Recapitulating Glioma Stem Cell Niches Using 3D Spheroid Models for Glioblastoma Research
Abstract
1. Introduction
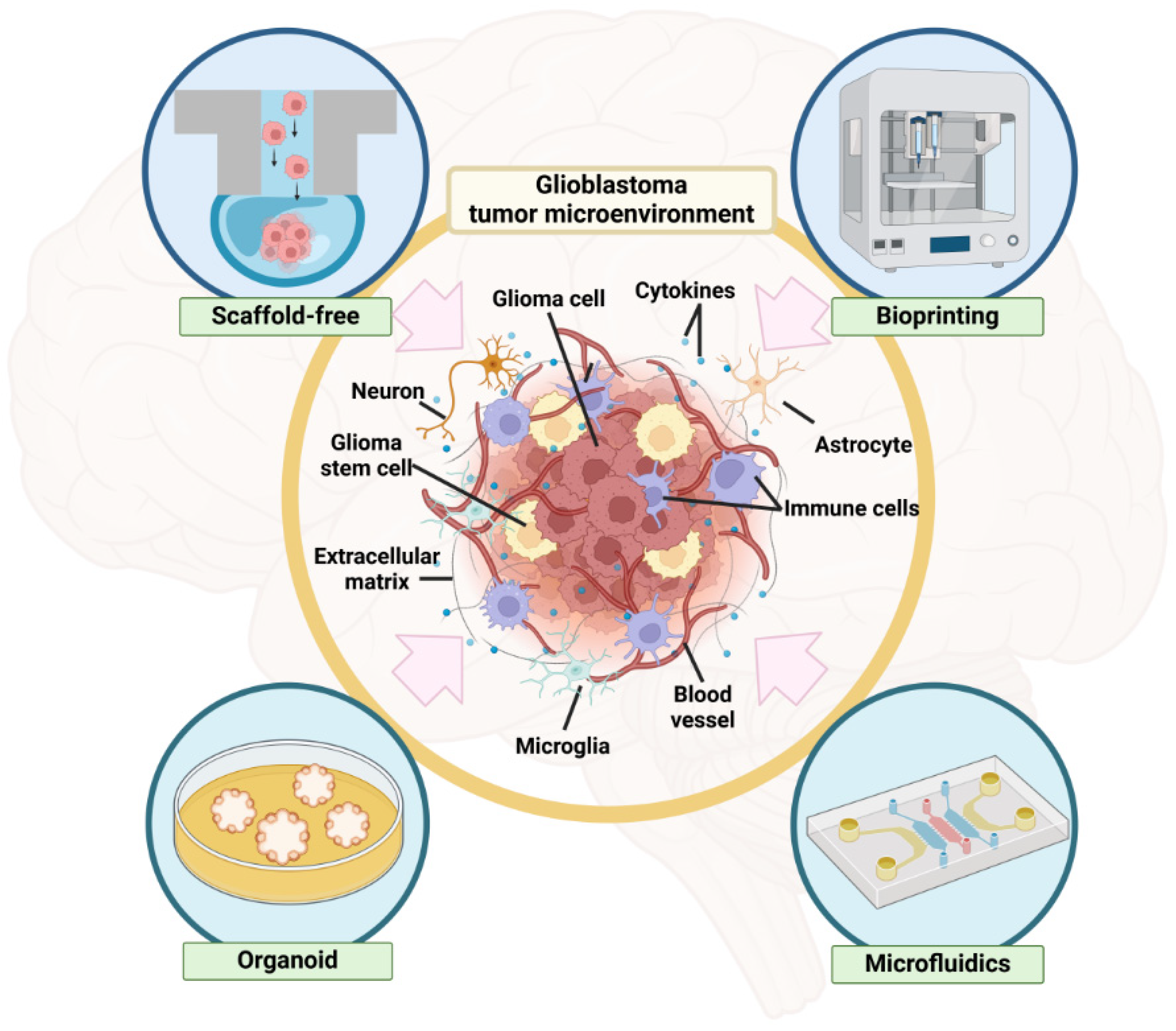
2. GSC Niche in GBM
3. GBM Spheroid Formation Methods to Study GSC Niche
3.1. Scaffold-Free Methods
3.1.1. Hanging Drop Method
3.1.2. Low-Adhesion Plates
3.1.3. Magnetic Levitation Method
3.2. Scaffold-Based Methods
3.2.1. Embedding GBM Spheroids in ECM Gels
3.2.2. 3D Bioprinting
3.3. Organoid Culture
3.4. Microfluidic Device
4. Current Challenges and Future Directions in GBM Tumor Modeling: Scaffold-Free, Scaffold-Based, Organoid, and Microfluidic Approaches
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Di Nunno, V.; Franceschi, E.; Tosoni, A.; Gatto, L.; Bartolini, S.; Brandes, A.A. Glioblastoma microenvironment: From an inviolable defense to a therapeutic chance. Front. Oncol. 2022, 12, 852950. [Google Scholar] [CrossRef] [PubMed]
- Giambra, M.; Messuti, E.; Di Cristofori, A.; Cavandoli, C.; Bruno, R.; Buonanno, R.; Marzorati, M.; Zambuto, M.; Rodriguez-Menendez, V.; Redaelli, S. Characterizing the genomic profile in high-grade gliomas: From tumor core to peritumoral brain zone, passing through glioma-derived tumorspheres. Biology 2021, 10, 1157. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Costa, A.; Osorio, L.; Lago, R.C.; Linhares, P.; Carvalho, B.; Caeiro, C. Current Standards of Care in Glioblastoma Therapy. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, AU, Australia, 2017. [Google Scholar]
- Naydenov, E.; Tzekov, C.; Minkin, K.; Nachev, S.; Romansky, K.; Bussarsky, V. Long-term survival with primary glioblastoma multiforme: A clinical study in bulgarian patients. Case Rep. Oncol. 2011, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Young, R.M.; Jamshidi, A.; Davis, G.; Sherman, J.H. Current trends in the surgical management and treatment of adult glioblastoma. Ann. Transl. Med. 2015, 3, 121. [Google Scholar] [PubMed]
- Sharma, P.; Aaroe, A.; Liang, J.; Puduvalli, V.K. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neurooncol. Adv. 2023, 5, vdad009. [Google Scholar] [CrossRef]
- Alifieris, C.; Trafalis, D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Yherapeutics 2015, 152, 63–82. [Google Scholar] [CrossRef]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef]
- Laranjo, M.; Carvalho, M.J.; Serambeque, B.; Alves, A.; Marto, C.M.; Silva, I.; Paiva, A.; Botelho, M.F. Obtaining cancer stem cell spheres from gynecological and breast cancer tumors. J. Vis. Rxperiments 2020, 157, e60022. [Google Scholar]
- Zhu, Z.-W.; Chen, L.; Liu, J.-X.; Huang, J.-W.; Wu, G.; Zheng, Y.-F.; Yao, K.-T. A novel three-dimensional tumorsphere culture system for the efficient and low-cost enrichment of cancer stem cells with natural polymers. Exp. Ther. Med. 2018, 15, 85–92. [Google Scholar] [CrossRef]
- Galli, R.; Binda, E.; Orfanelli, U.; Cipelletti, B.; Gritti, A.; De Vitis, S.; Fiocco, R.; Foroni, C.; Dimeco, F.; Vescovi, A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004, 64, 7011–7021. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, D.; Mellai, M.; Annovazzi, L.; Piazzi, A.; Monzeglio, O.; Caldera, V. Glioblastoma cancer stem cells: Basis for a functional hypothesis. Stem Cell Discov. 2012, 2, 122–131. [Google Scholar] [CrossRef]
- Alves, A.L.V.; Gomes, I.N.F.; Carloni, A.C.; Rosa, M.N.; da Silva, L.S.; Evangelista, A.F.; Reis, R.M.; Silva, V.A.O. Role of glioblastoma stem cells in cancer therapeutic resistance: A perspective on antineoplastic agents from natural sources and chemical derivatives. Stem Cell Res. Ther. 2021, 12, 206. [Google Scholar] [CrossRef]
- Horsman, M.R.; Vaupel, P. Pathophysiological Basis for the Formation of the Tumor Microenvironment. Front. Oncol. 2016, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 2003, 9, 685–693. [Google Scholar] [CrossRef]
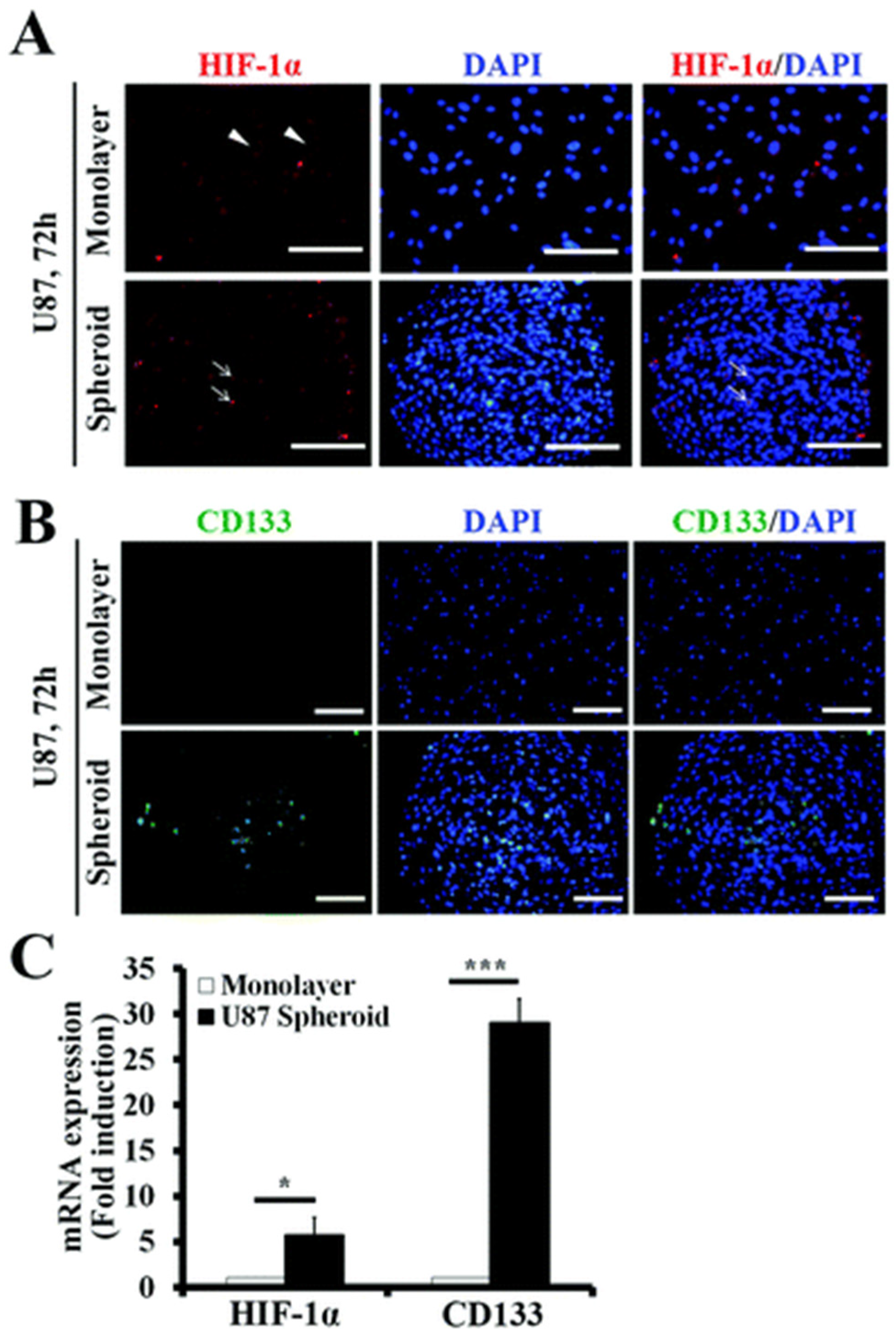
- Seidel, S.; Garvalov, B.K.; Wirta, V.; Von Stechow, L.; Schänzer, A.; Meletis, K.; Wolter, M.; Sommerlad, D.; Henze, A.-T.; Nister, M. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2α. Brain 2010, 133, 983–995. [Google Scholar] [CrossRef]
- Bar, E.E.; Lin, A.; Mahairaki, V.; Matsui, W.; Eberhart, C.G. Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am. J. Pathol. 2010, 177, 1491–1502. [Google Scholar] [CrossRef]
- Guerra-Rebollo, M.; Garrido, C.; Sánchez-Cid, L.; Soler-Botija, C.; Meca-Cortés, O.; Rubio, N.; Blanco, J. Targeting of replicating CD133 and OCT4/SOX2 expressing glioma stem cells selects a cell population that reinitiates tumors upon release of therapeutic pressure. Sci. Rep. 2019, 9, 9549. [Google Scholar] [CrossRef] [PubMed]
- Brescia, P.; Ortensi, B.; Fornasari, L.; Levi, D.; Broggi, G.; Pelicci, G. CD133 is essential for glioblastoma stem cell maintenance. Stem Cells 2013, 31, 857–869. [Google Scholar] [CrossRef]
- Kolenda, J.; Jensen, S.S.; Aaberg-Jessen, C.; Christensen, K.; Andersen, C.; Brunner, N.; Kristensen, B.W. Effects of hypoxia on expression of a panel of stem cell and chemoresistance markers in glioblastoma-derived spheroids. J. Neuro-Oncol. 2011, 103, 43–58. [Google Scholar] [CrossRef]
- Macharia, L.W.; Muriithi, W.; Heming, C.P.; Nyaga, D.K.; Aran, V.; Mureithi, M.W.; Ferrer, V.P.; Pane, A.; Filho, P.N.; Moura-Neto, V. The genotypic and phenotypic impact of hypoxia microenvironment on glioblastoma cell lines. BMC Cancer 2021, 21, 1248. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M.; Bandopadhyay, G.; Coyle, B.; Grabowska, A. A HIF-independent, CD133-mediated mechanism of cisplatin resistance in glioblastoma cells. Cell. Oncol. 2018, 41, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gong, S.; Pan, J.; Wang, J.; Zou, D.; Xiong, S.; Zhao, L.; Yan, Q.; Deng, Y.; Wu, N. Hyperbaric oxygen promotes not only glioblastoma proliferation but also chemosensitization by inhibiting HIF1α/HIF2α-Sox2. Cell Death Discov. 2021, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kotliarova, S.; Kotliarov, Y.; Li, A.; Su, Q.; Donin, N.M.; Pastorino, S.; Purow, B.W.; Christopher, N.; Zhang, W. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 2006, 9, 391–403. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef]
- Lazzari, G.; Couvreur, P.; Mura, S. Multicellular tumor spheroids: A relevant 3D model for the in vitro preclinical investigation of polymer nanomedicines. Polym. Chem. 2017, 8, 4947–4969. [Google Scholar] [CrossRef]
- Moharil, R.B.; Dive, A.; Khandekar, S.; Bodhade, A. Cancer stem cells: An insight. J. Oral Maxillofac. Pathol. 2017, 21, 463. [Google Scholar] [CrossRef]
- Persano, L.; Rampazzo, E.; Basso, G.; Viola, G. Glioblastoma cancer stem cells: Role of the microenvironment and therapeutic targeting. Biochem. Pharmacol. 2013, 85, 612–622. [Google Scholar] [CrossRef]
- Ivanov, D.P.; Parker, T.L.; Walker, D.A.; Alexander, C.; Ashford, M.B.; Gellert, P.R.; Garnett, M.C. Multiplexing spheroid volume, resazurin and acid phosphatase viability assays for high-throughput screening of tumour spheroids and stem cell neurospheres. PLoS ONE 2014, 9, e103817. [Google Scholar] [CrossRef]
- Mueller-Klieser, W. Tumor biology and experimental therapeutics. Crit. Rev. Oncol./Hematol. 2000, 36, 123–139. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; King, M. Gather round: In vitro tumor spheroids as improved models of in vivo tumors. J. Bioeng. Biomed. Sci. 2012, 2, e109. [Google Scholar] [CrossRef]
- Kelm, J.M.; Timmins, N.E.; Brown, C.J.; Fussenegger, M.; Nielsen, L.K. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol. Bioeng. 2003, 83, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Hofmann, S.; Ahmadi, R.; Becker, N.; Korshunov, A.; Engel, F.; Hartmann, C.; Felsberg, J.; Sabel, M.; Peterziel, H.; et al. Genomic and expression profiling of glioblastoma stem cell-like spheroid cultures identifies novel tumor-relevant genes associated with survival. Clin. Cancer Res. 2009, 15, 6541–6550. [Google Scholar] [CrossRef] [PubMed]
- Del Duca, D.; Werbowetski, T.; Del Maestro, R.F. Spheroid preparation from hanging drops: Characterization of a model of brain tumor invasion. J. Neuro-Oncol. 2004, 67, 295–303. [Google Scholar] [CrossRef]
- Nusblat, L.M.; Tanna, S.; Roth, C.M. Gene silencing of HIF-2α disrupts glioblastoma stem cell phenotype. Cancer Drug Resist. 2020, 3, 199. [Google Scholar] [CrossRef]
- Han, S.; Kim, S.; Hong, H.K.; Cho, Y.B.; Moon, H.E.; Paek, S.H.; Park, S. Multi-Inlet Spheroid Generator for High-Throughput Combinatorial Drug Screening Based on the Tumor Microenvironment. ACS Appl. Mater. Interfaces 2023, 15, 32087–32098. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, S.; Sanny, A.; Chan, D.L.-K.; van Noort, D.; Lim, W.; Tan, A.H.-M.; Park, S. 3D hanging spheroid plate for high-throughput CAR T cell cytotoxicity assay. J. Nanobiotechnol. 2022, 20, 30. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Kim, S.; Lee, C.; Sanny, A.; Tan, A.H.-M.; Park, S. A 3D hanging spheroid-filter plate for high-throughput drug testing and CAR T cell cytotoxicity assay. Analyst 2024, 149, 475–481. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, P.; Zhang, Q.; Man, X.; Xu, Y. Fabrication of heterocellular spheroids with controllable core-shell structure using inertial focusing effect for scaffold-free 3D cell culture models. Biofabrication 2024, 16, 045013. [Google Scholar] [CrossRef]
- Nath, S.; Devi, G.R. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef]
- Vinci, M.; Gowan, S.; Boxall, F.; Patterson, L.; Zimmermann, M.; Court, W.; Lomas, C.; Mendiola, M.; Hardisson, D.; Eccles, S.A. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Yuzhakova, D.; Lukina, M.; Sachkova, D.; Yusubalieva, G.; Dudenkova, V.; Gavrina, A.; Yashin, K.; Shirmanova, M. Development of a 3D Tumor Spheroid Model from the Patient’s Glioblastoma Cells and Its Study by Metabolic Fluorescence Lifetime Imaging. Сoвременные Mехнoлoгии В Mедицине 2023, 15, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Joo, C.; Park, K.H.; Kang, S.-W.; Huh, K.M.; Choi, J.S. Preparation and characterization of 3D human glioblastoma spheroids using an N-octanoyl glycol chitosan hydrogel. Int. J. Biol. Macromol. 2021, 185, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Hoang, H.-H.; You, D.; Han, J.; Lee, J.E.; Kim, S.; Park, S. Formation of size-controllable tumour spheroids using a microfluidic pillar array (μFPA) device. Analyst 2018, 143, 5841–5848. [Google Scholar] [CrossRef]
- Isogai, R.; Morio, H.; Okamoto, A.; Kitamura, K.; Furihata, T. Generation of a Human Conditionally Immortalized Cell-based Multicellular Spheroidal Blood-Brain Barrier Model for Permeability Evaluation of Macromolecules. Bio-Protocol 2022, 12, e4465. [Google Scholar] [CrossRef]
- Mathew-Schmitt, S.; Peindl, M.; Neundorf, P.; Dandekar, G.; Metzger, M.; Nickl, V.; Appelt-Menzel, A. Blood-tumor barrier in focus-investigation of glioblastoma-induced effects on the blood-brain barrier. J. Neuro-Oncol. 2024, 170, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Truong, T.T.; Park, J.-H.; Park, Y.; Kim, H.; Hyun, S.-A.; Shim, H.-E.; Mallick, S.; Park, H.-J.; Huh, K.M. Robust and customizable spheroid culture system for regenerative medicine. Biofabrication 2024, 16, 045016. [Google Scholar] [CrossRef]
- Durmus, N.G.; Tekin, H.C.; Guven, S.; Sridhar, K.; Arslan Yildiz, A.; Calibasi, G.; Ghiran, I.; Davis, R.W.; Steinmetz, L.M.; Demirci, U. Magnetic levitation of single cells. Proc. Natl. Acad. Sci. USA 2015, 112, E3661–E3668. [Google Scholar] [CrossRef]
- Souza, G.R.; Molina, J.R.; Raphael, R.M.; Ozawa, M.G.; Stark, D.J.; Levin, C.S.; Bronk, L.F.; Ananta, J.S.; Mandelin, J.; Georgescu, M.-M. Three-dimensional tissue culture based on magnetic cell levitation. Nat. Nanotechnol. 2010, 5, 291–296. [Google Scholar] [CrossRef]
- Haisler, W.L.; Timm, D.M.; Gage, J.A.; Tseng, H.; Killian, T.; Souza, G.R. Three-dimensional cell culturing by magnetic levitation. Nat. Protoc. 2013, 8, 1940–1949. [Google Scholar] [CrossRef]
- Jaganathan, H.; Gage, J.; Leonard, F.; Srinivasan, S.; Souza, G.R.; Dave, B.; Godin, B. Three-dimensional in vitro co-culture model of breast tumor using magnetic levitation. Sci. Rep. 2014, 4, 6468. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Hayashi, Y.; Stephens, C.; Georgescu, M.M. Invasive glioblastoma cells acquire stemness and increased Akt activation. Neoplasia 2010, 12, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Rijal, G.; Li, W. A versatile 3D tissue matrix scaffold system for tumor modeling and drug screening. Sci. Adv. 2017, 3, e1700764. [Google Scholar] [CrossRef]
- Goertzen, C.; Eymael, D.; Magalhaes, M. Three-dimensional quantification of spheroid degradation-dependent invasion and invadopodia formation. Biol. Proced. Online 2018, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Iazzolino, G.; Mendibil, U.; Arnaiz, B.; Ruiz-de-Angulo, A.; Azkargorta, M.; Uribe, K.B.; Khatami, N.; Elortza, F.; Olalde, B.; Gomez-Vallejo, V. Decellularization of xenografted tumors provides cell-specific in vitro 3D environment. Front. Oncol. 2022, 12, 956940. [Google Scholar] [CrossRef] [PubMed]
- Koh, I.; Cha, J.; Park, J.; Choi, J.; Kang, S.-G.; Kim, P. The mode and dynamics of glioblastoma cell invasion into a decellularized tissue-derived extracellular matrix-based three-dimensional tumor model. Sci. Rep. 2018, 8, 4608. [Google Scholar] [CrossRef]
- Tang, M.; Tiwari, S.K.; Agrawal, K.; Tan, M.; Dang, J.; Tam, T.; Tian, J.; Wan, X.; Schimelman, J.; You, S. Rapid 3D bioprinting of glioblastoma model mimicking native biophysical heterogeneity. Small 2021, 17, 2006050. [Google Scholar] [CrossRef]
- Hasselbach, L.A.; Irtenkauf, S.M.; Lemke, N.W.; Nelson, K.K.; Berezovsky, A.D.; Carlton, E.T.; Transou, A.D.; Mikkelsen, T.; deCarvalho, A.C. Optimization of high grade glioma cell culture from surgical specimens for use in clinically relevant animal models and 3D immunochemistry. J. Vis. Exp. 2014, 83, e51088. [Google Scholar]
- Guyon, J.; Andrique, L.; Pujol, N.; Røsland, G.V.; Recher, G.; Bikfalvi, A.; Daubon, T. A 3D spheroid model for glioblastoma. J. Vis. Exp. 2020, 158, e60998. [Google Scholar] [CrossRef]
- Amofa, K.Y.; Patterson, K.M.; Ortiz, J.; Kumar, S. Dissecting TGF-β-induced glioblastoma invasion with engineered hyaluronic acid hydrogels. APL Bioeng. 2024, 8, 026125. [Google Scholar] [CrossRef]
- Ning, L.; Shim, J.; Tomov, M.L.; Liu, R.; Mehta, R.; Mingee, A.; Hwang, B.; Jin, L.; Mantalaris, A.; Xu, C. A 3D bioprinted in vitro model of neuroblastoma recapitulates dynamic tumor-endothelial cell interactions contributing to solid tumor aggressive behavior. Adv. Sci. 2022, 9, 2200244. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T. Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol. 2015, 33, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Xie, Q.; Gimple, R.C.; Zhong, Z.; Tam, T.; Tian, J.; Kidwell, R.L.; Wu, Q.; Prager, B.C.; Qiu, Z. Three-dimensional bioprinted glioblastoma microenvironments model cellular dependencies and immune interactions. Cell Res. 2020, 30, 833–853. [Google Scholar] [CrossRef] [PubMed]
- Placone, J.K.; Engler, A.J. Recent advances in extrusion-based 3D printing for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1701161. [Google Scholar] [CrossRef] [PubMed]
- Negro, A.; Cherbuin, T.; Lutolf, M.P. 3D inkjet printing of complex, cell-laden hydrogel structures. Sci. Rep. 2018, 8, 17099. [Google Scholar] [CrossRef]
- Koo, S.; Santoni, S.M.; Gao, B.Z.; Grigoropoulos, C.P.; Ma, Z. Laser-assisted biofabrication in tissue engineering and regenerative medicine. J. Mater. Res. 2017, 32, 128–142. [Google Scholar] [CrossRef]
- Thakor, J.; Ahadian, S.; Niakan, A.; Banton, E.; Nasrollahi, F.; Hasani-Sadrabadi, M.M.; Khademhosseini, A. Engineered hydrogels for brain tumor culture and therapy. Bio-Des. Manuf. 2020, 3, 203–226. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Unal, S.; Arslan, S.; Yilmaz, B.K.; Oktar, F.N.; Ficai, D.; Ficai, A.; Gunduz, O. Polycaprolactone/gelatin/hyaluronic acid electrospun scaffolds to mimic glioblastoma extracellular matrix. Materials 2020, 13, 2661. [Google Scholar] [CrossRef]
- Palamà, I.E.; D’Amone, S.; Cortese, B. Microenvironmental rigidity of 3D scaffolds and influence on glioblastoma cells: A biomaterial design perspective. Front. Bioeng. Fiotechnol. 2018, 6, 131. [Google Scholar] [CrossRef]
- Rao, S.S.; DeJesus, J.; Short, A.R.; Otero, J.J.; Sarkar, A.; Winter, J.O. Glioblastoma behaviors in three-dimensional collagen-hyaluronan composite hydrogels. ACS Appl. Mater. Interfaces 2013, 5, 9276–9284. [Google Scholar] [CrossRef] [PubMed]
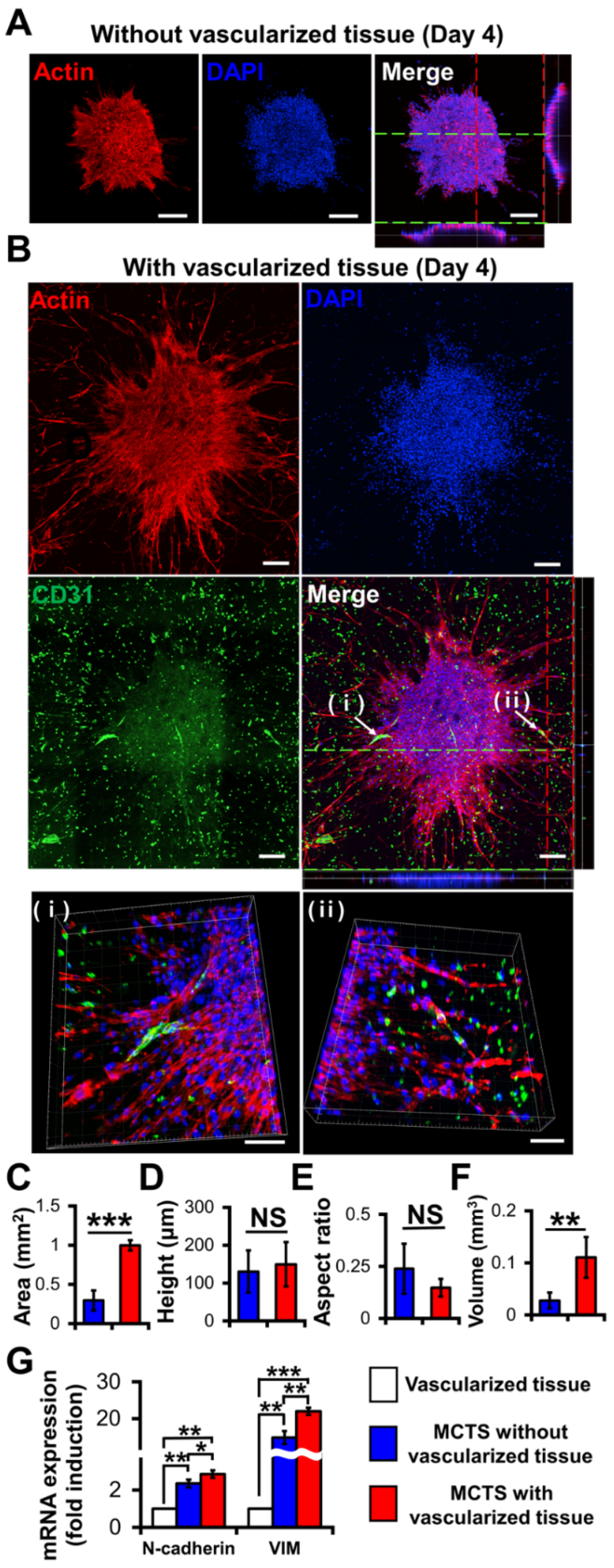
- Han, S.; Kim, S.; Chen, Z.; Shin, H.K.; Lee, S.Y.; Moon, H.E.; Paek, S.H.; Park, S. 3D Bioprinted Vascularized Tumour for Drug Testing. Int. J. Mol. Sci. 2020, 21, 2993. [Google Scholar] [CrossRef] [PubMed]
- Azzarelli, R. Organoid models of glioblastoma to study brain tumor stem cells. Front. Cell Dev. Biol. 2020, 8, 220. [Google Scholar] [CrossRef]
- Klein, E.; Hau, A.-C.; Oudin, A.; Golebiewska, A.; Niclou, S.P. Glioblastoma organoids: Pre-clinical applications and challenges in the context of immunotherapy. Front. Oncol. 2020, 10, 604121. [Google Scholar] [CrossRef]
- Rybin, M.J.; Ivan, M.E.; Ayad, N.G.; Zeier, Z. Organoid models of glioblastoma and their role in drug discovery. Front. Cell. Neurosci. 2021, 15, 605255. [Google Scholar] [CrossRef]
- Hubert, C.G.; Rivera, M.; Spangler, L.C.; Wu, Q.; Mack, S.C.; Prager, B.C.; Couce, M.; McLendon, R.E.; Sloan, A.E.; Rich, J.N. A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Cancer Res. 2016, 76, 2465–2477. [Google Scholar] [CrossRef]
- Sundar, S.J.; Shakya, S.; Barnett, A.; Wallace, L.C.; Jeon, H.; Sloan, A.; Recinos, V.; Hubert, C.G. Three-dimensional organoid culture unveils resistance to clinical therapies in adult and pediatric glioblastoma. Transl. Oncol. 2022, 15, 101251. [Google Scholar] [CrossRef]
- Mitchell, K.; Sprowls, S.A.; Arora, S.; Shakya, S.; Silver, D.J.; Goins, C.M.; Wallace, L.; Roversi, G.; Schafer, R.E.; Kay, K.; et al. WDR5 represents a therapeutically exploitable target for cancer stem cells in glioblastoma. Genes Dev. 2023, 37, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, K.; Kwapiszewski, R.; Brzózka, Z. Microfluidic devices as tools for mimicking the in vivo environment. New J. Chem. 2011, 35, 979–990. [Google Scholar] [CrossRef]
- Arneth, B. Tumor microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Torino, S.; Corrado, B.; Iodice, M.; Coppola, G. Pdms-based microfluidic devices for cell culture. Inventions 2018, 3, 65. [Google Scholar] [CrossRef]
- Akbari Kenari, M.; Rezvani Ghomi, E.; Akbari Kenari, A.; Arabi, S.M.S.; Deylami, J.; Ramakrishna, S. Biomedical applications of microfluidic devices: Achievements and challenges. Polym. Adv. Technol. 2022, 33, 3920–3934. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Ahn, S.I.; Sei, Y.J.; Park, H.J.; Kim, J.; Ryu, Y.; Choi, J.J.; Sung, H.J.; MacDonald, T.J.; Levey, A.I.; Kim, Y. Microengineered human blood-brain barrier platform for understanding nanoparticle transport mechanisms. Nat. Commun. 2020, 11, 175. [Google Scholar] [CrossRef]
- Straehla, J.P.; Hajal, C.; Safford, H.C.; Offeddu, G.S.; Boehnke, N.; Dacoba, T.G.; Wyckoff, J.; Kamm, R.D.; Hammond, P.T. A predictive microfluidic model of human glioblastoma to assess trafficking of blood–brain barrier-penetrant nanoparticles. Proc. Natl. Acad. Sci. USA 2022, 119, e2118697119. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Morales, R.-T.T.; Qian, W.; Wang, H.; Gagner, J.-P.; Dolgalev, I.; Placantonakis, D.; Zagzag, D.; Cimmino, L.; Snuderl, M. Hacking macrophage-associated immunosuppression for regulating glioblastoma angiogenesis. Biomaterials 2018, 161, 164–178. [Google Scholar] [CrossRef]
- Truong, D.; Fiorelli, R.; Barrientos, E.S.; Melendez, E.L.; Sanai, N.; Mehta, S.; Nikkhah, M. A three-dimensional (3D) organotypic microfluidic model for glioma stem cells–Vascular interactions. Biomaterials 2019, 198, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Ho, I.A.; Shim, W.S. Contribution of the microenvironmental niche to glioblastoma heterogeneity. BioMed Res. Int. 2017, 2017, 9634172. [Google Scholar] [CrossRef]
- Rape, A.; Ananthanarayanan, B.; Kumar, S. Engineering strategies to mimic the glioblastoma microenvironment. Adv. Drug Deliv. Rev. 2014, 79, 172–183. [Google Scholar] [CrossRef]
- Adjei-Sowah, E.A.; O’Connor, S.A.; Veldhuizen, J.; Lo Cascio, C.; Plaisier, C.; Mehta, S.; Nikkhah, M. Investigating the Interactions of Glioma Stem Cells in the Perivascular Niche at Single-Cell Resolution using a Microfluidic Tumor Microenvironment Model. Adv. Sci. 2022, 9, 2201436. [Google Scholar] [CrossRef]
- Chen, C.; Mehl, B.T.; Munshi, A.S.; Townsend, A.D.; Spence, D.M.; Martin, R.S. 3D-printed microfluidic devices: Fabrication, advantages and limitations—A mini review. Anal. Methods 2016, 8, 6005–6012. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dong, J.; Bhattacharjee, S.; Wijeratne, S.; Bruening, M.L.; Baker, G.L. Increased protein sorption in poly (acrylic acid)-containing films through incorporation of comb-like polymers and film adsorption at low pH and high ionic strength. Langmuir 2013, 29, 2946–2954. [Google Scholar] [CrossRef] [PubMed]
- Caballero, D.; Kaushik, S.; Correlo, V.; Oliveira, J.; Reis, R.; Kundu, S. Organ-on-chip models of cancer metastasis for future personalized medicine: From chip to the patient. Biomaterials 2017, 149, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, J.M.; Monge, R.; Martínez-González, A.; Virumbrales-Muñoz, M.; Llamazares, G.A.; Berganzo, J.; Hernández-Laín, A.; Santolaria, J.; Doblaré, M.; Hubert, C. Glioblastoma on a microfluidic chip: Generating pseudopalisades and enhancing aggressiveness through blood vessel obstruction events. Neuro-Oncology 2017, 19, 503–513. [Google Scholar] [CrossRef]
- Cai, X.; Briggs, R.G.; Homburg, H.B.; Young, I.M.; Davis, E.J.; Lin, Y.-H.; Battiste, J.D.; Sughrue, M.E. Application of microfluidic devices for glioblastoma study: Current status and future directions. Biomed. Microdevices 2020, 22, 60. [Google Scholar] [CrossRef]






| Method | Technique | Mechanism | Advantages | Limitations | Reference |
|---|---|---|---|---|---|
| Scaffold-free | Hanging Drop Method | Suspends small cell droplets; allows spheroid formation via gravity | Simple; cost-effective; uniform spheroids | Susceptible to droplet detachment; limited scalability | [33] |
| Low-Adhesion Plates | Uses non-adherent surfaces to prevent cell attachment to promote aggregation into spheroids | Easy to use; suitable for high-throughput screening | Variability in spheroid size; lacks ECM | [41,42] | |
| Magnetic Levitation Method | Uses magnetic nanoparticles to levitate and aggregate cells into spheroids | Forms large spheroids rapidly | Costly; potential biocompatibility issues | [49,50] | |
| Scaffold-based | ECM Gels | Embeds cells in hydrogels to mimic the natural tumor ECM microenvironment | High biocompatibility; mimics natural microenvironment | Limited mechanical strength; requires tuning of ECM | [57,59] |
| 3D Bioprinting | Layer-by-layer printing of bioinks to create complex 3D structures | Formation of functional tissue models; better mimics in vivo tumor traits | Expensive setup; limited bioink options | [62,63] | |
| Organoids | GBM Organoids | Self-assembly of cells to create 3D tumor models | Mimics tumor heterogeneity and stem cell niches | Long culture times; variability in size and structure | [77] |
| Microfluidics | Microfluidic Devices | Uses microchannels to create controlled, dynamic environments for cell growth | Real-time monitoring; precise control of microenvironments | Complex fabrication; scalability challenges | [80,81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Lee, S.; Kim, M.-H.; Park, S.; Lee, S.-Y. Recapitulating Glioma Stem Cell Niches Using 3D Spheroid Models for Glioblastoma Research. Biosensors 2024, 14, 539. https://doi.org/10.3390/bios14110539
Jo H, Lee S, Kim M-H, Park S, Lee S-Y. Recapitulating Glioma Stem Cell Niches Using 3D Spheroid Models for Glioblastoma Research. Biosensors. 2024; 14(11):539. https://doi.org/10.3390/bios14110539
Chicago/Turabian StyleJo, Hyunji, Seulgi Lee, Min-Hyeok Kim, Sungsu Park, and Seo-Yeon Lee. 2024. "Recapitulating Glioma Stem Cell Niches Using 3D Spheroid Models for Glioblastoma Research" Biosensors 14, no. 11: 539. https://doi.org/10.3390/bios14110539
APA StyleJo, H., Lee, S., Kim, M.-H., Park, S., & Lee, S.-Y. (2024). Recapitulating Glioma Stem Cell Niches Using 3D Spheroid Models for Glioblastoma Research. Biosensors, 14(11), 539. https://doi.org/10.3390/bios14110539






