Optical Detection of Cancer Cells Using Lab-on-a-Chip
Abstract
1. Introduction
2. Optical-Based Detection
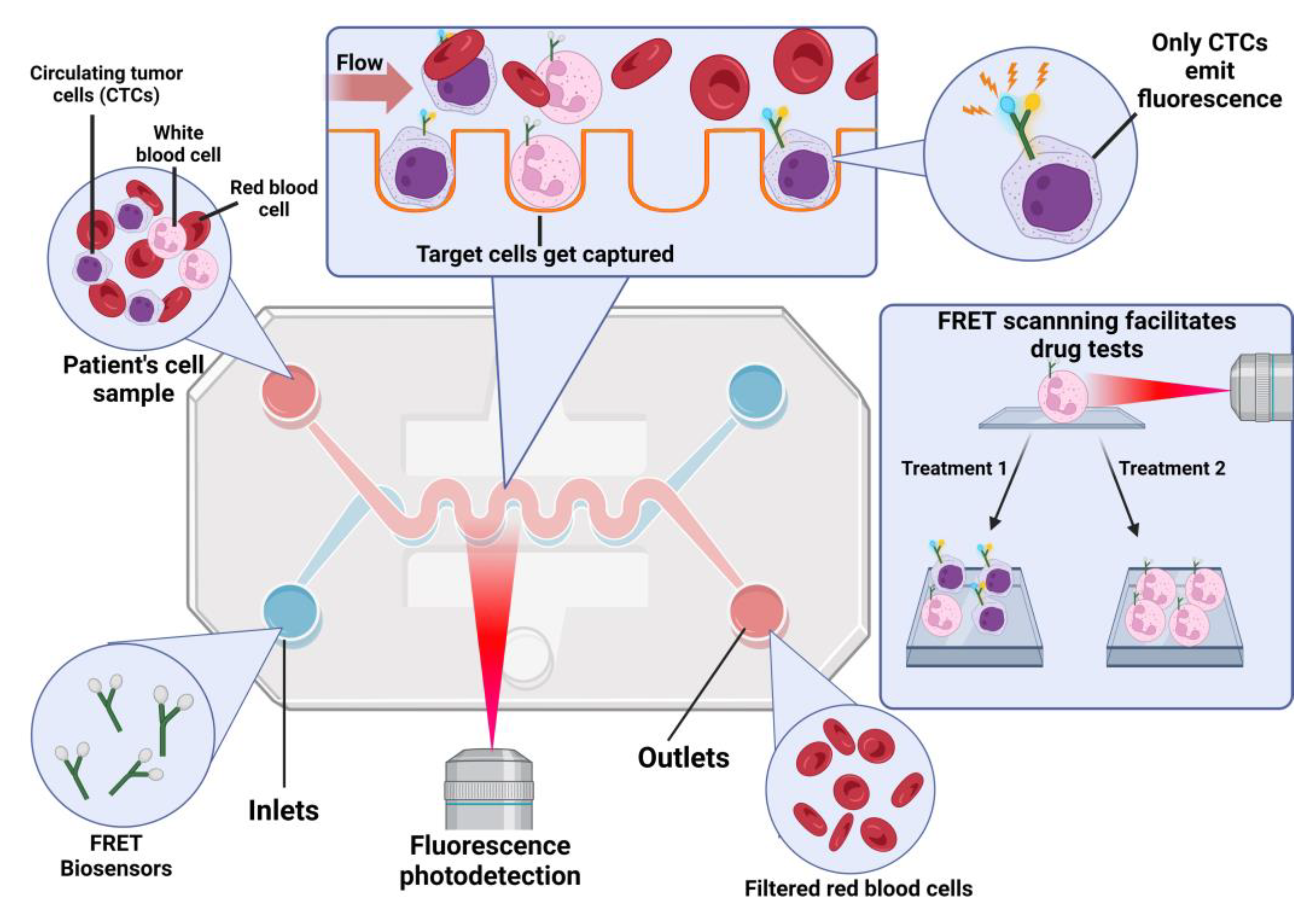
3. Fluorescence-Based Biosensors
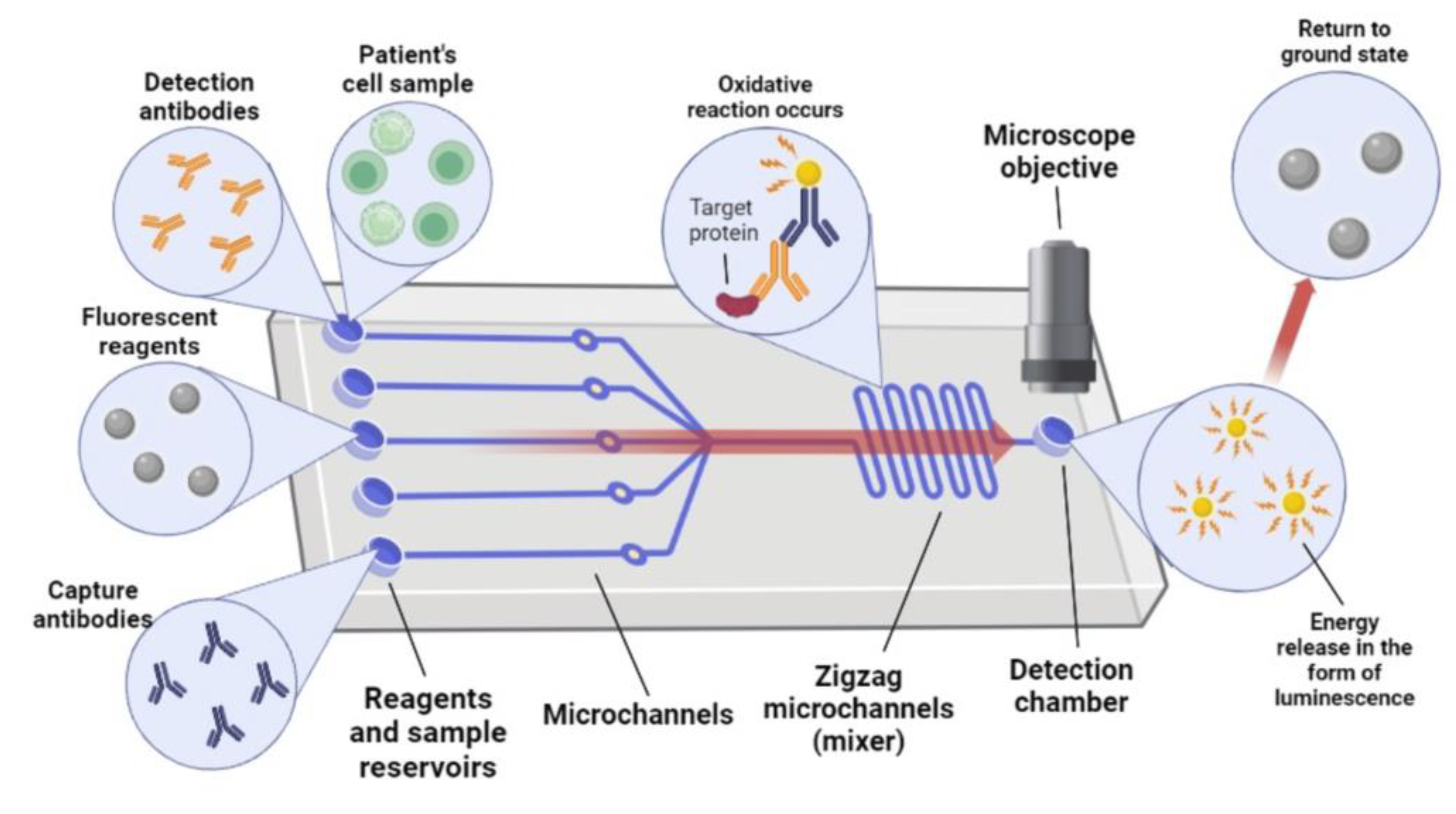
4. Chemiluminescence-Based Biosensors
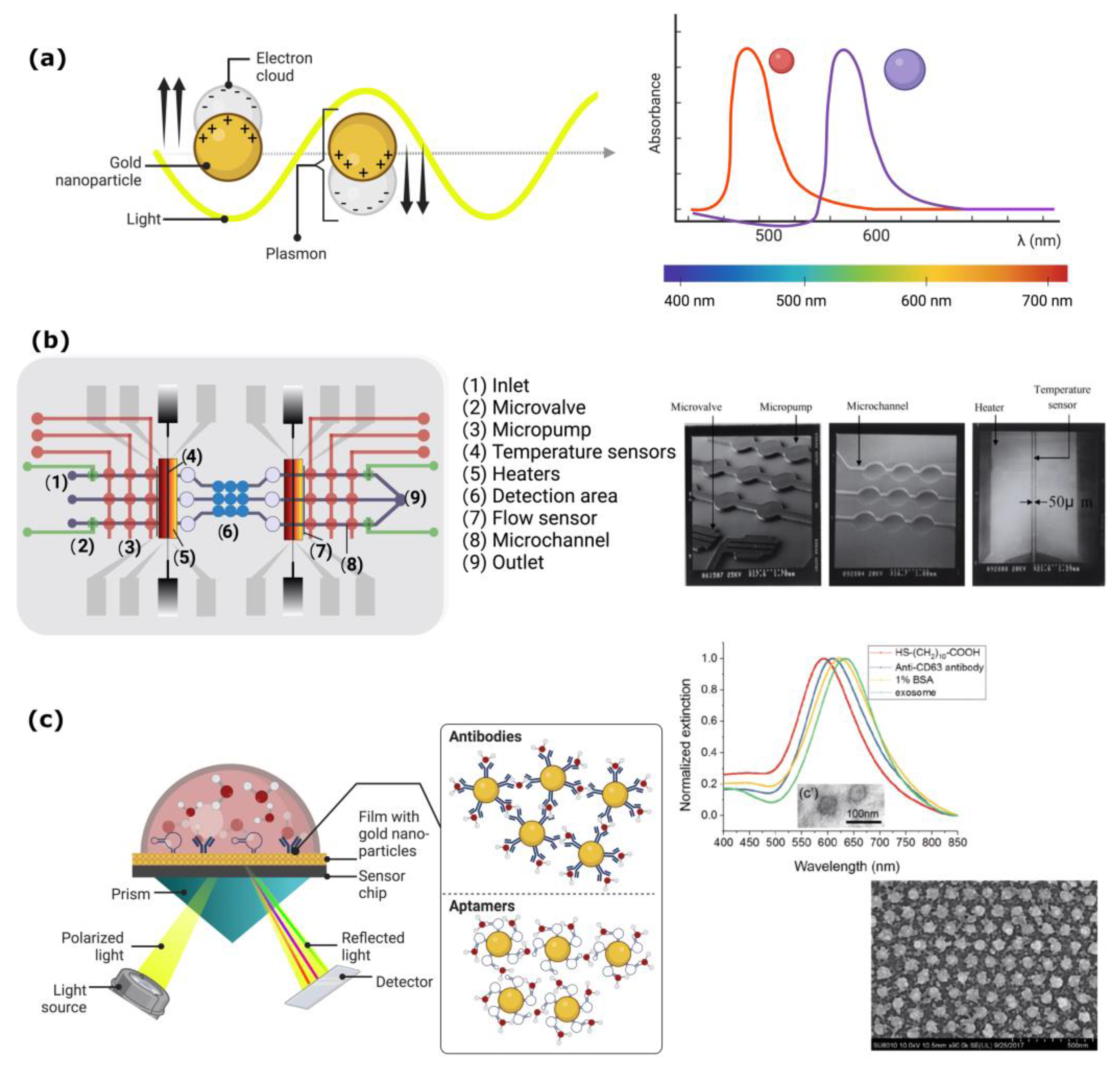
5. Plasmon-Based Biosensors
6. Surface-Enhanced Raman Scattering

7. Discussion
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Gao, L.; Chen, K.; Zhang, W.; Zhang, Q.; Li, Q.; Hu, K. Nanoparticles: A New Approach to Upgrade Cancer Diagnosis and Treatment. Nanoscale Res. Lett. 2021, 16, 88. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bargahi, N.; Ghasemali, S.; Jahandar-Lashaki, S.; Nazari, A. Recent advances for cancer detection and treatment by microfluidic technology, review and update. Biol. Proced. Online 2022, 24, 5. [Google Scholar] [CrossRef]
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early detection of pancreatic cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef]
- Rana, A.; Zhang, Y.; Esfandiari, L. Advancements in microfluidic technologies for isolation and early detection of circulating cancer-related biomarkers. Analyst 2018, 143, 2971–2991. [Google Scholar] [CrossRef]
- Zhou, J.; Kulasinghe, A.; Bogseth, A.; O’Byrne, K.; Punyadeera, C.; Papautsky, I. Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel. Microsyst. Nanoeng. 2019, 5, 8. [Google Scholar] [CrossRef]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef]
- Liu, J.; Lian, J.; Chen, Y.; Zhao, X.; Du, C.; Xu, Y.; Hu, H.; Rao, H.; Hong, X. Circulating Tumor Cells (CTCs): A Unique Model of Cancer Metastases and Non-invasive Biomarkers of Therapeutic Response. Front. Genet. 2021, 12, 734595. [Google Scholar] [CrossRef]
- Azizipour, N.; Avazpour, R.; Rosenzweig, D.H.; Sawan, M.; Ajji, A. Evolution of Biochip Technology: A Review from Lab-on-a-Chip to Organ-on-a-Chip. Micromachines 2020, 11, 599. [Google Scholar] [CrossRef]
- Caballero, D.; Kundu, S.C.; Reis, R.L. (Eds.) Microfluidics and Biosensors in Cancer Research; Springer International Publishing: Cham, Switzerland, 2022. [Google Scholar]
- Alves, P.U.; Vinhas, R.; Fernandes, A.R.; Birol, S.Z.; Trabzon, L.; Bernacka-Wojcik, I.; Igreja, R.; Lopes, P.; Baptista, P.V.; Águas, H.; et al. Multifunctional microfluidic chip for optical nanoprobe based RNA detection—Application to Chronic Myeloid Leukemia. Sci. Rep. 2018, 8, 381. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Tang, H.; Zong, N.; Jiang, X. Microfluidics for Biomedical Analysis. Small Methods 2020, 4, 1900451. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Akgönüllü, S.; Bakhshpour, M.; Pikin, A.K.; Denizli, A. Microfluidic Systems for Cancer Diagnosis and Applications. Micromachines 2021, 12, 1349. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, A.; Prickril, B. (Eds.) Biosensors and Biodetection; Springer: New York, NY, USA, 2017. [Google Scholar]
- Chen, C.; Wang, J. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Kumar, S.; Kaushik, B.K. Recent advancements in optical biosensors for cancer detection. Biosens. Bioelectron. 2022, 197, 113805. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Lee, Y.-C.; Lai, Y.-H.; Lim, J.-C.; Huang, N.-T.; Lin, C.-T.; Huang, J.-J. Review of Integrated Optical Biosensors for Point-of-Care Applications. Biosensors 2020, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Li, J.; Zhao, X.; Pu, K.; Zhang, R. Semiconducting Polymer Nanoreporters for Near-Infrared Chemiluminescence Imaging of Immunoactivation. Adv. Mater. 2020, 32, 1906314. [Google Scholar] [CrossRef]
- Masilamani, V.; Devanesan, S.; AlSalhi, M.S.; AlQahtany, F.S.; Farhat, K.H. Fluorescence spectral detection of acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML): A novel photodiagnosis strategy. Photodiagnosis Photodyn. Ther. 2020, 29, 101634. [Google Scholar] [CrossRef]
- Paidi, S.K.; Raj, P.; Bordett, R.; Zhang, C.; Karandikar, S.H.; Pandey, R.; Barman, I. Raman and quantitative phase imaging allow morpho-molecular recognition of malignancy and stages of B-cell acute lymphoblastic leukemia. Biosens. Bioelectron. 2021, 190, 113403. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Y. Fluorescence Resonance Energy Transfer Biosensors for Cancer Detection and Evaluation of Drug Efficacy. Clin. Cancer Res. 2010, 16, 3822–3824. [Google Scholar] [CrossRef] [PubMed]
- Nedbal, J.; Visitkul, V.; Ortiz-Zapater, E.; Weitsman, G.; Chana, P.; Matthews, D.R.; Ng, T.; Ameer-Beg, S.M. Time-domain microfluidic fluorescence lifetime flow cytometry for high-throughput Förster resonance energy transfer screening. Cytom. Part A 2015, 87, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhao, L.; Dong, H.; Zhao, W.; Liu, S.; Sui, G. Microfluidic Immunoassay System for Rapid Detection and Semi-Quantitative Determination of a Potential Serum Biomarker Mesothelin. ACS Sens. 2019, 4, 2952–2957. [Google Scholar] [CrossRef]
- Edwards, E.E.; Birmingham, K.G.; O’Melia, M.J.; Oh, J.; Thomas, S.N. Fluorometric Quantification of Single-Cell Velocities to Investigate Cancer Metastasis. Cell Syst. 2018, 7, 496–509.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wu, Z.; Zhang, W.; Yu, J.; Li, H.; Di, W.; Duan, Y. Substrate-Induced Growth of Micro/Nanostructured Zn(OH)F Arrays for Highly Sensitive Microfluidic Fluorescence Assays. ACS Appl. Mater. Interfaces 2021, 13, 28462–28471. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Q.; Hu, W.; Liao, J.; Zheng, G.; Su, M. Whole slide imaging of circulating tumor cells captured on a capillary microchannel device. Lab Chip 2019, 19, 3796–3803. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Stein, F.; Rouwkema, J.; Khalil, I.S.M.; Misra, S. Serial imaging of micro-agents and cancer cell spheroids in a microfluidic channel using multicolor fluorescence microscopy. PLoS ONE 2021, 16, e0253222. [Google Scholar] [CrossRef]
- Wang, B.; He, B.-S.; Ruan, X.-L.; Zhu, J.; Hu, R.; Wang, J.; Li, Y.; Yang, Y.-H.; Liu, M.-L. An integrated microfluidics platform with high-throughput single-cell cloning array and concentration gradient generator for efficient cancer drug effect screening. Mil. Med. Res. 2022, 9, 51. [Google Scholar] [CrossRef]
- Chiu, T.-K.; Lei, K.-F.; Hsieh, C.-H.; Hsiao, H.-B.; Wang, H.-M.; Wu, M.-H. Development of a Microfluidic-Based Optical Sensing Device for Label-Free Detection of Circulating Tumor Cells (CTCs) Through Their Lactic Acid Metabolism. Sensors 2015, 15, 6789–6806. [Google Scholar] [CrossRef]
- Ma, N.; Kamalakshakurup, G.; Aghaamoo, M.; Lee, A.P.; Digman, M.A. Label-Free Metabolic Classification of Single Cells in Droplets Using the Phasor Approach to Fluorescence Lifetime Imaging Microscopy. Cytometry A 2019, 95, 93–100. [Google Scholar] [CrossRef]
- Li, X.; Fan, B.; Liu, L.; Chen, D.; Cao, S.; Men, D.; Wang, J.; Chen, J. A Microfluidic Fluorescent Flow Cytometry Capable of Quantifying Cell Sizes and Numbers of Specific Cytosolic Proteins. Sci. Rep. 2018, 8, 14229. [Google Scholar] [CrossRef] [PubMed]
- Hamza, B.; Ng, S.R.; Prakadan, S.M.; Delgado, F.F.; Chin, C.R.; King, E.M.; Yang, L.F.; Davidson, S.M.; DeGouveia, K.L.; Cermak, N.; et al. Optofluidic real-time cell sorter for longitudinal CTC studies in mouse models of cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 2232–2236. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Mankar, S.; Maslova, A.; Ajiri, T.; Yotoriyama, T. Amplified piezoelectrically actuated on-chip flow switching for a rapid and stable microfluidic fluorescence activated cell sorter. RSC Adv. 2020, 10, 40395–40405. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Mankar, S.; Ajiri, T.; Shirai, K.; Yotoriyama, T. An integrated high-throughput microfluidic circulatory fluorescence-activated cell sorting system (μ-CFACS) for the enrichment of rare cells. Lab Chip 2021, 21, 3112–3127. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, C.; Liu, F.; Chen, X.; Liang, G.; Ren, F.; Liang, S.; Song, C.; Shi, J.; Qiu, W.; et al. On-Chip Multicolor Photoacoustic Imaging Flow Cytometry. Anal. Chem. 2021, 93, 8134–8142. [Google Scholar] [CrossRef]
- Isozaki, A.; Mikami, H.; Tezuka, H.; Matsumura, H.; Huang, K.; Akamine, M.; Hiramatsu, K.; Iino, T.; Ito, T.; Karakawa, H.; et al. Intelligent image-activated cell sorting 2.0. Lab Chip 2020, 20, 2263–2273. [Google Scholar] [CrossRef]
- Holzner, G.; Mateescu, B.; van Leeuwen, D.; Cereghetti, G.; Dechant, R.; Stavrakis, S.; deMello, A. High-throughput multiparametric imaging flow cytometry: Toward diffraction-limited sub-cellular detection and monitoring of sub-cellular processes. Cell Rep. 2021, 34, 108824. [Google Scholar] [CrossRef]
- Han, Y.; Lo, Y.-H. Imaging Cells in Flow Cytometer Using Spatial-Temporal Transformation. Sci. Rep. 2015, 5, 13267. [Google Scholar] [CrossRef]
- Mikami, H.; Kawaguchi, M.; Huang, C.-J.; Matsumura, H.; Sugimura, T.; Huang, K.; Lei, C.; Ueno, S.; Miura, T.; Ito, T.; et al. Virtual-freezing fluorescence imaging flow cytometry. Nat. Commun. 2020, 11, 1162. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, C. Electrogenerated Chemiluminescence Biosensing. Anal. Chem. 2020, 92, 524–534. [Google Scholar] [CrossRef]
- Yang, M.; Huang, J.; Fan, J.; Du, J.; Pu, K.; Peng, X. Chemiluminescence for bioimaging and therapeutics: Recent advances and challenges. Chem. Soc. Rev. 2020, 49, 6800–6815. [Google Scholar] [CrossRef] [PubMed]
- Fereja, T.H.; Hymete, A.; Gunasekaran, T. A Recent Review on Chemiluminescence Reaction, Principle and Application on Pharmaceutical Analysis. ISRN Spectrosc. 2013, 2013, 230858. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, J. Chemiluminescence-based aptasensors for various target analytes. Luminescence 2018, 33, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Kim, E.; Park, E.; Lee, J.H. A cost-effective chemiluminescent biosensor capable of early diagnosing cancer using a combination of magnetic beads and platinum nanoparticles. Talanta 2017, 162, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Su, Y.; Lv, Y. Advances in chemiluminescence and electrogenerated chemiluminescence based on silicon nanomaterials. Luminescence 2020, 35, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Laraib, U.; Sargazi, S.; Rahdar, A.; Khatami, M.; Pandey, S. Nanotechnology-based approaches for effective detection of tumor markers: A comprehensive state-of-the-art review. Int. J. Biol. Macromol. 2022, 195, 356–383. [Google Scholar] [CrossRef]
- Wang, S.; Ge, L.; Yan, M.; Yu, J.; Song, X.; Ge, S.; Huang, J. 3D microfluidic origami electrochemiluminescence immunodevice for sensitive point-of-care testing of carcinoma antigen 125. Sens. Actuators B Chem. 2013, 176, 1–8. [Google Scholar] [CrossRef]
- Razvi, S.; Bhana, S.; Mrabeti, S. Challenges in Interpreting Thyroid Stimulating Hormone Results in the Diagnosis of Thyroid Dysfunction. J. Thyroid Res. 2019, 2019, 4106816. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, Y.; Shin, H.S.; Lee, J.H. Highly sensitive chemiluminescence enzyme immunoassay for the quantification of carcinoembryonic antigen in the presence of an enhancer and a stabilizer. J. Immunol. Methods 2019, 471, 18–26. [Google Scholar] [CrossRef]
- Hu, B.; Li, J.; Mou, L.; Liu, Y.; Deng, J.; Qian, W.; Sun, J.; Cha, R.; Jiang, X. An automated and portable microfluidic chemiluminescence immunoassay for quantitative detection of biomarkers. Lab Chip 2017, 17, 2225–2234. [Google Scholar] [CrossRef]
- Yang, S.-M.; Lv, S.; Zhang, W.; Cui, Y. Microfluidic Point-of-Care (POC) Devices in Early Diagnosis: A Review of Opportunities and Challenges. Sensors 2022, 22, 1620. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, Y.; Qiu, R.; Foda, M.F.; Zhang, Y.; Wang, T.; Li, J. The fabrication of magnetic particle-based chemiluminescence immunoassay for human epididymis protein-4 detection in ovarian cancer. Biochem. Biophys. Rep. 2018, 13, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Wang, Y.; Tang, Y.; Zhao, D.; Guo, Q. A graphene quantum dots based electrochemiluminescence immunosensor for carcinoembryonic antigen detection using poly(5-formylindole)/reduced graphene oxide nanocomposite. Biosens. Bioelectron. 2018, 101, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Motaghi, H.; Ziyaee, S.; Mehrgardi, M.A.; Kajani, A.A.; Bordbar, A.-K. Electrochemiluminescence detection of human breast cancer cells using aptamer modified bipolar electrode mounted into 3D printed microchannel. Biosens. Bioelectron. 2018, 118, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Zhao, J.; Wang, S.; Lan, F.; Yan, M.; Yu, J. Ultrasensitive electrochemiluminescence assay of tumor cells and evaluation of H2O2 on a paper-based closed-bipolar electrode by in-situ hybridization chain reaction amplification. Biosens. Bioelectron. 2018, 102, 411–417. [Google Scholar] [CrossRef]
- Babamiri, B.; Bahari, D.; Salimi, A. Highly sensitive bioaffinity electrochemiluminescence sensors: Recent advances and future directions. Biosens. Bioelectron. 2019, 142, 111530. [Google Scholar] [CrossRef]
- Min, X.; Fu, D.; Zhang, J.; Zeng, J.; Weng, Z.; Chen, W.; Zhang, S.; Zhang, D.; Ge, S.; Zhang, J.; et al. An automated microfluidic chemiluminescence immunoassay platform for quantitative detection of biomarkers. Biomed. Microdevices 2018, 20, 91. [Google Scholar] [CrossRef]
- Roda, A.; Mirasoli, M.; Dolci, L.S.; Buragina, A.; Bonvicini, F.; Simoni, P.; Guardigli, M. Portable Device Based on Chemiluminescence Lensless Imaging for Personalized Diagnostics through Multiplex Bioanalysis. Anal. Chem. 2011, 83, 3178–3185. [Google Scholar] [CrossRef]
- Tang, C.K.; Vaze, A.; Rusling, J.F. Automated 3D-printed unibody immunoarray for chemiluminescence detection of cancer biomarker proteins. Lab Chip 2017, 17, 484–489. [Google Scholar] [CrossRef]
- Bellassai, N.; D’Agata, R.; Jungbluth, V.; Spoto, G. Surface Plasmon Resonance for Biomarker Detection: Advances in Non-invasive Cancer Diagnosis. Front. Chem. 2019, 7, 570. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, Z.-H.; Zhao, W.-M.; Wang, L.; Yan, X.; Zhu, A.-s.; Qiu, F.-m.; Zhang, K.-K. Research advances on surface plasmon resonance biosensors. Nanoscale 2022, 14, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Sina, A.A.I.; Vaidyanathan, R.; Wuethrich, A.; Carrascosa, L.G.; Trau, M. Label-free detection of exosomes using a surface plasmon resonance biosensor. Anal. Bioanal. Chem. 2019, 411, 1311–1318. [Google Scholar] [CrossRef]
- Homola, J. Present and future of surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Kou, X.; Yang, Z.; Wang, J. Tailoring Longitudinal Surface Plasmon Wavelengths, Scattering and Absorption Cross Sections of Gold Nanorods. ACS Nano 2008, 2, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Bouhelier, A.; Bachelot, R.; Lerondel, G.; Kostcheev, S.; Royer, P.; Wiederrecht, G.P. Surface Plasmon Characteristics of Tunable Photoluminescence in Single Gold Nanorods. Phys. Rev. Lett. 2005, 95, 267405. [Google Scholar] [CrossRef]
- Klar, T.; Perner, M.; Grosse, S.; von Plessen, G.; Spirkl, W.; Feldmann, J. Surface-Plasmon Resonances in Single Metallic Nanoparticles. Phys. Rev. Lett. 1998, 80, 4249–4252. [Google Scholar] [CrossRef]
- Camley, R.E.; Mills, D.L. Collective excitations of semi-infinite superlattice structures: Surface plasmons, bulk plasmons, and the electron-energy-loss spectrum. Phys. Rev. B 1984, 29, 1695–1706. [Google Scholar] [CrossRef]
- Kim, D.M.; Park, J.S.; Jung, S.-W.; Yeom, J.; Yoo, S.M. Biosensing Applications Using Nanostructure-Based Localized Surface Plasmon Resonance Sensors. Sensors 2021, 21, 3191. [Google Scholar] [CrossRef]
- García Hernández, L.A. Light scattering and plasmonic response of Au–Fe3O4 nanoparticles. SN Appl. Sci. 2020, 2, 1844. [Google Scholar] [CrossRef]
- Falkowski, P.; Lukaszewski, Z.; Gorodkiewicz, E. Potential of surface plasmon resonance biosensors in cancer detection. J. Pharm. Biomed. Anal. 2021, 194, 113802. [Google Scholar] [CrossRef]
- Šípová, H.; Homola, J. Surface plasmon resonance sensing of nucleic acids: A review. Anal. Chim. Acta 2013, 773, 9–23. [Google Scholar] [CrossRef]
- Lee, K.-H.; Su, Y.-D.; Chen, S.-J.; Tseng, F.-G.; Lee, G.-B. Microfluidic systems integrated with two-dimensional surface plasmon resonance phase imaging systems for microarray immunoassay. Biosens. Bioelectron. 2007, 23, 466–472. [Google Scholar] [CrossRef]
- Lv, X.; Geng, Z.; Su, Y.; Fan, Z.; Wang, S.; Fang, W.; Chen, H. Label-Free Exosome Detection Based on a Low-Cost Plasmonic Biosensor Array Integrated with Microfluidics. Langmuir 2019, 35, 9816–9824. [Google Scholar] [CrossRef] [PubMed]
- Liedberg, B.; Nylander, C.; Lundström, I. Biosensing with surface plasmon resonance—How it all started. Biosens. Bioelectron. 1995, 10, i–ix. [Google Scholar] [CrossRef] [PubMed]
- Jebelli, A.; Oroojalian, F.; Fathi, F.; Mokhtarzadeh, A.; Guardia, M.d.l. Recent advances in surface plasmon resonance biosensors for microRNAs detection. Biosens. Bioelectron. 2020, 169, 112599. [Google Scholar] [CrossRef]
- Zeng, S.; Yong, K.-T.; Roy, I.; Dinh, X.-Q.; Yu, X.; Luan, F. A Review on Functionalized Gold Nanoparticles for Biosensing Applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Guo, L.; Jackman, J.A.; Yang, H.-H.; Chen, P.; Cho, N.-J.; Kim, D.-H. Strategies for enhancing the sensitivity of plasmonic nanosensors. Nano Today 2015, 10, 213–239. [Google Scholar] [CrossRef]
- Sannomiya, T.; Vörös, J. Single plasmonic nanoparticles for biosensing. Trends Biotechnol. 2011, 29, 343–351. [Google Scholar] [CrossRef]
- Wang, D.-S.; Fan, S.-K. Microfluidic Surface Plasmon Resonance Sensors: From Principles to Point-of-Care Applications. Sensors 2016, 16, 1175. [Google Scholar] [CrossRef]
- Loyez, M.; Lobry, M.; Hassan, E.M.; DeRosa, M.C.; Caucheteur, C.; Wattiez, R. HER2 breast cancer biomarker detection using a sandwich optical fiber assay. Talanta 2021, 221, 121452. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, S.; Liu, F.-Z.; Shuang, C.; Zhang, B.; Jha, R.; Kaushik, B.K. Etched multicore fiber sensor using copper oxide and gold nanoparticles decorated graphene oxide structure for cancer cells detection. Biosens. Bioelectron. 2020, 168, 112557. [Google Scholar] [CrossRef] [PubMed]
- Raghu, D.; Christodoulides, J.A.; Christophersen, M.; Liu, J.L.; Anderson, G.P.; Robitaille, M.; Byers, J.M.; Raphael, M.P. Nanoplasmonic pillars engineered for single exosome detection. PLoS ONE 2018, 13, e0202773. [Google Scholar] [CrossRef] [PubMed]
- Focsan, M.; Craciun, A.M.; Potara, M.; Leordean, C.; Vulpoi, A.; Maniu, D.; Astilean, S. Flexible and Tunable 3D Gold Nanocups Platform as Plasmonic Biosensor for Specific Dual LSPR-SERS Immuno-Detection. Sci. Rep. 2017, 7, 14240. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef]
- Campion, A.; Kambhampati, P. Surface-enhanced Raman scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Birke, R.L.; Lombardi, J.R. Surface-Enhanced Raman Scattering. In Spectroelectrochemistry: Theory and Practice; Springer US: Boston, MA, USA, 1988; pp. 263–348. [Google Scholar]
- Zhang, R.; Zhang, Y.; Dong, Z.C.; Jiang, S.; Zhang, C.; Chen, L.G.; Zhang, L.; Liao, Y.; Aizpurua, J.; Luo, Y.; et al. Chemical mapping of a single molecule by plasmon-enhanced Raman scattering. Nature 2013, 498, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Jeon, T.; Kim, D.; Park, S.; Kim, S.; Kim, D. Nanostructured plasmonic substrates for use as SERS sensors. Nano Converg. 2016, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Fang, J.; Xu, Z. Advances in droplet microfluidics for SERS and Raman analysis. Biosens. Bioelectron. 2022, 198, 113822. [Google Scholar] [CrossRef]
- Bantz, K.C.; Meyer, A.F.; Wittenberg, N.J.; Im, H.; Kurtuluş, Ö.; Lee, S.H.; Lindquist, N.C.; Oh, S.-H.; Haynes, C.L. Recent progress in SERS biosensing. Phys. Chem. Chem. Phys. 2011, 13, 11551–11567. [Google Scholar] [CrossRef]
- Lin, J.; Zheng, J.; Wu, A. An efficient strategy for circulating tumor cell detection: Surface-enhanced Raman spectroscopy. J. Mater. Chem. B 2020, 8, 3316–3326. [Google Scholar] [CrossRef]
- Xiong, Q.; Lim, C.Y.; Ren, J.; Zhou, J.; Pu, K.; Chan-Park, M.B.; Mao, H.; Lam, Y.C.; Duan, H. Magnetic nanochain integrated microfluidic biochips. Nat. Commun. 2018, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-Y.; Hossain, M.K.; Lee, J.-H.; Han, J.; Lee, H.J.; Kim, K.-J.; Kim, J.-H.; Lee, K.-B.; Choi, J.-W. Selective isolation and noninvasive analysis of circulating cancer stem cells through Raman imaging. Biosens. Bioelectron. 2018, 102, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Willner, M.R.; McMillan, K.S.; Graham, D.; Vikesland, P.J.; Zagnoni, M. Surface-Enhanced Raman Scattering Based Microfluidics for Single-Cell Analysis. Anal. Chem. 2018, 90, 12004–12010. [Google Scholar] [CrossRef]
- Pallaoro, A.; Hoonejani, M.R.; Braun, G.B.; Meinhart, C.D.; Moskovits, M. Rapid Identification by Surface-Enhanced Raman Spectroscopy of Cancer Cells at Low Concentrations Flowing in a Microfluidic Channel. ACS Nano 2015, 9, 4328–4336. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Nguyen, J.T.; Rutledge, S.; Zhang, J.; Wang, C.; Walker, G.C. Detection of chronic lymphocytic leukemia cell surface markers using surface enhanced Raman scattering gold nanoparticles. Cancer Lett. 2010, 292, 91–97. [Google Scholar] [CrossRef]
- Dochow, S.; Krafft, C.; Neugebauer, U.; Bocklitz, T.; Henkel, T.; Mayer, G.; Albert, J.; Popp, J. Tumour cell identification by means of Raman spectroscopy in combination with optical traps and microfluidic environments. Lab Chip 2011, 11, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Ramser, K.; Wenseleers, W.; Dewilde, S.; Doorslaer, S.V.; Moens, L. The combination of resonance Raman spectroscopy, optical tweezers and microfluidic systems applied to the study of various heme-containing single cells. Spectroscopy 2008, 22, 463191. [Google Scholar] [CrossRef]
- Zachariah, E.; Bankapur, A.; Santhosh, C.; Valiathan, M.; Mathur, D. Probing oxidative stress in single erythrocytes with Raman Tweezers. J. Photochem. Photobiol. B Biol. 2010, 100, 113–116. [Google Scholar] [CrossRef]
- Ramser, K.; Enger, J.; Goksör, M.; Hanstorp, D.; Logg, K.; Käll, M. A microfluidic system enabling Raman measurements of the oxygenation cycle in single optically trapped red blood cells. Lab Chip 2005, 5, 431–436. [Google Scholar] [CrossRef]
- Arano-Martinez, J.A.; Martínez-González, C.L.; Salazar, M.I.; Torres-Torres, C. A Framework for Biosensors Assisted by Multiphoton Effects and Machine Learning. Biosensors 2022, 12, 710. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, F.; Zhao, J.; Fu, L.; Gu, Y.; Qu, L.; Zhu, C.; Zhu, J.-J.; Lin, Y. MXenes-based nanomaterials for biosensing and biomedicine. Coord. Chem. Rev. 2023, 479, 215002. [Google Scholar] [CrossRef]
- Du, L.; Chen, W.; Wang, J.; Cai, W.; Kong, S.; Wu, C. Folic acid-functionalized zirconium metal-organic frameworks based electrochemical impedance biosensor for the cancer cell detection. Sens. Actuators B Chem. 2019, 301, 127073. [Google Scholar] [CrossRef]
- Abdullah, A.; Dastider, S.G.; Jasim, I.; Shen, Z.; Yuksek, N.; Zhang, S.; Dweik, M.; Almasri, M. Microfluidic based impedance biosensor for pathogens detection in food products. Electrophoresis 2019, 40, 508–520. [Google Scholar] [CrossRef]
- Dkhar, D.S.; Kumari, R.; Malode, S.J.; Shetti, N.P.; Chandra, P. Integrated lab-on-a-chip devices: Fabrication methodologies, transduction system for sensing purposes. J. Pharm. Biomed. Anal. 2023, 223, 115120. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-care diagnostics for infectious diseases: From methods to devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef]
- Kolluri, N.; Klapperich, C.M.; Cabodi, M. Towards lab-on-a-chip diagnostics for malaria elimination. Lab A Chip 2018, 18, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Dekker, S.; Isgor, P.K.; Feijten, T.; Segerink, L.I.; Odijk, M. From chip-in-a-lab to lab-on-a-chip: A portable Coulter counter using a modular platform. Microsyst. Nanoeng. 2018, 4, 34. [Google Scholar] [CrossRef]
- Chauhan, P.; Bhargava, A.; Kumari, R.; Ratre, P.; Tiwari, R.; Kumar Srivastava, R.; Goryacheva, I.Y.; Kumar Mishra, P. Surface-enhanced Raman scattering biosensors for detection of oncomiRs in breast cancer. Drug Discov. Today 2022, 27, 2121–2136. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Mak, W.C.; Pui Ho, A.H. Chapter 10—Microfluidic-based plasmonic biosensors. In Microfluidic Biosensors; Academic Press: Cambridge, MA, USA, 2023; pp. 287–312. [Google Scholar]
- Jalali, M.; Isaac Hosseini, I.; AbdelFatah, T.; Montermini, L.; Wachsmann Hogiu, S.; Rak, J.; Mahshid, S. Plasmonic nanobowtiefluidic device for sensitive detection of glioma extracellular vesicles by Raman spectrometry. Lab Chip 2021, 21, 855–866. [Google Scholar] [CrossRef]

| Luminophore Type | Sensor Model | Analyte | Sensing System | Dynamic Range | Detection Limit | Ref. |
|---|---|---|---|---|---|---|
| Graphene quantum dots | Sandwich-type immunosensor | carcinoembryonic antigen | signal amplification strategy based on P5FIn/erGO | 0.1 pgmL−1–10 ng mL−1 | 3.78 fg mL−1 | [55] |
| Luminol | ECL sensor | human breast cancer cells (MCF-7) | bipolar electrode mounted into 3D printed microchannel | 100–700 cells | 10 cells | [56] |
| paper-based closed bipolar electrode | 1.0 × 102–1.0 × 107 cells mL−1 | 40 cells mL−1 | [57] |
| SPR Sensor Type | Microfluidic Formats | Detection Limits | Required Volume | Analysis Time |
|---|---|---|---|---|
| Prism-based SPR sensor | Flow-through cell | 0.2 µg/mL | 100–1000 µL | 5–20 min |
| Digital microfluidic | 1 µg/mL | 0.2–1 µL | 1 min | |
| Waveguide, fiber-optic SPR sensor | Flow-through cell | 100 µg/mL | ~200 µL | 10 min |
| Grating-based SPR sensor | Flow-through cell | 100 µg/mL | - | 10 min |
| CD-based | 200 µg/mL | 20–40 µL | 5 min | |
| Localized SPR sensor using nanostructures | Capillary-driven (paper and membrane-based) | - | - | - |
| Flow-through cell | 0.3–1 µg/mL | 30–200 µL | 10 min |
| Classification | Substrate | Receptor | Analyte | Linear Range, LOD | Assay Time | Real Sample | Features | Ref. |
|---|---|---|---|---|---|---|---|---|
| NP-coated optic fiber-based platform | Au film-coated optical fiber | Aptamer, HER2 antibody | Breast cancer HER2 protein | 9.3 ng/mL (77.4 pM) | 10 min | ND | HER2 biomarker detection using sandwich assay with anti-HER2 ssDNA aptamer and HER2 antibody. | [82] |
| Optical fiber with copper oxide nanoflower (CuO-NF) and Au NPs-coated Gox structure | 2-deoxy-D-glucose (2-DG) | Cancer cell | 1 × 10−2–1 × 106 cells/mL, 2–10 cells/mL | ND | ND | Use of multi-core fiber structure. Coating of optical fiber with GOx and CuO-NF: increasing surface area and adsorption capability. Discrimination of cancer cells using 2-DG that binds to GULP receptor: the presence of more GULP receptors on cancer cell, inducing a peak shift. Reusable through washing with PBS. | [83] | |
| Solid-based nanopatterned flatform | Au nanopillars on quartz coverslips | Anti-CD63 antibody | Exosome | ND | ND | MCF7 breast adenocarcinoma cells | Fabrication of Au nanopillar array by electron beam lithography. Enabled multiplexed measurement using LSPRi. | [84] |
| Au nano-ellipsoid array on quartz substrate | Anti-CD63 antibody | Exosome | 1 ng/mL | <4 h | ND | Fabrication of nanostructures via AAO-templated Au deposition on a quartz substrate. Integration of LSPR and microfluidic systems. | [75] | |
| Metal-insulator metal (MIM) nanodisks on PDMS | none | Cancer cell (adherent cell) | NA | ND | ND | Construction of a MIM nanodisk consisting of Au-SiO2-Au on an InP substrate. Fabrication of a flexible sensor by transferring a MIM nanodisk onto PDMS. | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Hernández, L.A.; Martínez-Martínez, E.; Pazos-Solís, D.; Aguado-Preciado, J.; Dutt, A.; Chávez-Ramírez, A.U.; Korgel, B.; Sharma, A.; Oza, G. Optical Detection of Cancer Cells Using Lab-on-a-Chip. Biosensors 2023, 13, 439. https://doi.org/10.3390/bios13040439
García-Hernández LA, Martínez-Martínez E, Pazos-Solís D, Aguado-Preciado J, Dutt A, Chávez-Ramírez AU, Korgel B, Sharma A, Oza G. Optical Detection of Cancer Cells Using Lab-on-a-Chip. Biosensors. 2023; 13(4):439. https://doi.org/10.3390/bios13040439
Chicago/Turabian StyleGarcía-Hernández, Luis Abraham, Eduardo Martínez-Martínez, Denni Pazos-Solís, Javier Aguado-Preciado, Ateet Dutt, Abraham Ulises Chávez-Ramírez, Brian Korgel, Ashutosh Sharma, and Goldie Oza. 2023. "Optical Detection of Cancer Cells Using Lab-on-a-Chip" Biosensors 13, no. 4: 439. https://doi.org/10.3390/bios13040439
APA StyleGarcía-Hernández, L. A., Martínez-Martínez, E., Pazos-Solís, D., Aguado-Preciado, J., Dutt, A., Chávez-Ramírez, A. U., Korgel, B., Sharma, A., & Oza, G. (2023). Optical Detection of Cancer Cells Using Lab-on-a-Chip. Biosensors, 13(4), 439. https://doi.org/10.3390/bios13040439







