Scope of Onsite, Portable Prevention Diagnostic Strategies for Alternaria Infections in Medicinal Plants
Abstract
1. Introduction
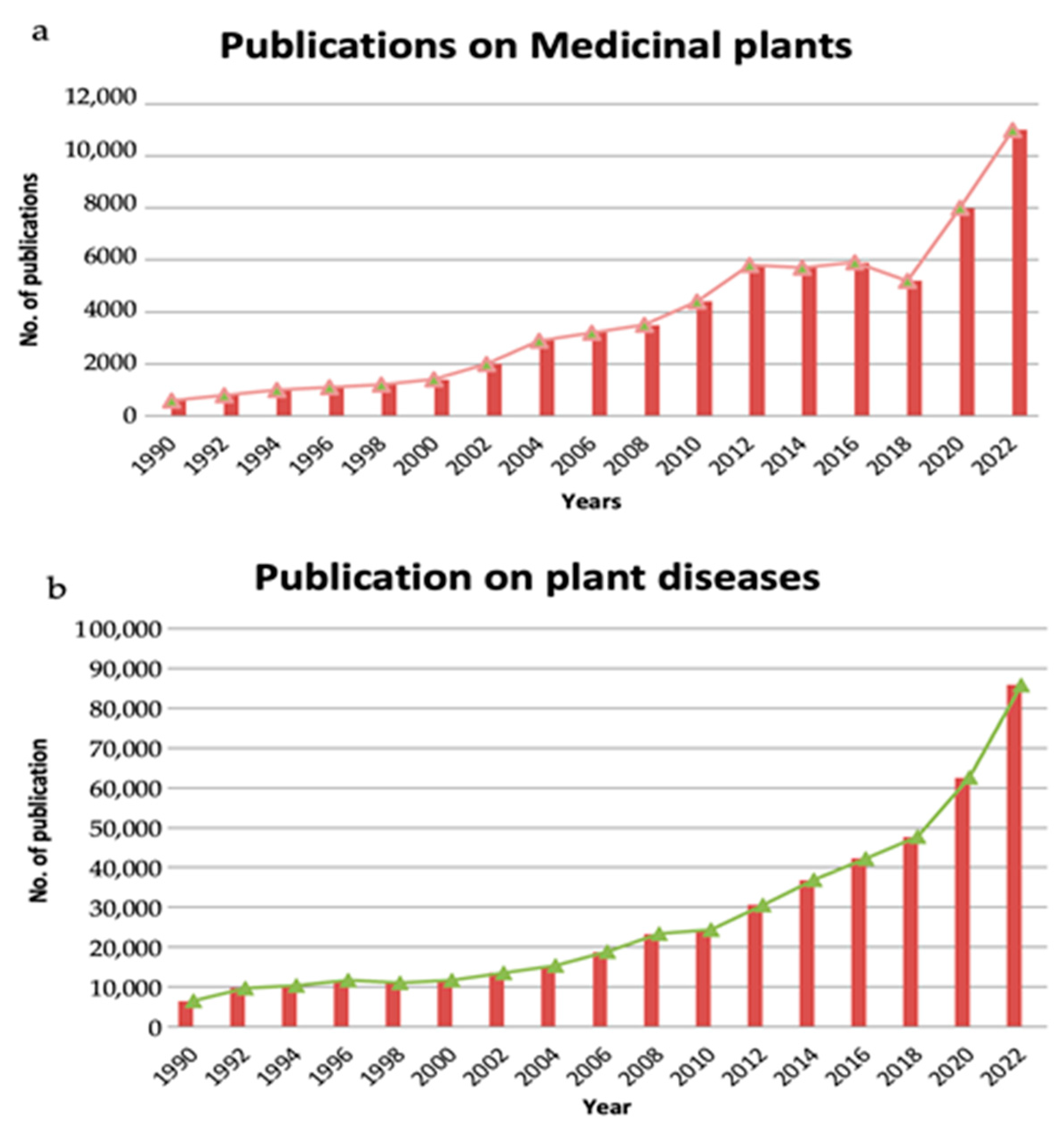
2. Global Status of Disease Burden on Medicinal Plants
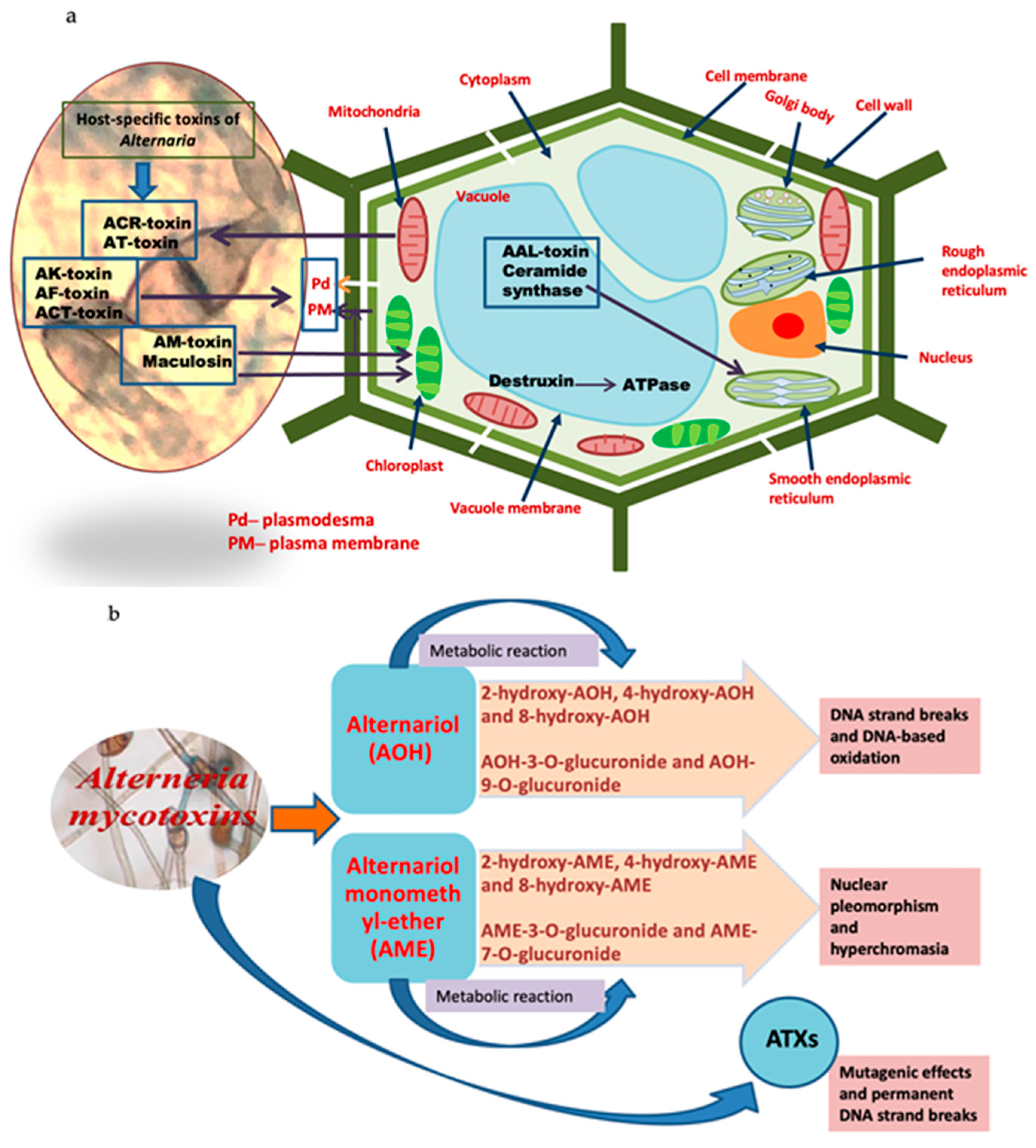
Alternaria Toxins in Medicinal Plants and Their Effects on Humans
3. Preventive Measures for Alternaria Infections in Medicinal Plants
4. Common Available Diagnostic Strategies
4.1. Conventional Methods
4.1.1. Visual Observation
4.1.2. Microscopy
4.1.3. Mycological Diagnosis
4.1.4. Biological Assays or Indicator Plant Tests
4.2. Rapid Lab-Based Diagnostic Methods
4.2.1. Antigen-/Antibody-Based Diagnostics
4.2.2. Molecular Genetic Identification
4.2.3. Flow Cytometry (FCM)
4.2.4. Gas Chromatography
5. Portable Diagnosis Techniques
5.1. PCR-Based Systems
5.2. Lateral Flow Assays (LFAs)
5.3. Microsphere Immunoassays (MIA)
5.4. Hyperspectral Imaging Techniques
5.5. Fluorescence Imaging
5.6. Loop-Mediated Isothermal Amplification (LAMP)
6. Smartphone-Based Portable Device Diagnoses
7. Nanomaterial-Based Biosensing Strategies for Plant Pathogen Detection
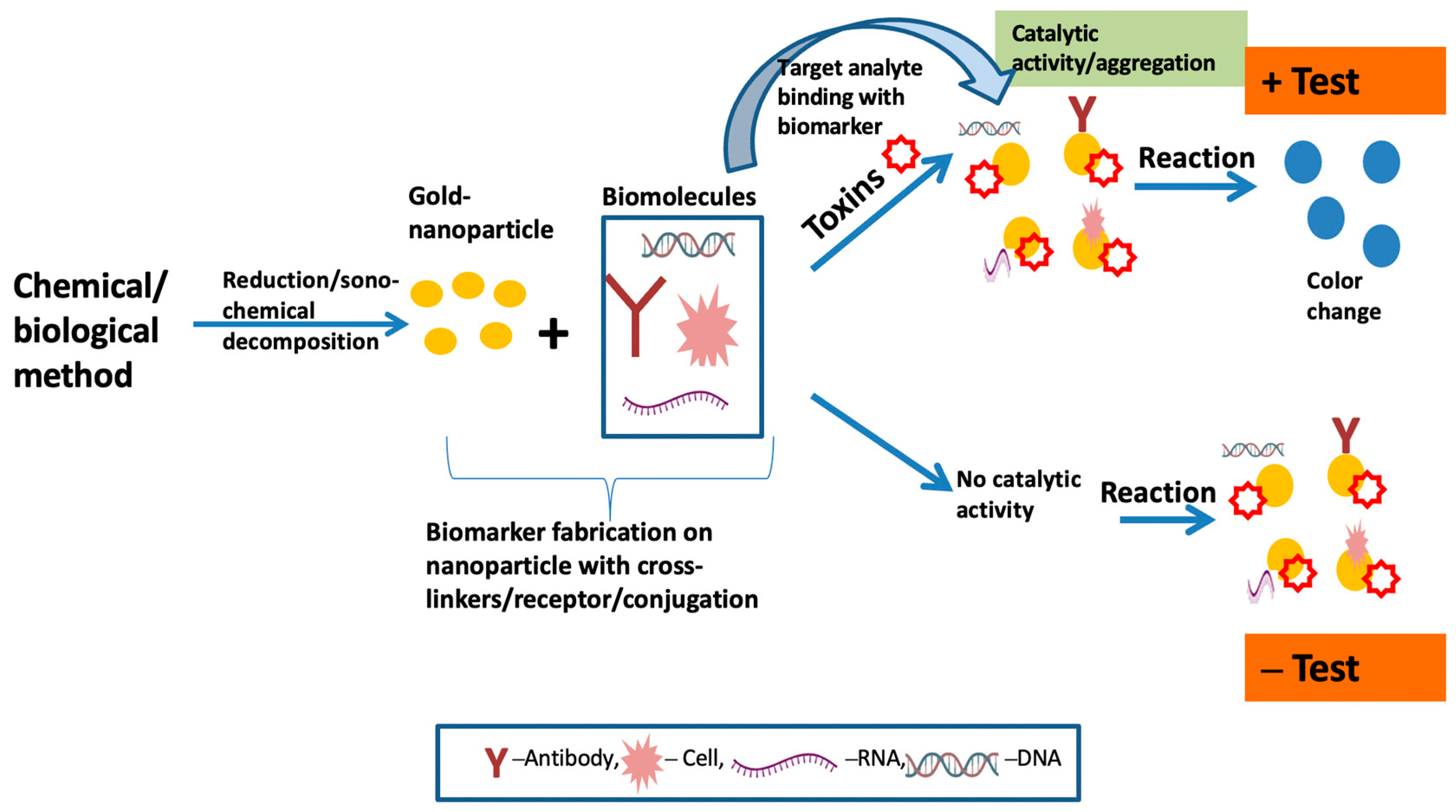
7.1. Colorimetric Sensing
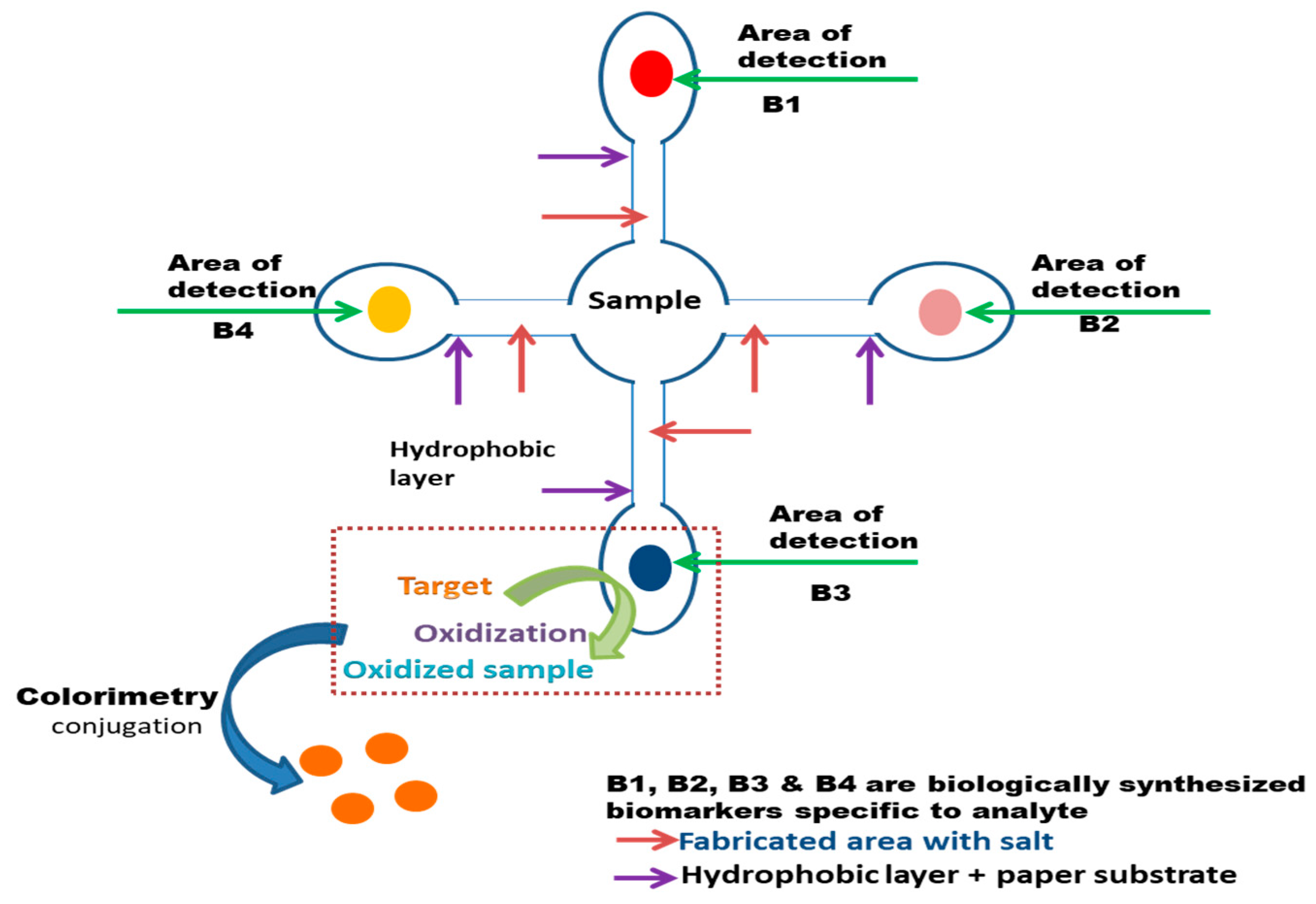
7.2. Paper-Based Sensing
7.3. Surface Plasmon Resonance (SPR)-Based Biosensing
7.4. Chip-Based Rapid Sensing
7.5. Electrochemical Sensing
8. Idea for Suitable Future Strategies in the Detection of Alternaria Species
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahmoudi, R. Application of Medicinal Plants: From Past to Present. MOJ Biol. Med. 2017, 1, 80. [Google Scholar] [CrossRef]
- Pešić, M. Development of Natural Product Drugs in a Sustainable Manner. Brief for United Nations Global Sustainable Development Report 2015. 2015. Available online: https://sustainabledevelopment.un.org/content/documents/6544118_Pesic_Development%20of%20natural%20product%20drugs%20in%20a%20%20sustainable%20manner.pdf (accessed on 16 November 2022).
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal Plant Analysis: A Historical and Regional Discussion of Emergent Complex Techniques. Front. Pharmacol. 2019, 10, 1480. [Google Scholar] [CrossRef] [PubMed]
- Swetha, P.; Sundararaj, R. Diseases of Medicinal Plants Cultivated in Karnataka and Their Management. In Medicinal Plants; Intechopen: London, UK, 2022. [Google Scholar] [CrossRef]
- Roberson, E.B.; Frances, A.; Havens, K.; Maschinski, J.; Meyer, A.; Ott, L. Fund plant conservation to solve biodiversity crisis. Science 2020, 367, 258. [Google Scholar] [CrossRef] [PubMed]
- Salmeron-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide Research Trends on Medicinal Plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, D.; Yang, X.; Zhang, L.; Yang, M. Detection of seven Alternaria toxins in edible and medicinal herbs using ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. X 2022, 13, 100186. [Google Scholar] [CrossRef]
- De Berardis, S.; De Paola, E.L.; Montevecchi, G.; Garbini, D.; Masino, F.; Antonelli, A.; Melucci, D. Determination of four Alternaria alternata mycotoxins by QuEChERS approach coupled with liquid chromatography-tandem mass spectrometry in tomato-based and fruit-based products. Food Res. Int. 2018, 106, 677–685. [Google Scholar] [CrossRef] [PubMed]
- FAO Launches 2020 as the UN’s International Year of Plant Health 2020. 2 December 2019. Available online: http://www.fao.org/news/story/en/item/1253551/icode/ (accessed on 3 December 2022).
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Buja, I.; Sabella, E.; Monteduro, A.G.; Chiriacò, M.S.; De Bellis, L.; Luvisi, A.; Maruccio, G. Advances in Plant Disease Detection and Monitoring: From Traditional Assays to In-Field Diagnostics. Sensors 2021, 21, 2129. [Google Scholar] [CrossRef]
- Cai, H.Y.; Caswell, J.; Prescott, J.F. Nonculture Molecular Techniques for Diagnosis of Bacterial Disease in Animals: A Diagnostic Laboratory Perspective. Veter. Pathol. 2014, 51, 341–350. [Google Scholar] [CrossRef]
- Kliot, A.; Kontsedalov, S.; Lebedev, G.; Brumin, M.; Cathrin, P.B.; Marubayashi, J.M.; Škaljac, M.; Belausov, E.; Czosnek, H.; Ghanim, M. Fluorescence in situ Hybridizations (FISH) for the Localization of Viruses and Endosymbiotic Bacteria in Plant and Insect Tissues. J. Vis. Exp. 2014, 84, e51030. [Google Scholar] [CrossRef]
- López, M.M.; Llop, P.; Cubero, J.; Penyalver, R.; Caruso, P.; Bertolini, E.; Peñalver, J.; Gorris, M.T.; Cambra, M. Strategies for Improving Serological and Molecular Detection of Plant Pathogenic Bacteria. In Plant Pathogenic Bacteria; Springer: Berlin/Heidelberg, Germany, 2001; pp. 83–86. [Google Scholar] [CrossRef]
- Ward, E.; Foster, S.J.; A Fraaije, B.; Mccartney, H.A. Plant pathogen diagnostics: Immunological and nucleic acid-based approaches. Ann. Appl. Biol. 2004, 145, 1–16. [Google Scholar] [CrossRef]
- Chitarra, L.G.; Bulk, R.W.V.D. The Application of Flow Cytometry and Fluorescent Probe Technology for Detection and Assessment of Viability of Plant Pathogenic Bacteria. Eur. J. Plant Pathol. 2003, 109, 407–417. [Google Scholar] [CrossRef]
- Fang, Y.; Ramasamy, R.P. Current and Prospective Methods for Plant Disease Detection. Biosensors 2015, 5, 537–561. [Google Scholar] [CrossRef]
- Sinha, P.; Govil, J.N.; Singh, V.K. (Eds.) Recent Progress in Medicinal Plants. Diseases and Their Management; SCI Tech Publishing LLC.: Bradford, PA, USA, 2002; pp. 1–6. [Google Scholar]
- Scholes, J.D.; Rolfe, S.A. Chlorophyll fluorescence imaging as tool for understanding the impact of fungal diseases on plant performance: A phenomics perspective. Funct. Plant Biol. 2009, 36, 880–892. [Google Scholar] [CrossRef]
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the Support in Gold-Containing Nanoparticles as Heterogeneous Catalysts. Chem. Rev. 2020, 120, 3890–3938. [Google Scholar] [CrossRef] [PubMed]
- Shivashankarappa, A.; Sanjay, K.R. Escherichia coli-based synthesis of cadmium sulfide nanoparticles, characterization, antimicrobial and cytotoxicity studies. Braz. J. Microbiol. 2020, 51, 939–948. [Google Scholar] [CrossRef]
- Chen, A.; Mao, X.; Sun, Q.; Wei, Z.; Li, J.; You, Y.; Zhao, J.; Jiang, G.; Wu, Y.; Wang, L.; et al. Alternaria Mycotoxins: An Overview of Toxicity, Metabolism, and Analysis in Food. J. Agric. Food Chem. 2021, 69, 7817–7830. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011, 9, 2407. [Google Scholar] [CrossRef]
- Sun, C.; Li, F.; Wei, M.; Xiang, Z.; Chen, C.; Xu, D. Detection and Biological Characteristics of Alternaria alternata Resistant to Difenoconazole from Paris polyphylla var. chinensis, an Indigenous Medicinal Herb. Plant Dis. 2021, 105, 1546–1554. [Google Scholar] [CrossRef]
- Apnikheti. SafedMusli. 2022. Available online: https://www.apnikheti.com/en/pn/agriculture/horticulture/medicinal-plants/safed-musli (accessed on 12 December 2022).
- Manjunath, H.; Nakkeeran, S.; Raguchander, T. First Report of Anthracnose on noni Caused by Colletotrichum gloeosporioides in India. Arch. Phytopathol. Plant Prot. 2012, 45, 276–279. [Google Scholar]
- Barman, S.; Ghosh, R.; Dalal, D.; Mandal, N.C. Suppression of Leaf Blight of Ocimum sanctum L. Using Lactic Acid Bacteria as Novel Bio-control Agent. Proc. Natl. Acad. Sci. USA India Sect. B Boil. Sci. 2018, 88, 1389–1397. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Banerjee, S. First Report of Alternaria brassicae Leaf Spot Disease of Aloe vera and It’s Disease Intensity in West Benga. Eur. J. Biotechnol. Biosci. 2014, 2, 37–43. [Google Scholar]
- Shivanna, M.B.; Parashurama, T.R.; Achar, K.G.S.; Vasanthakumari, M.M. Fungal foliar diseases in Withania somnifera and its effect on secondary metabolites. Plant Biosyst. Int. J. Deal. all Asp. Plant Biol. 2014, 148, 907–916. [Google Scholar] [CrossRef]
- Kulkarni, M.S.; Prashanthi, S.K. Diseases Medicinal Plants Vlll- Holybasil, Mint and lpecac. In Becent Progress in Medicinal Plants. Diseasesand Their Management; Sinha, P., Govil, J.N., Singh, V.K., Eds.; SCI Tech Publishing LLC.: Bradford, PA, USA, 2002; pp. 127–136. [Google Scholar]
- Kalra, A.; Singh, H.B.; Pandey, R.; Samad, A.; Patra, N.K.; Kumar, S. Diseases in Mint: Causal Organisms, Distribution, and Control Measures. J. Herbs, Spices Med. Plants 2005, 11, 71–91. [Google Scholar] [CrossRef]
- Prajapati, P.; Sharma, K. A Hand Book of Medicinal Plants; Agrobios: Jodhpur, India, 2003; p. 81. (In Indian) [Google Scholar]
- Gupta, M.L.; Misra, H.O.; Kalra, A.; Khanuja, S.P.S. Root-Rot and Wilt: A New Disease of Ashwagandha (Withania Somnifera) Caused by Fusarium solani. J. Med. Aromat. Plant Sci. 2004, 26, 285–287. [Google Scholar]
- Chandel, S.; Dubey, K.; Kaushal, P. Major Diseases of Medicinal and Aromatic Plants Recorded in Himachal Pradesh-India. J. Plant Dis. Sci. 2014, 9, 145–153. [Google Scholar]
- Sarkar, S.; Dasgupta, B. First Report of Leaf Spot of Aswagandha (Withania Somnifera Dunal) caused by Colletotrichum gloeosporioides from West Bengal, India. J. Mycopathol. Res. 2017, 55, 257–259. [Google Scholar]
- Achar, K.G.S.; Parashurama, T.R.; Shivanna, M.B. A New Record of Leaf Spot Caused by Xanthomonas campestris in Tinospora cordifolia in India. Int. J. Curr. Microbiol. Appl. Sci. 2014, 1, 269–273. [Google Scholar]
- Kawuri, R.; Suprapta, D.N.; Nitta, Y.; Homma, T. Destructive Leaf Rot Disease Caused by Fusarium oxysporum on Aloe barbadensis Miller in Bali. Agril. Sci. Res. J. 2012, 2, 295–301. [Google Scholar]
- Reddy, P.P. Fungal Diseases and Their Management in Horticultural Crops; Scientific Publishing: New Delhi, India, 2010; Volume 342001, 359p. [Google Scholar]
- Morrison, W.R.; Linderman, S.; Hausbeck, M.K.; Werling, B.P.; Szendrei, Z. Disease and Insect Pests of Asparagus. Extension Bulletin e3219, Michigan State University. 2014. Available online: http://msue.anr.msu.edu/uploads/resources/pdfs/Disease_and_Insect_Pests_of_Asparagus_(E3219) (accessed on 12 December 2022).
- Mondal, G. Occurrence of Synchytrium Inducing Gall on Punarnava (Boerhavia diffusa) from West Bengal, India. In Proceedings of the 6th International Conference on “Plant, Pathogens and People” with the Mission “Challenges in Plant Pathologyto Benefit Humankind”, New Delhi, India, 23–27 February 2016; Volume 27, p. 110012. [Google Scholar]
- Jetawat, R.P.S.; Mathur, K.; Singh, K.; Singh, A. Effect of Fungicides against Fusarium solani and Rhizoctonia solani Infecting Ashwagandha. Ecoscan 2015, 7, 75–78. [Google Scholar]
- de Figueirêdo, G.S.; de Figueirêdo, L.C.; Cavalcanti, F.C.N.; dos Santos, A.C.; da Costa, A.F.; de Oliveira, N.T. Biological and chemical control of Sclerotinia sclerotiorum using Trichoderma spp. and Ulocladium atrum and pathogenicity to bean plants. Braz. Arch. Biol. Technol. 2010, 53, 1–9. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, J.; Xuan, Z.; Li, L.; Wang, H.; Wang, S.; Liu, H.; Wang, S. Development and validation of a rapid and efficient method for simultaneous determination of mycotoxins in coix seed using one-step extraction and UHPLC-HRMS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2021, 38, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zou, L.; Luo, R.; Wang, Y. Determination of five Alternaria toxins in wolfberry using modified QuEChERS and ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2020, 311, 125975. [Google Scholar] [CrossRef] [PubMed]
- Dall’asta, C.; Cirlini, M.; Falavigna, C. Chapter Three—Mycotoxins from Alternaria: Toxicological Implications. In Advances in Molecular Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 8, pp. 107–121. [Google Scholar] [CrossRef]
- Dai, J.; Peng, H.; Chen, W.; Cheng, J.; Wu, Y. Development of multiplex real-time PCR for simultaneous detection of three Potyviruses in tobacco plants. J. Appl. Microbiol. 2013, 114, 502–508. [Google Scholar] [CrossRef]
- Zhao, J.; Bao, S.; Ma, G.; Wu, X. Characterization of Alternaria species associated with muskmelon foliar diseases in Beijing municipality of China. J. Gen. Plant Pathol. 2016, 82, 29–32. [Google Scholar] [CrossRef]
- Blagojević, J.; Vukojević, J.; Ivanović, B.; Ivanović, Ž. Characterization of Alternaria Species Associated with Leaf Spot Disease of Armoracia rusticana in Serbia. Plant Dis. 2020, 104, 1378–1389. [Google Scholar] [CrossRef] [PubMed]
- Matić, S.; Tabone, G.; Garibaldi, A.; Gullino, M.L. Alternaria Leaf Spot Caused by Alternaria Species: An Emerging Problem on Ornamental Plants in Italy. Plant Dis. 2020, 104, 2275–2287. [Google Scholar] [CrossRef]
- Ghosh, R.; Barman, S.; Khatun, J.; Mandal, N.C. Biological control of Alternaria alternata causing leaf spot disease of Aloe vera using two strains of rhizobacteria. Biol. Control 2016, 97, 102–108. [Google Scholar] [CrossRef]
- Pinto, V.E.F.; Patriarca, A. Alternaria Species and Their Associated Mycotoxins. Methods Mol. Biol. 2017, 1542, 13–32. [Google Scholar] [CrossRef]
- Goessens, T.; De Baere, S.; De Troyer, N.; Deknock, A.; Goethals, P.; Lens, L.; Pasmans, F.; Croubels, S. Multi-residue analysis of 20 mycotoxins including major metabolites and emerging mycotoxins in freshwater using UHPLC-MS/MS and application to freshwater ponds in flanders, Belgium. Environ. Res. 2021, 196, 110366. [Google Scholar] [CrossRef]
- Mujahid, C.; Savoy, M.-C.; Baslé, Q.; Woo, P.M.; Ee, E.C.Y.; Mottier, P.; Bessaire, T. Levels of Alternaria Toxins in Selected Food Commodities Including Green Coffee. Toxins 2020, 12, 595. [Google Scholar] [CrossRef]
- Lehmann, L.; Esch, H.; Wagner, J.; Rohnstock, L.; Metzler, M. Estrogenic and genotoxic potential of equol and two hydroxylated metabolites of Daidzein in cultured human Ishikawa cells. Toxicol. Lett. 2005, 158, 72–86. [Google Scholar] [CrossRef]
- Bensassi, F.; Gallerne, C.; El Dein, O.S.; Hajlaoui, M.R.; Bacha, H.; Lemaire, C. Cell death induced by the Alternaria mycotoxin Alternariol. Toxicol. In Vitro 2012, 26, 915–923. [Google Scholar] [CrossRef]
- Pfeiffer, E.; Eschbach, S.; Metzler, M. Alternaria toxins: DNA strand-breaking activity in mammalian cellsin vitro. Mycotoxin Res. 2007, 23, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Neils, A.L.; Brisco-McCann, E.I.; Harlan, B.R.; Hausbeck, M.K. Management strategies for Alternaria leaf blight on American ginseng. Crop Prot. 2021, 139, 105302. [Google Scholar] [CrossRef]
- Waterworth, K. Treatment for Alternaria—Recognizing and Preventing Alternaria Symptoms. Gardening Know How. 20 July 2022. Available online: https://www.gardeningknowhow.com/plant-problems/disease/alternaria-leaf-spot.htm (accessed on 31 January 2023).
- Viriyasuthee, W.; Jogloy, S.; Saksirirat, W.; Saepaisan, S.; Gleason, M.L.; Chen, R.S. Biological Control of Alternaria Leaf Spot Caused by Alternaria spp. in Jerusalem Artichoke (Helianthus tuberosus L.) under Two Fertilization Regimes. Plants 2019, 8, 463. [Google Scholar] [CrossRef]
- Kgatle, M.; Flett, B.; Truter, M.; Aveling, T. Control of Alternaria leaf blight caused by Alternaria alternata on sunflower using fungicides and Bacillus amyloliquefaciens. Crop Prot. 2020, 132, 105146. [Google Scholar] [CrossRef]
- Nielsen, L. Alternaria Leaf Spot Prevention and Treatment, Epic Gardening. 2021. Available online: https://www.epicgardening.com/Alternaria-leaf-spot/ (accessed on 28 December 2022).
- Dyussembayev, K.; Sambasivam, P.; Bar, I.; Brownlie, J.C.; Shiddiky, M.J.A.; Ford, R. Biosensor Technologies for Early Detection and Quantification of Plant Pathogens. Front. Chem. 2021, 9, 636245. [Google Scholar] [CrossRef] [PubMed]
- Chase, A.R. Advanced Treatment of Alternaria. Greenhouse Product News. 11 February 2021. Available online: https://gpnmag.com/article/advanced-treatment-Alternaria/ (accessed on 28 December 2022).
- Mahlein, A.-K. Plant Disease Detection by Imaging Sensors—Parallels and Specific Demands for Precision Agriculture and Plant Phenotyping. Plant Dis. 2016, 100, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.-C. Remote Sensing of Diseases. Annu. Rev. Phytopathol. 2020, 58, 225–252. [Google Scholar] [CrossRef]
- Şensoy, K.G.; Muti, M. The Novel Nanomaterials Based Biosensors and Their Applications. In Novel Nanomaterials; Intechopen: London, UK, 2021. [Google Scholar] [CrossRef]
- Ray, M.; Ray, A.; Dash, S.; Mishra, A.; Achary, K.G.; Nayak, S.; Singh, S. Fungal disease detection in plants: Traditional assays, novel diagnostic techniques and biosensors. Biosens. Bioelectron. 2017, 87, 708–723. [Google Scholar] [CrossRef]
- Lau, H.Y.; Wu, H.; Wee, E.J.H.; Trau, M.; Wang, Y.; Botella, J.R. Specific and Sensitive Isothermal Electrochemical Biosensor for Plant Pathogen DNA Detection with Colloidal Gold Nanoparticles as Probes. Sci. Rep. 2017, 7, 38896. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Sun, Y.; Chen, B.; Hu, F.; Guo, C.; Yang, T. Recent Advances in Colorimetric Sensors Based on Gold Nanoparticles for Pathogen Detection. Biosensors 2022, 13, 29. [Google Scholar] [CrossRef]
- Chane, T.B.; Boyraz, N. Biotechnological Tools for Detection, Identification and Management of Plant Diseases. Afr. J. Biotechnol. 2019, 18, 798–805. [Google Scholar]
- Kaur, L.; Sharma, S.G. Identification of plant diseases and distinct approaches for their management. Bull. Natl. Res. Cent. 2021, 45, 169. [Google Scholar] [CrossRef]
- Liu, L.; Dong, Y.; Huang, W.; Du, X.; Ren, B.; Huang, L.; Zheng, Q.; Ma, H. A Disease Index for Efficiently Detecting Wheat Fusarium Head Blight Using Sentinel-2 Multispectral Imagery. IEEE Access 2020, 8, 52181–52191. [Google Scholar] [CrossRef]
- Patil, P.; Narayankar, P.; Mulimani, D.; Patil, M. Using Microscopic Images to Predict Plant Diseases in a Deep Learning Environment. In ICT for Competitive Strategies; CRC Press: Boca Raton, FL, USA, 2020; pp. 807–813. [Google Scholar] [CrossRef]
- Nezhad, A.S. Future of portable devices for plant pathogen diagnosis. Lab Chip 2014, 14, 2887–2904. [Google Scholar] [CrossRef]
- Khakimov, A.; Salakhutdinov, I.; Omolikov, A.; Utaganov, S. Traditional and current-prospective methods of agricultural plant diseases detection: A review. IOP Conf. Ser. Earth Environ. Sci. 2022, 951, 012002. [Google Scholar] [CrossRef]
- Krug, J.C.; Müller, G.M.; Bills, G.F.; Foster, M.S. Moist chambers for the development of fungi. In Biodiversity of Fungi: Inventory and Monitoring Methods; Elsevier: Amsterdam, The Netherlands, 2004; pp. 589–593. [Google Scholar]
- Khasanov, B.A. Diseases of Agricultural Crops and Measures to Combat Them. Textbook for Graduate Students; TashSAU Publishing Department: Tashkent, Uzbekistan, 2011. [Google Scholar]
- Sharma, R. Diseases of Medicinal & Aromatic Plants and Their Management. Recent Approaches Manag. Plant Dis. 2018, 251, 283. [Google Scholar]
- Dyakov, Y.T.; Elansky, S.N. General Phytopathology; Yurayt Publishing House: Moscow, Russia, 2019. [Google Scholar]
- Bunsevich, L.L.; Kostyuk, M.A.; Danilyuk, Y.P. Fruit Growing and Viticulture of the South of Russia [Electronic Resource]; NCZRIHV: Krasnodar, Russia, 2012; Volume 5, p. 6. [Google Scholar]
- Upadyshev, M.T. Heat resistance of pear plants during recovery from latent viruses using dry-air thermotherapy. Hortic. Vitic. 2022, 1, 44–51. (In Russian) [Google Scholar] [CrossRef]
- Singh, A. Techniques, to Identify Plant Pathogens (Part-1). Plant Cell Technology. Your Partner in Plant Tissue Culture. Available online: https://www.plantcelltechnology.com/blog/techniques-to-identify-plant-pathogens-part1-2239da (accessed on 23 March 2022).
- Narayanasamy, P. Microbial Plant Pathogens-Detection and Disease Diagnosis: Fungal Pathogens; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Schaad, N.W.; Opgenorth, D.; Gaush, P. Real-Time Polymerase Chain Reaction for One-Hour On-Site Diagnosis of Pierce’s Disease of Grape in Early Season Asymptomatic Vines. Phytopathology 2002, 92, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Capote, N.; Pastrana, A.M.; Aguado, A.; Sánchez-Torres, P. Molecular Tools for Detection of Plant Pathogenic Fungi and Fungicide Resistance. Plant Pathol. 2012, 59, 151–202. [Google Scholar]
- Egamberdiev, S.S.; Salakhutdinov, I.B.; Radjabov, F.S.; Kurbanov, A.Y.; Abdurakhmonov, I.Y. Methodical Instruction on the Detection of Pathogen of Fusarium Genera, Identifying More Aggressive Forms and Their Control Measures; Center of Genomics and Bioinformatics of SA of RUz “Fan va talim poligraf” LLC.: Tashkent, Uzbekistan, 2017. [Google Scholar]
- Shapiro, H.M. Practical Flow Cytometry; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Greilhuber, J.; Temsch, E.M.; Loureiro, J.C.M. Nuclear DNA Content Measurement. In Flow Cytometry with Plant Cells; Doležel, J., Greilhuber, J., Suda, J., Eds.; Wiley-VCH Press: Hoboken, NJ, USA, 2007; pp. 67–101. [Google Scholar]
- D’Hondt, L.; Höfte, M.; VAN Bockstaele, E.; Leus, L. Applications of flow cytometry in plant pathology for genome size determination, detection and physiological status. Mol. Plant Pathol. 2011, 12, 815–828. [Google Scholar] [CrossRef]
- Nicolì, F.; Negro, C.; Nutricati, E.; Vergine, M.; Aprile, A.; Sabella, E.; Damiano, G.; De Bellis, L.; Luvisi, A. Accumulation of Azelaic Acid in Xylella fastidiosa-Infected Olive Trees: A Mobile Metabolite for Health Screening. Phytopathology 2019, 109, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Dorokhov, Y.; Komarova, T.; Sheshukova, E. Volatile organic compounds and plant virus–host interaction. In Plant Virus–Host Interactio; Academic Press: Cambridge, MA, USA, 2014; pp. 241–262. [Google Scholar] [CrossRef]
- Agustika, D.K.; Mercuriani, I.S.; Ariyanti, N.A.; Purnomo, C.W.; Triyana, K.; Iliescu, D.D.; Leeson, M.S. Gas Chromatography-Mass Spectrometry Analysis of Compounds Emitted by Pepper Yellow Leaf Curl Virus-Infected Chili Plants: A Preliminary Study. Separations 2021, 8, 136. [Google Scholar] [CrossRef]
- Eid, K.; El-Sayed, A.-N.; Shoala, T. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Sugar Beet Leaf Extracts in Response to Exogenous Application of Resistance Inducers to Manage Sugar Beet Powdery Mildew. Egypt. J. Phytopathol. 2018, 46, 257–277. [Google Scholar] [CrossRef]
- Tholl, D.; Hossain, O.; Weinhold, A.; Röse, U.S.R.; Wei, Q. Trends and applications in plant volatile sampling and analysis. Plant J. 2021, 106, 314–325. [Google Scholar] [CrossRef]
- Luna-Moreno, D.; Sánchez-Álvarez, A.; Islas-Flores, I.; Canto-Canche, B.; Carrillo-Pech, M.; Villarreal-Chiu, J.F.; Rodríguez-Delgado, M. Early Detection of the Fungal Banana Black Sigatoka Pathogen Pseudocercospora fijiensis by an SPR Immunosensor Method. Sensors 2019, 19, 465. [Google Scholar] [CrossRef]
- Gueidan, C.; Aptroot, A.; Cáceres, M.E.D.S.; Binh, N.Q. Erratum to: Molecular phylogeny of the tropical lichen family Pyrenulaceae: Contribution from dried herbarium specimens and FTA card samples. Mycol. Prog. 2016, 15, 14. [Google Scholar] [CrossRef]
- Monis, P.T.; Giglio, S. Nucleic acid amplification-based techniques for pathogen detection and identification. Infect. Genet. Evol. 2006, 6, 2–12. [Google Scholar] [CrossRef]
- Khiyami, M.A.; Almoammar, H.; Awad, Y.; Alghuthaymi, M.A.; Abd-Elsalam, K.A. Plant pathogen nafnodiagnostic techniques: Forthcoming changes? Biotechnol. Biotechnol. Equip. 2014, 28, 775–785. [Google Scholar] [CrossRef]
- Miles, T.D.; Martin, F.N.; Coffey, M.D. Development of Rapid Isothermal Amplification Assays for Detection of Phytophthora spp. in Plant Tissue. Phytopathology 2015, 105, 265–278. [Google Scholar] [CrossRef]
- Donoso, A.; Valenzuela, S. In-field molecular diagnosis of plant pathogens: Recent trends and future perspectives. Plant Pathol. 2018, 67, 1451–1461. [Google Scholar] [CrossRef]
- De Boer, S.H.; López, M.M. New Grower-Friendly Methods for Plant Pathogen Monitoring. Annu. Rev. Phytopathol. 2012, 50, 197–218. [Google Scholar] [CrossRef]
- Pöhlmann, C.; Dieser, I.; Sprinzl, M. A lateral flow assay for identification of Escherichia coli by ribosomal RNA hybridisation. Analyst 2014, 139, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Charlermroj, R.; Himananto, O.; Seepiban, C.; Kumpoosiri, M.; Warin, N.; Oplatowska, M.; Gajanandana, O.; Grant, I.R.; Karoonuthaisiri, N.; Elliott, C.T. Multiplex Detection of Plant Pathogens Using a Microsphere Immunoassay Technology. PLoS ONE 2013, 8, e62344. [Google Scholar] [CrossRef] [PubMed]
- Vignali, D.A. Multiplexed particle-based flow cytometric assays. J. Immunol. Methods 2000, 243, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Mahlein, A.-K.; Steiner, U.; Hillnhütter, C.; Dehne, H.-W.; Oerke, E.-C. Hyperspectral imaging for small-scale analysis of symptoms caused by different sugar beet diseases. Plant Methods 2012, 8, 3. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, G.; Pan, Y.; Yang, X.; Chen, L.; Zhao, C. A Review of Advanced Technologies and Development for Hyperspectral-Based Plant Disease Detection in the Past Three Decades. Remote Sens. 2020, 12, 3188. [Google Scholar] [CrossRef]
- Cheshkova, A.F. A review of hyperspectral image analysis techniques for plant disease detection and identif ication. Vavilovskii Zhurnal Genet. Selektsii 2022, 26, 202–213. [Google Scholar] [CrossRef]
- Gao, Z.; Shao, Y.; Xuan, G.; Wang, Y.; Liu, Y.; Han, X. Real-time hyperspectral imaging for the in-field estimation of strawberry ripeness with deep learning. Artif. Intell. Agric. 2020, 4, 31–38. [Google Scholar] [CrossRef]
- Pan, T.-T.; Chyngyz, E.; Sun, D.-W.; Paliwal, J.; Pu, H. Pathogenetic process monitoring and early detection of pear black spot disease caused by Alternaria alternata using hyperspectral imaging. Postharvest Biol. Technol. 2019, 154, 96–104. [Google Scholar] [CrossRef]
- Bauriegel, E.; Brabandt, H.; Gärber, U.; Herppich, W. Chlorophyll fluorescence imaging to facilitate breeding of Bremia lactucae-resistant lettuce cultivars. Comput. Electron. Agric. 2014, 105, 74–82. [Google Scholar] [CrossRef]
- Chaerle, L.; Hagenbeek, D.; De Bruyne, E.; Valcke, R.; Van Der Straeten, D. Thermal and Chlorophyll-Fluorescence Imaging Distinguish Plant-Pathogen Interactions at an Early Stage. Plant Cell Physiol. 2004, 45, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Konanz, J.; Herrle, F.; Weiss, C.; Post, S.; Kienle, P. Quality of life of patients after low anterior, intersphincteric, and abdominoperineal resection for rectal cancer—A matched-pair analysis. Int. J. Color. Dis. 2013, 28, 679–688. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef]
- Meng, X. Determination of Porosity of Lignocellulosic Biomass before and after Pretreatment by Using Simons’ Stain and NMR Techniques Bioresource Technology. Bioresour. Technol. 2013, 144, 467–476. [Google Scholar] [CrossRef]
- Castaldi, S.; Zorrilla, J.G.; Petrillo, C.; Russo, M.T.; Ambrosino, P.; Masi, M.; Cimmino, A.; Isticato, R. Alternaria alternata Isolated from Infected Pears (Pyrus communis) in Italy Produces Non-Host Toxins and Hydrolytic Enzymes as Infection Mechanisms and Exhibits Competitive Exclusion against Botrytis cinerea in Co-Infected Host Fruits. J. Fungi 2023, 9, 326. [Google Scholar] [CrossRef]
- Malewar, A. Smartphone-Based Portable Device Diagnoses Plant Diseases in the Field. Tech Explorist—Latest Science News. 2019. Available online: https://www.techexplorist.com (accessed on 11 June 2023).
- Verma, S.; Chug, A.; Singh, A.P.; Sharma, S.; Rajvanshi, P. Deep Learning-Based Mobile Application for Plant Disease Diagnosis: A Proof of Concept with a Case Study on Tomato Plant. In Advances in Environmental Engineering and Green Technologies; IGI Global: Hershey, PA, USA, 2019; pp. 242–271. [Google Scholar]
- Lu, J.; Hu, J.; Zhao, G.; Mei, F.; Zhang, C. An in-field automatic wheat disease diagnosis system. Comput. Electron. Agric. 2017, 142, 369–379. [Google Scholar] [CrossRef]
- Ramcharan, A.; McCloskey, P.; Baranowski, K.; Mbilinyi, N.; Mrisho, L.; Ndalahwa, M.; Legg, J.; Hughes, D.P. A Mobile-Based Deep Learning Model for Cassava Disease Diagnosis. Front. Plant Sci. 2019, 10, 272. [Google Scholar] [CrossRef]
- Janarthan, S.; Thuseethan, S.; Rajasegarar, S.; Yearwood, J. P2OP—Plant Pathology on Palms: A deep learning-based mobile solution for in-field plant disease detection. Comput. Electron. Agric. 2022, 202, 107371. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Chen, M.; Hou, C.; Huo, D.; Fa, H.; Zhao, Y.; Shen, C. A sensitive electrochemical DNA biosensor based on three-dimensional nitrogen-doped graphene and Fe3O4 nanoparticles. Sens. Actuators B Chem. 2017, 239, 421–429. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Che Marzuki, N.H.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xiong, E.; Zhang, X.; Zhang, X.; Chen, J. Nanomaterials as signal amplification elements in DNA-based electrochemical sensing. Nano Today 2014, 9, 197–211. [Google Scholar] [CrossRef]
- Chauhan, N.; Saxena, K.; Jain, U. Single molecule detection; from microscopy to sensors. Int. J. Biol. Macromol. 2022, 209, 1389–1401. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, A.S.; Hassani, S.; Mirnia, K.; Abdollahi, M. Recent Advances in Nanotechnology-Based Biosensors Development for Detection of Arsenic, Lead, Mercury, and Cadmium. Int. J. Nanomed. 2021, 16, 803–832. [Google Scholar] [CrossRef]
- Zheng, K.; Zhou, D.; Wu, L.; Li, J.; Zhao, B.; Zhang, S.; He, R.; Xiao, L.; Zoya, I.; Yu, L.; et al. Gold-Nanoparticle-Based Multistage Drug Delivery System for Antitumor Therapy. Drug Deliv. 2022, 29, 3186–3196. [Google Scholar] [CrossRef]
- Goyal, R.N.; Gupta, V.K.; Chatterjee, S. Voltammetric biosensors for the determination of paracetamol at carbon nanotube modified pyrolytic graphite electrode. Sens. Actuators B Chem. 2010, 149, 252–258. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Ali, M.A. Nanomaterials in Biosensors. In Nanomaterials for Biosensors; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–74. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, M.; Wang, Y.; Liu, J. Nanozyme and aptamer- based immunosorbent assay for aflatoxin B1. J. Hazard. Mater. 2020, 399, 123154. [Google Scholar] [CrossRef]
- Jain, S.; Nehra, M.; Kumar, R.; Dilbaghi, N.; Hu, T.; Kumar, S.; Kaushik, A.; Li, C.-Z. Internet of medical things (IoMT)-integrated biosensors for point-of-care testing of infectious diseases. Biosens. Bioelectron. 2021, 179, 113074. [Google Scholar] [CrossRef]
- Reich, M.; Bosshard, P.P.; Stark, M.; Beyser, K.; Borgmann, S. Species Identification of Bacteria and Fungi from Solid and Liquid Culture Media by MALDI-TOF Mass Spectrometry. J. Bacteriol. Parasitol. 2013, 10. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, M.; Lee, T.; Choi, J.-W. Highly Sensitive Biosensors Based on Biomolecules and Functional Nanomaterials Depending on the Types of Nanomaterials: A Perspective Review. Materials 2020, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Wang, Y.; Zhao, L.; Ji, C.; Chen, D.; Nie, L. Applications of Gold Nanoparticles in Non-Optical Biosensors. Nanomaterials 2018, 8, 977. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Chen, C.-P.; Wu, T.-H.; Yang, C.-H.; Lin, C.-W.; Chen, C.-Y. Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef] [PubMed]
- Bülbül, G.; Hayat, A.; Andreescu, S. Portable Nanoparticle-Based Sensors for Food Safety Assessment. Sensors 2015, 15, 30736–30758. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Gupta, R.; Malik, V.; Ameta, R.K. Emerging nanomaterials for improved biosensing. Meas. Sens. 2021, 16, 100050. [Google Scholar] [CrossRef]
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef]
- Ebralidze, I.I.; Laschuk, N.O.; Poisson, J.; Zenkina, O.V. Colorimetric Sensors and Sensor Arrays. In Nanomaterials Design for Sensing Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–39. [Google Scholar]
- Dester, E.; Kao, K.; Alocilja, E.C. Detection of Unamplified E. coli O157 DNA Extracted from Large Food Samples Using a Gold Nanoparticle Colorimetric Biosensor. Biosensors 2022, 12, 274. [Google Scholar] [CrossRef]
- Lau, H.Y.; Botella, J.R. Advanced DNA-Based Point-of-Care Diagnostic Methods for Plant Diseases Detection. Front. Plant Sci. 2017, 8, 2016. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, T.; de la Guardia, M.; Baradaran, B. Lateral flow assays towards point-of-care cancer detection: A review of current progress and future trends. TrAC Trends Anal. Chem. 2020, 125, 115842. [Google Scholar] [CrossRef]
- Hristov, D.R.; Rodriguez-Quijada, C.; Gomez-Marquez, J.; Hamad-Schifferli, K. Designing Paper-Based Immunoassays for Biomedical Applications. Sensors 2019, 19, 554. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Kim, M.I. Nanomaterial-mediated paper-based biosensors for colorimetric pathogen detection. TrAC Trends Anal. Chem. 2020, 132, 116038. [Google Scholar] [CrossRef]
- Hu, J.; Wang, S.; Wang, L.; Li, F.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Advances in paper-based point-of-care diagnostics. Biosens. Bioelectron. 2014, 54, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-S.; Lee, M.R.; Yang, K.-Y.; Kim, C.S.; Lee, K.-H. Paper-based colorimetric sensor for easy and simple detection of polygalacturonase activity aiming for diagnosis of Allium white rot disease. Anal. Chim. Acta 2020, 1113, 1–8. [Google Scholar] [CrossRef]
- Jang, H.; Park, J.-H.; Oh, J.; Kim, K.; Kim, M.-G. Advanced Colorimetric Paper Sensors Using Color Focusing Effect Based on Asymmetric Flow of Fluid. ACS Sens. 2019, 4, 1103–1108. [Google Scholar] [CrossRef]
- Florschütz, K.; Schröter, A.; Schmieder, S.; Chen, W.; Schweizer, P.; Sonntag, F.; Danz, N.; Baronian, K.; Kunze, G. ‘Phytochip’: On-chip detection of phytopathogenic RNA viruses by a new surface plasmon resonance platform. J. Virol. Methods 2013, 189, 80–86. [Google Scholar] [CrossRef]
- Li, Z.; Yu, T.; Paul, R.; Fan, J.; Yang, Y.; Wei, Q. Agricultural nanodiagnostics for plant diseases: Recent advances and challenges. Nanoscale Adv. 2020, 2, 3083–3094. [Google Scholar] [CrossRef]
- Abdulhalim, I.; Zourob, M.; Lakhtakia, A. Surface Plasmon Resonance for Biosensing: A Mini-Review. Electromagnetics 2008, 28, 214–242. [Google Scholar] [CrossRef]
- Shpacovitch, V.; Hergenröder, R. Surface Plasmon Resonance (SPR)-Based Biosensors as Instruments with High Versatility and Sensitivity. Sensors 2020, 20, 3010. [Google Scholar] [CrossRef]
- Li, M.; Cushing, S.K.; Wu, N. Plasmon-enhanced optical sensors: A review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, E.; Chandler, D.J.; Nussio, M.; Mamotte, C.D. Real-time and Label-free Bio-sensing of Molecular Interactions by Surface Plasmon Resonance: A Laboratory Medicine Perspective. Clin. Biochem. Rev. 2012, 33, 161–173. [Google Scholar] [PubMed]
- Shrivastav, A.M.; Cvelbar, U.; Abdulhalim, I. A comprehensive review on plasmonic-based biosensors used in viral diagnostics. Commun. Biol. 2021, 4, 70. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, P.; Zalnezhad, E.; Sekaran, S.D.; Adikan, F.R.M. Rapid Immunoglobulin M-Based Dengue Diagnostic Test Using Surface Plasmon Resonance Biosensor. Sci. Rep. 2014, 4, 3851. [Google Scholar] [CrossRef]
- Patel, R.; Mitra, B.; Vinchurkar, M.; Adami, A.; Patkar, R.; Giacomozzi, F.; Lorenzelli, L.; Baghini, M.S. A review of recent advances in plant-pathogen detection systems. Heliyon 2022, 8, e11855. [Google Scholar] [CrossRef] [PubMed]
- Elmer, W.; White, J.C. The Future of Nanotechnology in Plant Pathology. Annu. Rev. Phytopathol. 2018, 56, 111–133. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Topkaya, S.N.; Azimzadeh, M.; Ozsoz, M. Electrochemical Biosensors for Cancer Biomarkers Detection: Recent Advances and Challenges. Electroanalysis 2016, 28, 1402–1419. [Google Scholar] [CrossRef]
- Yazdanparast, S.; Benvidi, A.; Banaei, M.; Nikukar, H.; Tezerjani, M.D.; Azimzadeh, M. Dual-aptamer based electrochemical sandwich biosensor for MCF-7 human breast cancer cells using silver nanoparticle labels and a poly(glutamic acid)/MWNT nanocomposite. Microchim. Acta 2018, 185, 405. [Google Scholar] [CrossRef] [PubMed]
- Krivitsky, V.; Granot, E.; Avidor, Y.; Borberg, E.; Voegele, R.T.; Patolsky, F. Rapid Collection and Aptamer-Based Sensitive Electrochemical Detection of Soybean Rust Fungi Airborne Urediniospores. ACS Sens. 2021, 6, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Güner, A.; Çevik, E.; Şenel, M.; Alpsoy, L. An electrochemical immunosensor for sensitive detection of Escherichia coli O157:H7 by using chitosan, MWCNT, polypyrrole with gold nanoparticles hybrid sensing platform. Food Chem. 2017, 229, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Bekir, K.; Barhoumi, H.; Braiek, M.; Chrouda, A.; Zine, N.; Abid, N.; Maaref, A.; Bakhrouf, A.; Ouada, H.B.; Jaffrezic-Renault, N.; et al. Electrochemical impedance immunosensor for rapid detection of stressed pathogenic Staphylococcus aureus bacteria. Environ. Sci. Pollut. Res. 2015, 22, 15796–15803. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, X.; Dai, H.; Wu, Q. State Estimation of Lithium Ion Battery Based on Electrochemical Impedance Spectroscopy with On-Board Impedance Measurement System. In Proceedings of the 2015 IEEE Vehicle Power and Propulsion Conference, Montreal, QC, Canada, 19–22 October 2015; pp. 1–5. [Google Scholar] [CrossRef]
- Alatraktchi, F.A.; Svendsen, W.E.; Molin, S. Electrochemical Detection of Pyocyanin as a Biomarker for Pseudomonas aeruginosa: A Focused Review. Sensors 2020, 20, 5218. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A. An introduction to sensors and biosensors. In Electrochemical Biosensors; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–10. [Google Scholar]
- Kaya, H.O.; Cetin, A.E.; Azimzadeh, M.; Topkaya, S.N. Pathogen detection with electrochemical biosensors: Advantages, challenges and future perspectives. J. Electroanal. Chem. 2021, 882, 114989. [Google Scholar] [CrossRef]
- Liu, C.; Xu, C.; Xue, N.; Sun, J.H.; Cai, H.; Li, T.; Liu, Y.; Wang, J. Enzyme Biosensors for Point-of-Care Testing. In MEMS Sensors—Design and Application; Intechopen: London, UK, 2018. [Google Scholar] [CrossRef]
- Sawant, S.N. Development of Biosensors From Biopolymer Composites. In Biopolymer Composites in Electronics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 353–383. [Google Scholar]
- Zarei, A.; Zamani, Z.; Fattahi, R.; Salami, A.; Mousavi, A. Analysis of the Phenylpropanoid Enzyme Activities and Products in the Soft- and Hard-Seeded Pomegranate Genotypes during Fruit Development. Int. J. Fruit Sci. 2015, 16, 242–258. [Google Scholar] [CrossRef]
- Nouri, B.; Mohtasebi, S.S.; Rafiee, S. Quality detection of pomegranate fruit infected with fungal disease. Int. J. Food Prop. 2020, 23, 9–21. [Google Scholar] [CrossRef]
- MacDougall, S.; Bayansal, F.; Ahmadi, A. Emerging Methods of Monitoring Volatile Organic Compounds for Detection of Plant Pests and Disease. Biosensors 2022, 12, 239. [Google Scholar] [CrossRef]
- Allison, J.D.; Marcotte, M.; Noseworthy, M.; Ramsfield, T. Forest Biosecurity in Canad-An Integrated Multi-Agency Approach. Front. For. Glob. Chang. 2021, 144. [Google Scholar] [CrossRef]
- Azfar, S.; Nadeem, A.; Basit, A. Pest detection and control techniques using wireless sensor network: A review. J. Entomol. Zool. Stud. JEZS 2015, 3, 92–99. [Google Scholar]
- Effah, E.; Holopainen, J.K.; McCormick, A.C. Potential roles of volatile organic compounds in plant competition. Perspect. Plant Ecol. Evol. Syst. 2019, 38, 58–63. [Google Scholar] [CrossRef]
- He, Y.; Zhou, J.; Fu, R.; Liu, Y.; Wang, Y.; Liu, H.; Zhao, J.; Cui, Y.; Jiao, B. The application of DNA-HRP functionalized AuNP probes in colorimetric detection of citrus-associated Alternaria genes. Talanta 2022, 237, 122917. [Google Scholar] [CrossRef]
- Fethi, A. Novel materials for electrochemical sensing platforms. Sens. Int. 2020, 1, 100035. [Google Scholar] [CrossRef]
- Yoo, S.M.; Lee, S.Y. Optical Biosensor for the Detection of Pathogenic Micro-organisms. Trends Biotechnol. 2016, 34, 7–25. [Google Scholar] [CrossRef]
- Crudo, F.; Barilli, A.; Mena, P.; Rotoli, B.M.; Del Rio, D.; Dall’asta, C.; Dellafiora, L. An in vitro study on the transport and phase II metabolism of the mycotoxin alternariol in combination with the structurally related gut microbial metabolite urolithin C. Toxicol. Lett. 2021, 340, 15–22. [Google Scholar] [CrossRef]
- Mehrannia, L.; Khalilzadeh, B.; Rahbarghazi, R.; Milani, M.; Kanberoglu, G.S.; Yousefi, H.; Erk, N. Electrochemical Biosensors as a Novel Platform in the Identification of Listeriosis Infection. Biosensors 2023, 13, 216. [Google Scholar] [CrossRef]

| Disease | Strain | Family | Infection in Medicinal Plants | Region | References |
|---|---|---|---|---|---|
| Black spot | A. alternata | Pleosporaceae | Paris polyphylla var. chinensis | China | [24] |
| Leaf spot | A. alternata | Pleosporaceae | Adosa (L.) Nees | Bangalore | [4] |
| Leaf spot | A. alternata | Pleosporaceae | Safed musli (Chlorophytum borivilianum) | Karnataka | [25] |
| Leaf blight | A. alternata | Pleosporaceae | Noni (Morinda citrifolia L.) | Tamil Nadu and Karnataka | [26] |
| Leaf blight | A. alternata | Pleosporaceae | Tulsi (Ocimum sanctum L.) | West Bengal | [27] |
| Leaf spot | A. brassicae | Pleosporaceae | Aloe vera (Aloe barbadensis Mill.) | Parganas, West Bengal | [28] |
| Leaf blight | A. tenuis | Pleosporaceae | Sarpagandha (Rauwolfia serpentina (L.) Benth. Ex Kurz) | Bengaluru | [29] |
| Leaf blight | A. alternata | Pleosporaceae | African basil (Ocimum gratissimum L.) | Karnataka | [30] |
| Leaf spot | A. alternata | Pleosporaceae | Mint (Mentha arvensis L.) | Jammu and Kashmir | [31] |
| Leaf blight | A. alternata | Pleosporaceae | Catharanthus roseus L. G. Don. | Haryana | [32] |
| Leaf spot | A. tenuis | Pleosporaceae | Ashwagandha (Withania somnifera) | West Bengal | [33] |
| Leaf blight | A. alternata | Pleosporaceae | Senna (Cassia angustifolia) | Himachal Pradesh | [34] |
| Leaf spot | Colletotrichum gloeosporioides | Glomerellaceae | Ashwagandha (Withania somnifera) | West Bengal | [35] |
| Leaf spot | Xanthomonas campestris | Xanthomonadaceae | Giloy (Tinospora cordifolia (Thunb.) Miers) | Karnataka | [36] |
| Basal stem rot | Fusarium oxysporum | Nectriaceae | Aloe vera (Aloe barbadensis Mill.) | Bali | [37] |
| Damping off | Phytophthora parasitica and Rhizoctinia solani | Peronosporaceae and Ceratobasidiaceae | Belladonna (Atropa belladonna L.) | Jodhpur | [38] |
| Rust | Puccinia asparagi | Pucciniaceae | Asparagus (Asparagus officinalis L.) | Michigan State | [39] |
| Wart | Synchytrium lepidagathidis | Synchytriaceae | Kalmegh (Andrographis paniculata (Burm. f.) Wall. ex Nees) | West Bengal | [40] |
| Damping off | Rhizoctonia solani | Ceratobasidiaceae | Ashwagandha (Withania somnifera) | Udaipur | [41] |
| Sclerotinia blight | Sclerotinia sclerotiorum | Sclerotiniaceae | Mint (Mentha arvensis L.) | Jammu and Kashmir | [31,42] |
| Wilt | Fusarium solani | Nectriaceae | Ashwagandha (Withania somnifera) | Lucknow | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shukla, S.; Singh, P.; Shukla, S.; Ali, S.; Didwania, N. Scope of Onsite, Portable Prevention Diagnostic Strategies for Alternaria Infections in Medicinal Plants. Biosensors 2023, 13, 701. https://doi.org/10.3390/bios13070701
Shukla S, Singh P, Shukla S, Ali S, Didwania N. Scope of Onsite, Portable Prevention Diagnostic Strategies for Alternaria Infections in Medicinal Plants. Biosensors. 2023; 13(7):701. https://doi.org/10.3390/bios13070701
Chicago/Turabian StyleShukla, Sadhana, Pushplata Singh, Shruti Shukla, Sajad Ali, and Nidhi Didwania. 2023. "Scope of Onsite, Portable Prevention Diagnostic Strategies for Alternaria Infections in Medicinal Plants" Biosensors 13, no. 7: 701. https://doi.org/10.3390/bios13070701
APA StyleShukla, S., Singh, P., Shukla, S., Ali, S., & Didwania, N. (2023). Scope of Onsite, Portable Prevention Diagnostic Strategies for Alternaria Infections in Medicinal Plants. Biosensors, 13(7), 701. https://doi.org/10.3390/bios13070701





