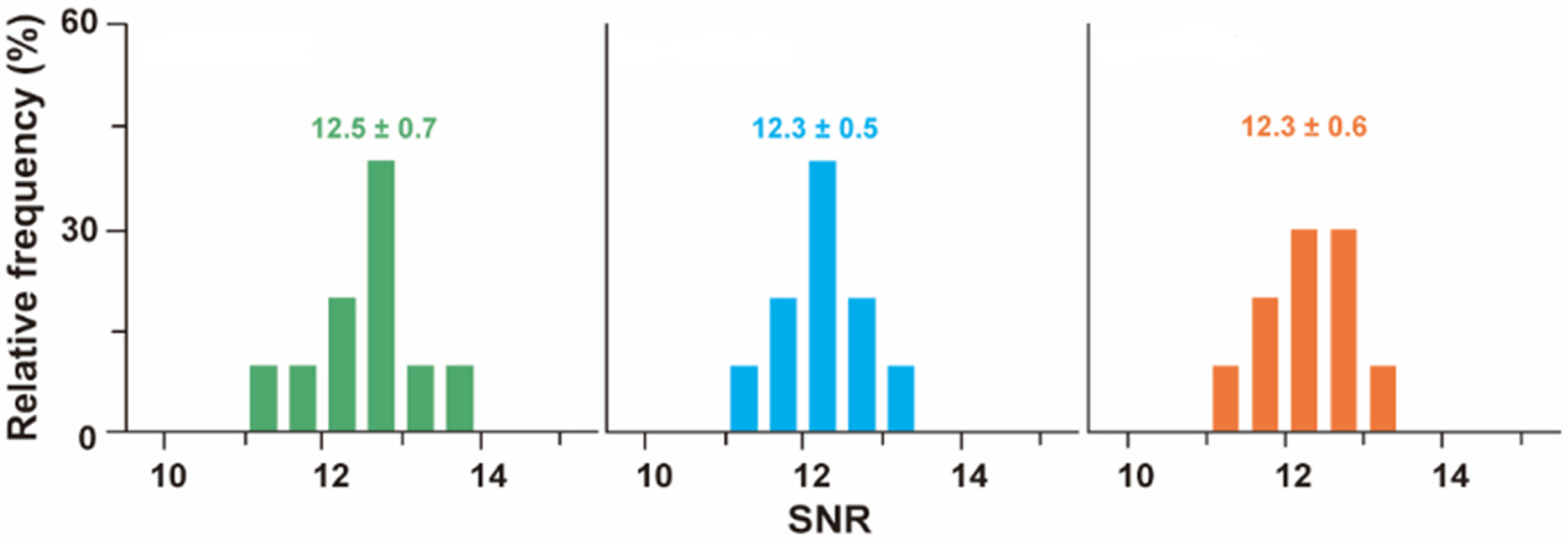
3.1. Remarks Concerning the Monitoring of Molecular Motion
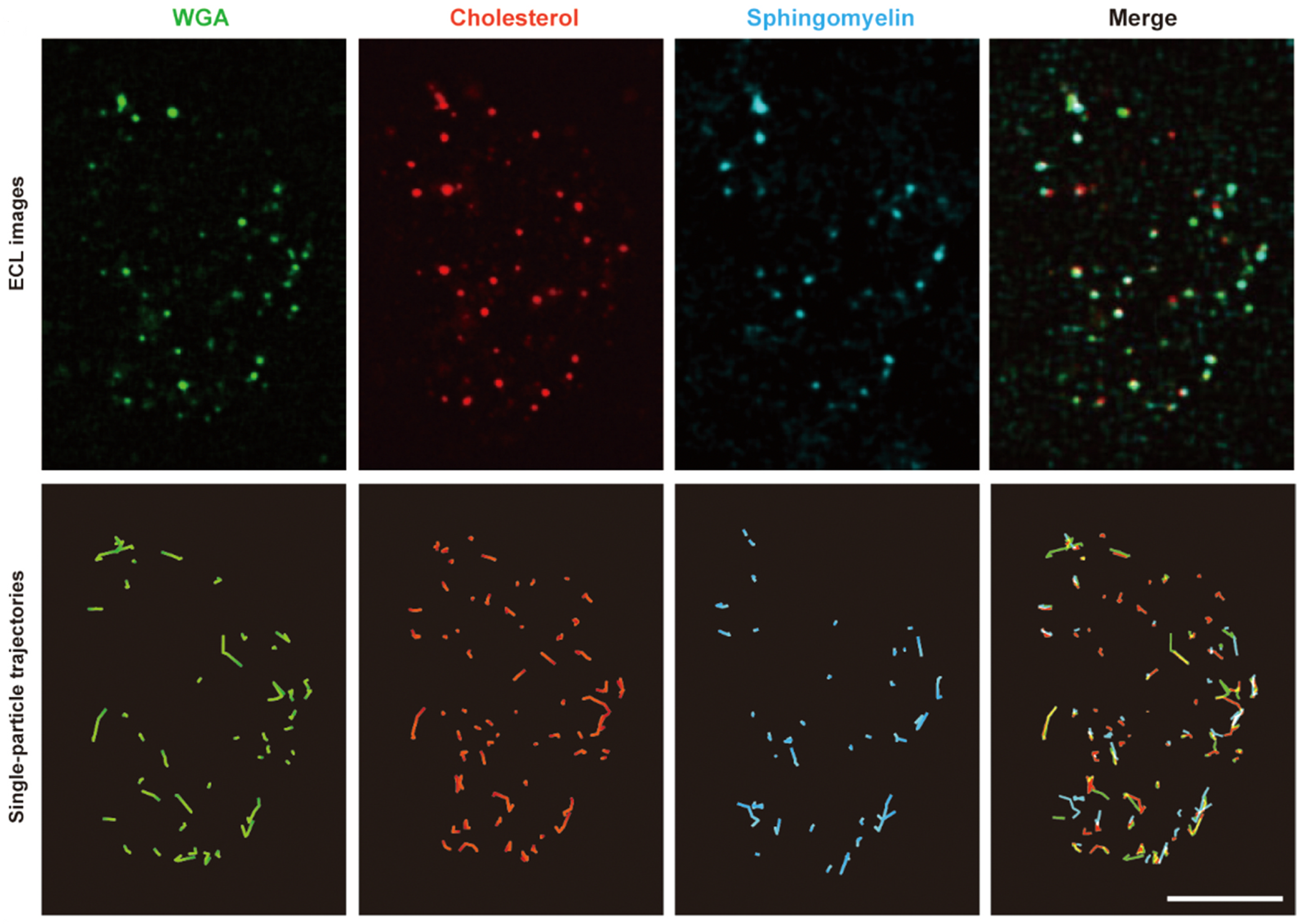
3.2. Evaluation of Intermolecular Interactions Using Proposed TIP Tracking Approach
3.3. Contextual Overview Concerning the Merits of Reported Contribution
- It considers readily available organic, inorganic, electrical, and electronic components to design and implement the required infrastructure.
- It can accurately detect the motion of biomolecules on the target cells’ surface.
- Consequently, it precisely monitors the time-dependent evolution of the biomolecules that exist on the target cells’ surface.
- It generates consistently sharp and clear image frames over time.
- It can produce color images while the respective color details are rendered precisely in accordance with their biomolecular role.
- The generated image frames’ resolution is sufficient to precisely capture the motion of the analyzed biomolecules.
- Consequently, it is possible to accurately represent the paths of the observed biomolecules on the surface of the respective cells.
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chitwood, P.J.; Hegde, R.S. An intramembrane chaperone complex facilitates membrane protein biogenesis. Nature 2020, 584, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Min, D.; DiMaio, F.; Wei, K.Y.; Vahey, M.D.; Boyken, S.E.; Chen, Z.; Fallas, J.A.; Ueda, G.; Sheffler, W.; et al. Accurate computational design of multipass transmembrane proteins. Science 2018, 359, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Graber, Z.T.; Baumgart, T.; Stone, H.A.; Cohen, A.E. Cell Membranes Resist Flow. Cell 2018, 175, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Wang, Z.G.; Xie, H.Y.; Liu, A.A.; Lamb, D.C.; Pang, D.W. Single-Virus Tracking: From Imaging Methodologies to Virological Applications. Chem. Rev. 2020, 120, 1936–1979. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Tauzin, L.J.; Baiyasi, R.; Wang, W.; Moringo, N.; Shuang, B.; Landes, C.F. Single Particle Tracking: From Theory to Biophysical Applications. Chem. Rev. 2017, 117, 7331–7376. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; An, H.J.; Kim, T.; Lee, C.; Lee, N.K. Mechanism of Cyanine5 to Cyanine3 Photoconversion and Its Application for High-Density Single-Particle Tracking in a Living Cell. J. Am. Chem. Soc. 2021, 143, 14125–14135. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Arumugam, S.; Nasilowski, M.; Joshi, H.; Wunder, C.; Chambon, V.; Prakash, V.; Grazon, C.; Nadal, B.; Maiti, P.K.; et al. Quantum dot-loaded monofunctionalized DNA icosahedra for single-particle tracking of endocytic pathways. Nat. Nanotechnol. 2016, 11, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Zhang, D.; Cui, X.; Wei, M.; Zhang, T.; Wang, J.; Xiao, L.; Xia, Y. Direct Monitoring of Cell Membrane Vesiculation with 2D AuNP@MnO2 Nanosheet Supraparticles at the Single-Particle Level. Angew. Chem. Int. Ed. 2019, 58, 10542–10546. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, W. Single-molecule fluorescence imaging for probing nanocatalytic process. Chem 2023, 9, 16–28. [Google Scholar] [CrossRef]
- Hsiang, J.C.; Jablonski, A.E.; Dickson, R.M. Optically Modulated Fluorescence Bioimaging: Visualizing Obscured Fluorophores in High Background. Acc. Chem. Res. 2014, 47, 1545–1554. [Google Scholar] [CrossRef]
- Han, D.; Goudeau, B.; Manojlovic, D.; Jiang, D.; Fang, D.; Sojic, N. Electrochemiluminescence Loss in Photobleaching. Angew. Chem. Int. Ed. 2021, 60, 7686–7690. [Google Scholar] [CrossRef] [PubMed]
- Zanut, A.; Fiorani, A.; Canola, S.; Saito, T.; Ziebart, N.; Rapino, S.; Rebeccani, S.; Barbon, A.; Irie, T.; Josel, H.-P.; et al. Insights into the mechanism of coreactant electrochemiluminescence facilitating enhanced bioanalytical performance. Nat. Commun. 2020, 11, 2668. [Google Scholar] [CrossRef]
- Wang, N.; Gao, H.; Li, Y.; Li, G.; Chen, W.; Jin, Z.; Lei, J.; Wei, Q.; Ju, H. Dual Intramolecular Electron Transfer for In Situ Coreactant-Embedded Electrochemiluminescence Microimaging of Membrane Protein. Angew. Chem. Int. Ed. 2021, 60, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhou, P.; Fu, W.; Ding, L.; Guo, W.; Su, B. Spatially Selective Imaging of Cell–Matrix and Cell–Cell Junctions by Electrochemiluminescence. Angew. Chem. Int. Ed. 2021, 60, 11769–11773. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; Li, B.; Liu, J.; Jiang, D.; Liu, B.; Sojic, N. Single Biomolecule Imaging by Electrochemiluminescence. J. Am. Chem. Soc. 2021, 143, 17910–17914. [Google Scholar] [CrossRef] [PubMed]
- Voci, S.; Goudeau, B.; Valenti, G.; Lesch, A.; Jović, M.; Rapino, S.; Paolucci, F.; Arbault, S.; Sojic, N. Surface-Confined Electrochemiluminescence Microscopy of Cell Membranes. J. Am. Chem. Soc. 2018, 140, 14753–14760. [Google Scholar] [CrossRef] [PubMed]
- Miao, W. Electrogenerated Chemiluminescence and Its Biorelated Applications. Chem. Rev. 2008, 108, 2506–2553. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xu, G. Applications and trends in electrochemiluminescence. Chem. Soc. Rev. 2010, 39, 3275–3304. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Liu, A.A.; Fu, H.; Cheng, Q.Y.; Zhang, M.Y.; Pan, M.M.; Liu, L.P.; Luo, M.Y.; Tang, B.; Zhao, W.; et al. Breaking through the Size Control Dilemma of Silver Chalcogenide Quantum Dots via Trialkylphosphine-Induced Ripening: Leading to Ag2Te Emitting from 950 to 2100 nm. J. Am. Chem. Soc. 2021, 143, 12867–12877. [Google Scholar] [CrossRef]
- Cao, Z.; Shu, Y.; Qin, H.; Su, B.; Peng, X. Quantum Dots with Highly Efficient, Stable, and Multicolor Electrochemiluminescence. ACS Cent. Sci. 2020, 6, 1129–1137. [Google Scholar] [CrossRef]
- Zhang, J.; Arbault, S.; Sojic, N.; Jiang, D. Electrochemiluminescence Imaging for Bioanalysis. Annu. Rev. Anal. Chem. 2019, 12, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Y.; Wang, Z.G.; Hu, H.; Zhang, Z.L.; Tang, B.; Luo, M.Y.; Yang, L.L.; Wang, B.; Pang, D.W. How different are the surfaces of semiconductor Ag2Se quantum dots with various sizes? Sci. Bull. 2022, 67, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Hou, X.; Xu, J.J.; Chen, H.-Y. Electrochemically Generated versus Photoexcited Luminescence from Semiconductor Nanomaterials: Bridging the Valley between Two Worlds. Chem. Rev. 2014, 114, 11027–11059. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hou, X.; Dai, X.; Yao, Z.; Lv, L.; Jin, Y.; Peng, X. Stoichiometry-Controlled InP-Based Quantum Dots: Synthesis, Photoluminescence, and Electroluminescence. J. Am. Chem. Soc. 2019, 141, 6448–6452. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, R.; Sojic, N.; Jiang, D.; Chen, H.Y. Intracellular Wireless Analysis of Single Cells by Bipolar Electrochemiluminescence Confined in a Nanopipette. Angew. Chem. Int. Ed. 2020, 59, 10416–10420. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.T.; Jiang, H.; Wu, W.T.; Zhang, F.L.; Tian, S.Y.; Fan, W.T.; Liu, Y.L.; Amatore, C.; Huang, W.H. Homeostasis inside Single Activated Phagolysosomes: Quantitative and Selective Measurements of Submillisecond Dynamics of Reactive Oxygen and Nitrogen Species Production with a Nanoelectrochemical Sensor. J. Am. Chem. Soc. 2022, 144, 9723–9733. [Google Scholar] [CrossRef]
- Wang, Z.G.; Liu, S.L.; Tian, Z.Q.; Zhang, Z.L.; Tang, H.W.; Pang, D.W. Myosin-Driven Intercellular Transportation of Wheat Germ Agglutinin Mediated by Membrane Nanotubes between Human Lung Cancer Cells. ACS Nano 2012, 6, 10033–10041. [Google Scholar] [CrossRef] [PubMed]
- Schwefel, D.; Maierhofer, C.; Beck, J.G.; Seeberger, S.; Diederichs, K.; Möller, H.M.; Welte, W.; Wittmann, V. Structural Basis of Multivalent Binding to Wheat Germ Agglutinin. J. Am. Chem. Soc. 2010, 132, 8704–8719. [Google Scholar] [CrossRef]
- Lin, L.S.; Song, J.; Song, L.; Ke, K.; Liu, Y.; Zhou, Z.; Shen, Z.; Li, J.; Yang, Z.; Tang, W.; et al. Simultaneous Fenton-like Ion Delivery and Glutathione Depletion by MnO2-Based Nanoagent to Enhance Chemodynamic Therapy. Angew. Chem. Int. Ed. 2018, 57, 4902–4906. [Google Scholar] [CrossRef]
- Liu, S.L.; Sheng, R.; Jung, J.H.; Wang, L.; Stec, E.; O’Connor, M.J.; Song, S.; Bikkavilli, R.K.; Winn, R.A.; Lee, D.; et al. Orthogonal lipid sensors identify transbilayer asymmetry of plasma membrane cholesterol. Nat. Chem. Biol. 2017, 13, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, A.; Sekizawa, Y.; Emoto, K.; Sakuraba, H.; Inoue, K.; Kobayashi, H.; Umeda, M. Lysenin, a Novel Sphingomyelin-specific Binding Protein. J. Biol. Chem. 1998, 273, 5300–5306. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocu, R. Dynamic Monitoring of Time-Dependent Evolution of Biomolecules Using Quantum Dots-Based Biosensors Assemblies. Biosensors 2024, 14, 380. https://doi.org/10.3390/bios14080380
Bocu R. Dynamic Monitoring of Time-Dependent Evolution of Biomolecules Using Quantum Dots-Based Biosensors Assemblies. Biosensors. 2024; 14(8):380. https://doi.org/10.3390/bios14080380
Chicago/Turabian StyleBocu, Razvan. 2024. "Dynamic Monitoring of Time-Dependent Evolution of Biomolecules Using Quantum Dots-Based Biosensors Assemblies" Biosensors 14, no. 8: 380. https://doi.org/10.3390/bios14080380
APA StyleBocu, R. (2024). Dynamic Monitoring of Time-Dependent Evolution of Biomolecules Using Quantum Dots-Based Biosensors Assemblies. Biosensors, 14(8), 380. https://doi.org/10.3390/bios14080380




