Recent Advancements of LSPR Fiber-Optic Biosensing: Combination Methods, Structure, and Prospects
Abstract
1. Introduction
2. Sensing Principle and Combination
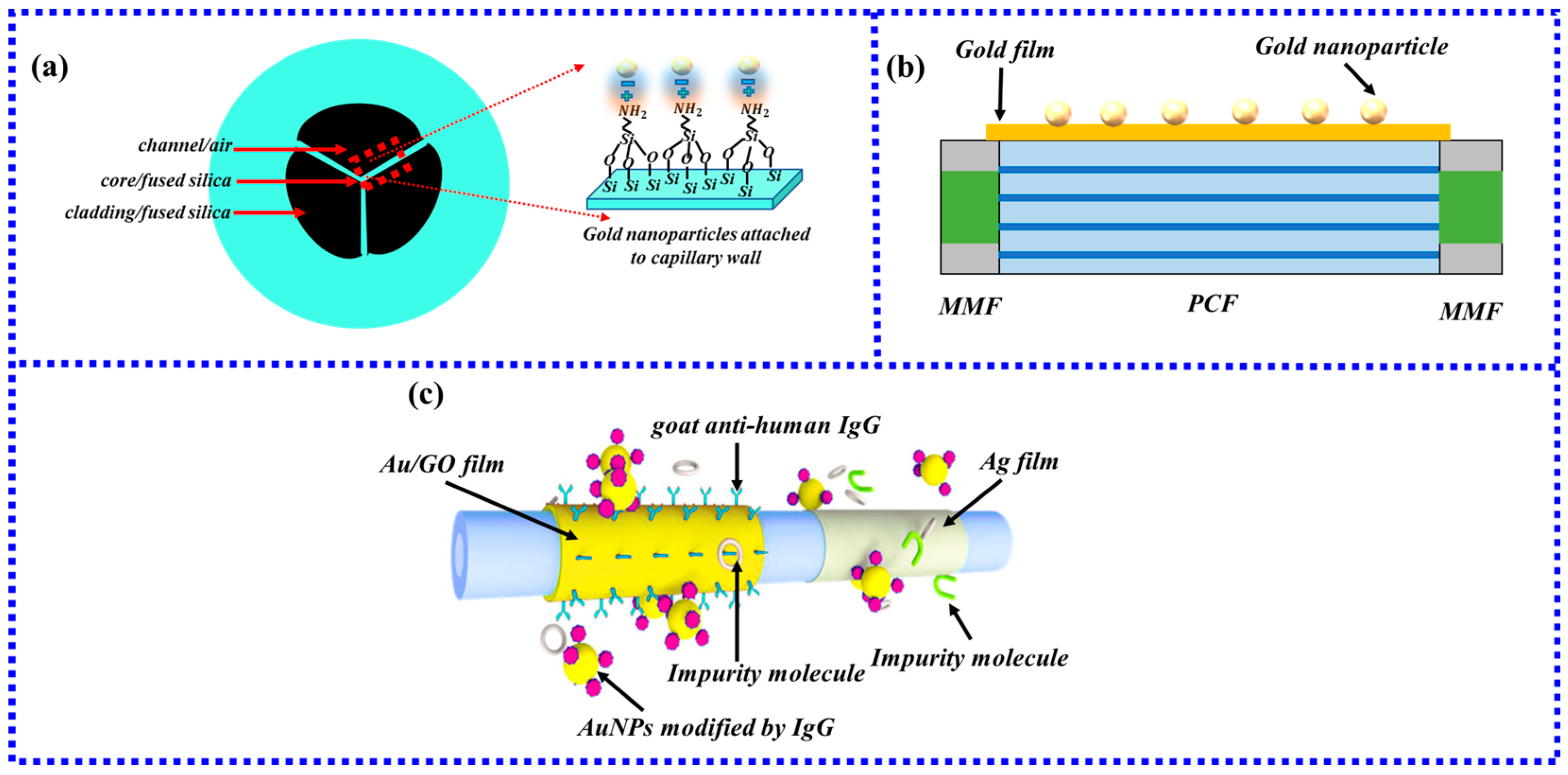
2.1. Ways of Optical Fiber Generate LSPR
2.2. Ways of Biomass Binding to Gold Nanoparticles
2.3. Combination of Gold Nanoparticles to Optical Fiber
2.3.1. Chemical Bond Method
2.3.2. Non-Chemical Bond Method
3. Structure Classification and Application of LSPR Fiber-Optic Biosensors
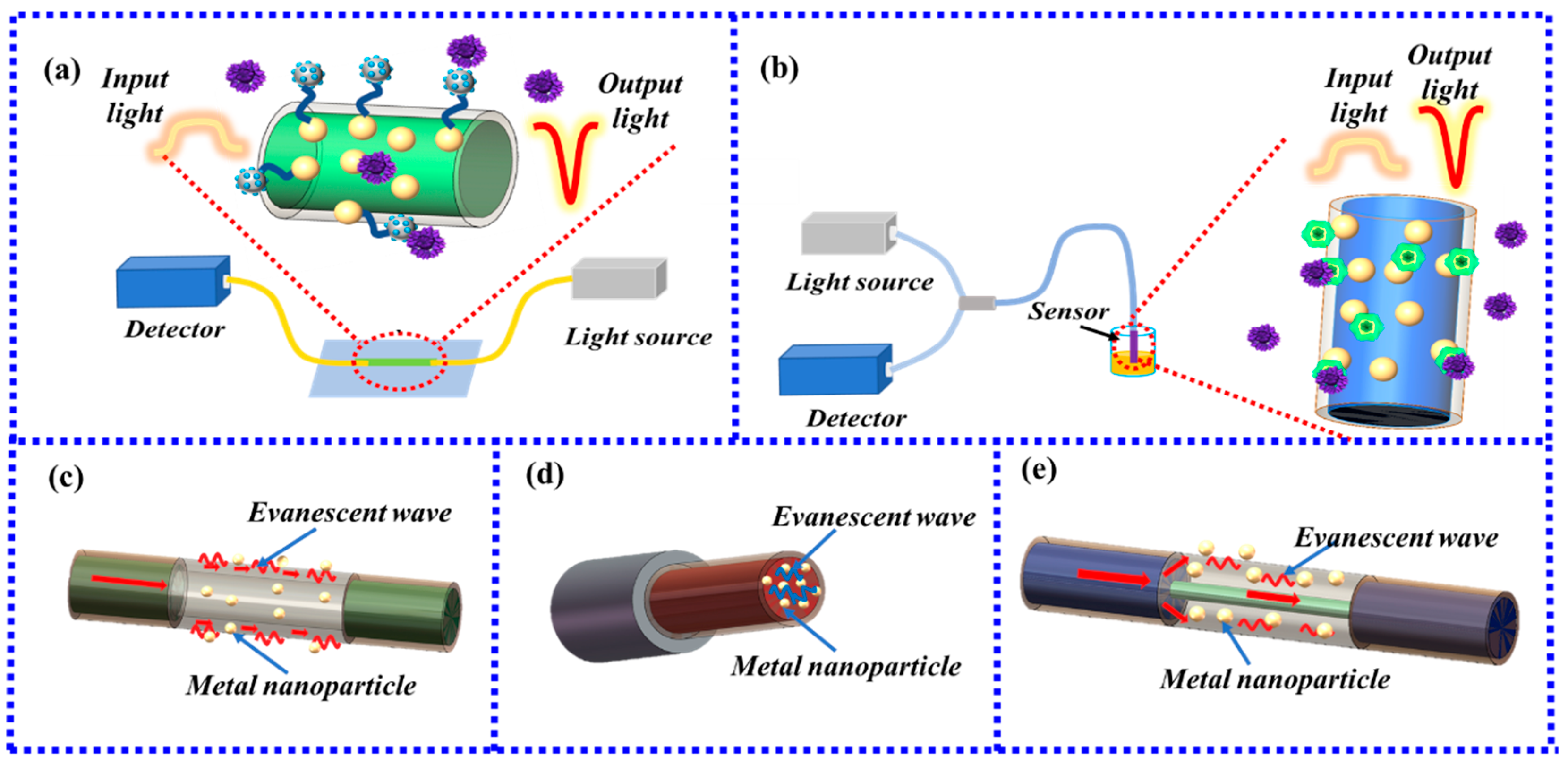
3.1. LSPR Biosensors Based on Ordinary Optical Fibers

3.2. LSPR Biosensors Based on Special Shape Fibers
3.2.1. LSPR Fiber-Optic Biosensor Based on Tapered Fiber
3.2.2. LSPR Fiber-Optic Biosensor Based on U-Type Fiber
3.2.3. LSPR Fiber-Optic Biosensor Based on Ω-Type Fiber
3.2.4. LSPR Fiber-Optic Biosensor Based on S-Type Fiber
3.2.5. LSPR Fiber-Optic Biosensor Based on D-Type Fiber
3.3. LSPR Biosensors Based on Specialty Optical Fibers
3.3.1. LSPR Fiber-Optic Biosensor Based on HCF
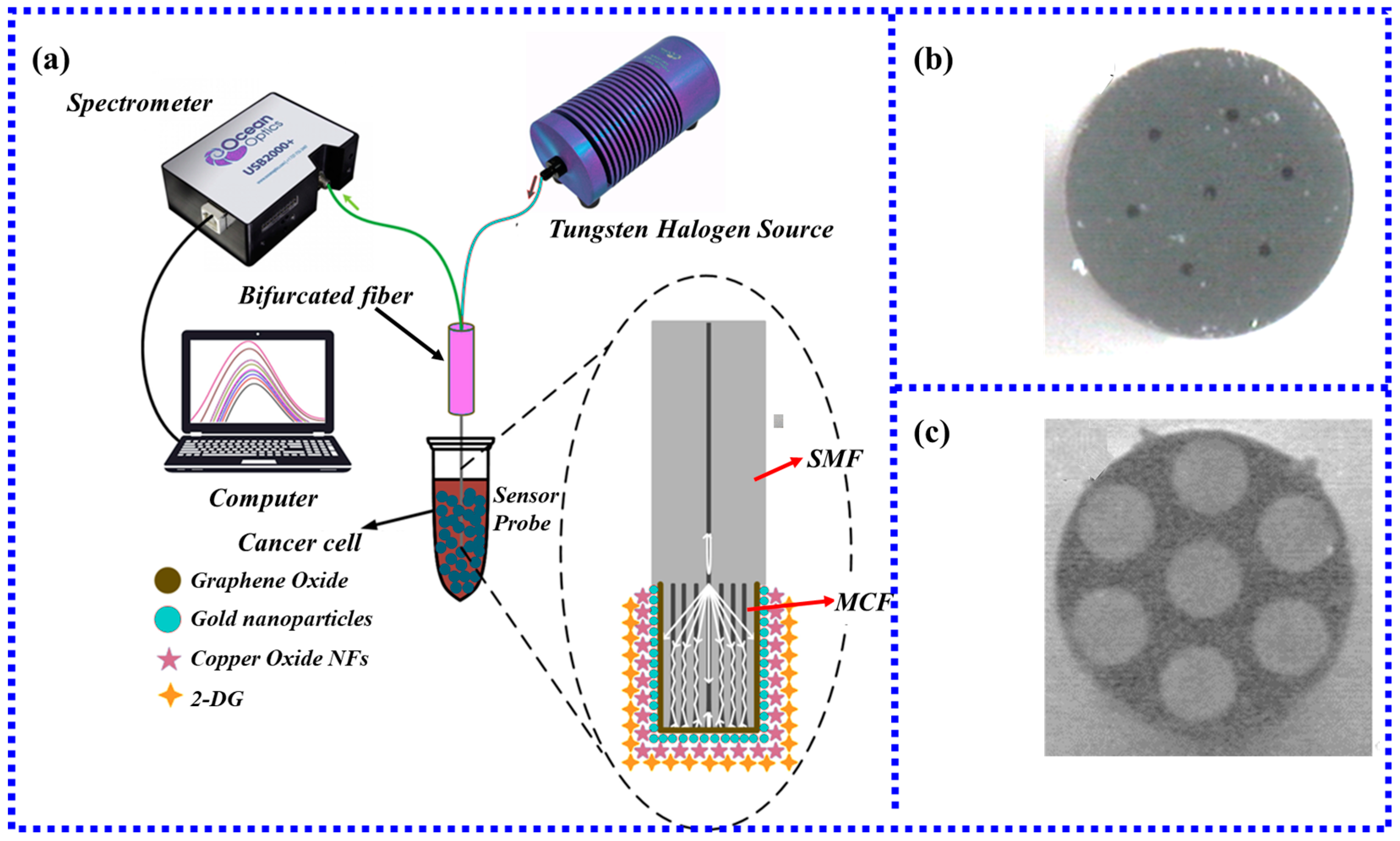
3.3.2. LSPR Fiber-Optic Biosensor Based on MCF
3.3.3. LSPR Fiber-Optic Biosensor Based on MOF
3.3.4. LSPR Fiber-Optic Biosensor Based on POF
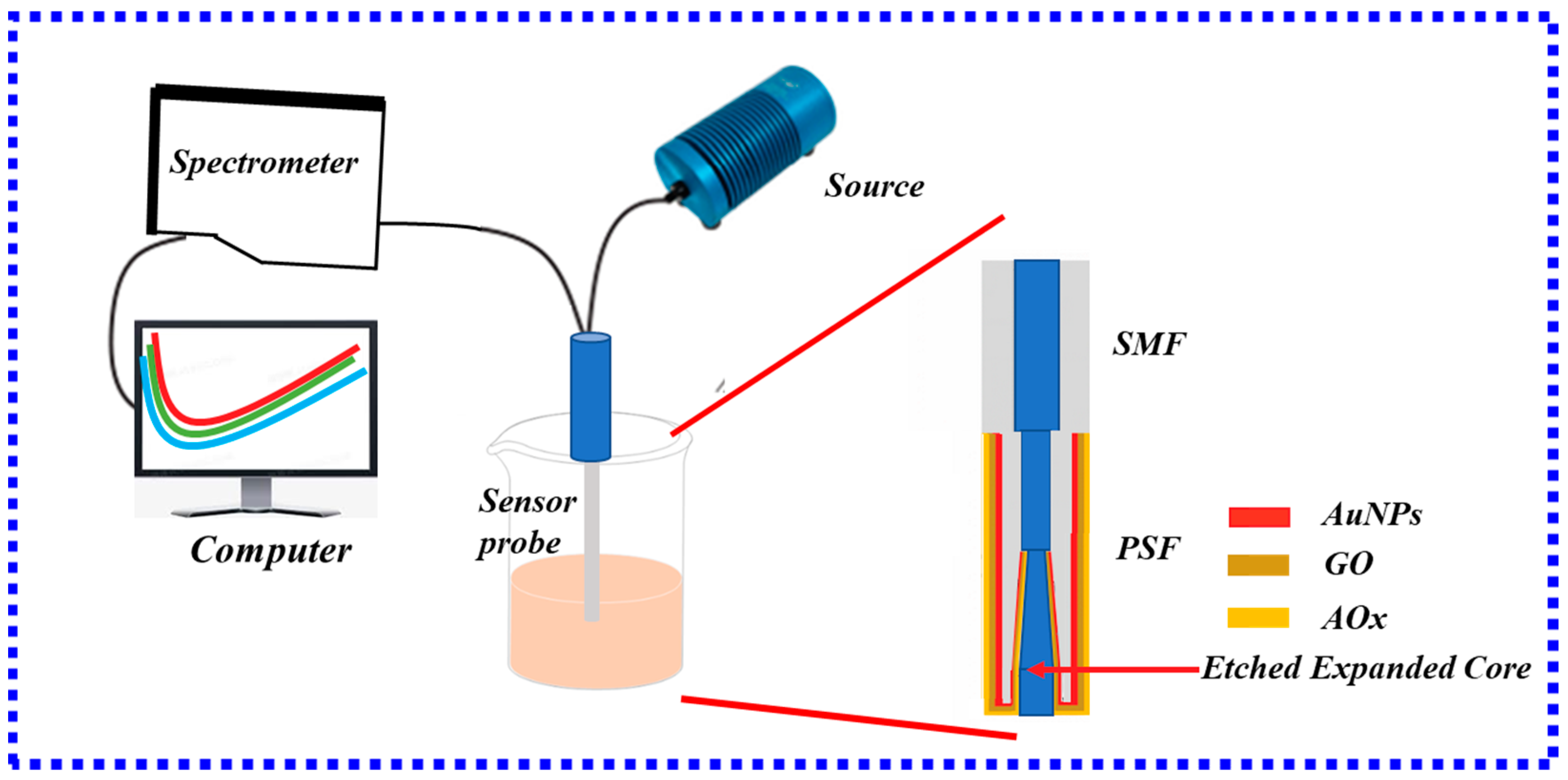
3.3.5. LSPR Fiber-Optic Biosensor Based on PSF
4. Outlook
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, T.-C.; Sun, A.Y.; Huang, Y.-C.; Wang, C.-H.; Wang, S.-C.; Chau, L.-K. Integration of Power-Free and Self-Contained Microfluidic Chip with Fiber Optic Particle Plasmon Resonance Aptasensor for Rapid Detection of SARS-CoV-2 Nucleocapsid Protein. Biosensors 2022, 12, 785. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, N.; Zhou, X.; Zhang, Y.; Zhao, Y.; Nguyen, L.V.; Ebendorff-Heidepriem, H.; Warren-Smith, S.C.J.S.; Chemical, A.B. In-situ DNA detection with an interferometric-type optical sensor based on tapered exposed core microstructured optical fiber. Sens. Actuators B Chem. 2022, 351, 130942. [Google Scholar] [CrossRef]
- Sypabekova, M.; Aitkulov, A.; Blanc, W.; Tosi, D. Reflector-less nanoparticles doped optical fiber biosensor for the detection of Case thrombin. Biosens. Bioelectron. 2020, 165, 112365. [Google Scholar] [CrossRef]
- Arjmand, M.; Aray, A.; Saghafifar, H.; Alijanianzadeh, M. Quantitative Analysis of Methyl-Parathion Pesticide in Presence of Enzyme Substrate Using Tapered Fiber Optic Biosensor. IEEE Sens. J. 2020, 20, 5243–5250. [Google Scholar] [CrossRef]
- Mahani, M.; Alimohamadi, F.; Torkzadeh-Mahani, M.; Hassani, Z.; Khakbaz, F.; Divsar, F.; Yoosefian, M. LSPR biosensing for the early-stage prostate cancer detection using hydrogen bonds between PSA and antibody: Molecular dynamic and experimental study. J. Mol. Liq. 2021, 324, 114736. [Google Scholar] [CrossRef]
- Fang, S.; Song, D.; Zhu, A.; Long, F. Nanoporous layer fiber biosensing platform for real time culture- and separation-free detecting bacterial pathogens and measuring their susceptibility to antibiotics. Sens. Actuators B-Chem. 2020, 325, 128748. [Google Scholar] [CrossRef]
- Han, S.; Zhou, X.; Tang, Y.; He, M.; Zhang, X.; Shi, H.; Xiang, Y. Practical, highly sensitive, and regenerable evanescent-wave biosensor for detection of Hg2+ and Pb2+ in water. Biosens. Bioelectron. 2016, 80, 265–272. [Google Scholar] [CrossRef]
- Sharma, P.; Semwal, V.; Gupta, B.D. A highly selective LSPR biosensor for the detection of taurine realized on optical fiber substrate and gold nanoparticles. Opt. Fiber Technol. 2019, 52, 101962. [Google Scholar] [CrossRef]
- Wang, B.-T.; Wang, Q. An interferometric optical fiber biosensor with high sensitivity for IgG/anti-IgG immunosensing. Opt. Commun. 2018, 426, 388–394. [Google Scholar] [CrossRef]
- Li, X.; Li, F.; Zhou, X.; Zhang, Y.; Nguyen, L.V.; Warren-Smith, S.C.; Zhao, Y. Measurement. Optical fiber DNA biosensor with temperature monitoring based on double microcavities Fabry-Perot interference and Vernier combined effect. IEEE Trans. Instrum. Meas. 2022, 72, 7001208. [Google Scholar]
- Guo, Z.; Qin, Y.; Chen, P.; Hu, J.; Zhou, Y.; Zhao, X.; Liu, Z.; Fei, Y.; Jiang, X.; Wu, X. Hyperboloid-Drum Microdisk Laser Biosensors for Ultrasensitive Detection of Human IgG. Small 2020, 16, 202000239. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jing, J.-Y.; Wang, B.-T. Highly Sensitive SPR Biosensor Based on Graphene Oxide and Staphylococcal Protein A Co-Modified TFBG for Human IgG Detection. IEEE Trans. Instrum. Meas. 2019, 68, 3350–3357. [Google Scholar] [CrossRef]
- Li, X.; Gong, P.; Zhao, Q.; Zhou, X.; Zhang, Y.; Zhao, Y.J.S.; Chemical, A.B. Plug-in optical fiber SPR biosensor for lung cancer gene detection with temperature and pH compensation. Sens. Actuators B Chem. 2022, 359, 131596. [Google Scholar] [CrossRef]
- Semwal, V.; Gupta, B.D. Localized Surface Plasmon Resonance Based Tapered Fiber Optic Ethanol Sensor. In Proceedings of the Conference on Lasers and Electro-Optics Europe/European Quantum Electronics Conference (CLEO/Europe-EQEC), Munich, Germany, 23–27 June 2019. [Google Scholar]
- You, Z.; Qiu, Q.; Chen, H.; Feng, Y.; Wang, X.; Wang, Y.; Ying, Y. Laser-induced noble metal nanoparticle-graphene composites enabled flexible biosensor for pathogen detection. Biosens. Bioelectron. 2020, 150, 111896. [Google Scholar] [CrossRef]
- Agnarsson, B.; Ingthorsson, S.; Gudjonsson, T.; Leosson, K. Evanescent-wave fluorescence microscopy using symmetric planar waveguides. Opt. Express 2009, 17, 5075–5082. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zhang, F.; Yu, S. Probing the binding affinity of plasma proteins adsorbed on Au nanoparticles. Nanoscale 2017, 9, 4787–4792. [Google Scholar] [CrossRef]
- Velusamy, P.; Kumar, G.V.; Jeyanthi, V.; Das, J.; Pachaiappan, R. Bio-Inspired Green Nanoparticles: Synthesis, Mechanism, and Antibacterial Application. Toxicol. Res. 2016, 32, 95–102. [Google Scholar] [CrossRef]
- Luo, B.; Xu, Y.; Wu, S.; Zhao, M.; Jiang, P.; Shi, S.; Zhang, Z.; Wang, Y.; Wang, L.; Liu, Y. A novel immunosensor based on excessively tilted fiber grating coated with gold nanospheres improves the detection limit of Newcastle disease virus. Biosens. Bioelectron. 2018, 100, 169–175. [Google Scholar] [CrossRef]
- Paul, D.; Dutta, S.; Saha, D.; Biswas, R. LSPR based Ultra-sensitive low cost U-bent optical fiber for volatile liquid sensing. Sens. Actuators B-Chem. 2017, 250, 198–207. [Google Scholar] [CrossRef]
- Xu, Y.; Xiong, M.; Yan, H. A portable optical fiber biosensor for the detection of zearalenone based on the localized surface plasmon resonance. Sens. Actuators B-Chem. 2021, 336, 129752. [Google Scholar] [CrossRef]
- Zhou, X.; Gong, P.; Ai, Y.; Hou, Y.; Li, X. Miniature optical fiber DNA hybridization sensor with temperature compensation using a gold nanofilm-coated optical fiber. IEEE Sens. J. 2023. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, C.; Wang, J.; Li, N.; Song, Y.; Wu, H.; Liu, Y. A Fiber Bragg Grating Sensor Based on Cladding Mode Resonance for Label-Free Biosensing. Biosensors 2023, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Abakah, B. Non-Destructive Imaging of Phytosulfokine Trafficking in Plants Using Fiber-Optic Fluorescence Microscopy. Master’s Thesis, East Tennessee State University, Johnson City, TN, USA, 2023. [Google Scholar]
- Liu, Z.; Zhang, W.; Zhang, X.; Wang, S.; Xia, Z.; Guo, X.; Zhao, Y.; Wang, P.; Wang, X.-H. Microstructured Optical Fiber-Enhanced Light–Matter Interaction Enables Highly Sensitive Exosome-Based Liquid Biopsy of Breast Cancer. Anal. Chem. 2023, 95, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Ranathunga, C.; Banerjee, P.P.; Chen, X.; Zhong, J.F.; Sinha, U.K. Practical tapered optical fiber system for in-situ label-free sensing of various antigens. Opt. Eng. 2022, 61, 074102. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, J.; Li, D.; Yang, L. Aptamer-based chemiluminescent optical fiber immunosensor with enhanced signal amplification for ultrasensitive detection of tumor biomarkers. Biosens. Bioelectron. 2022, 214, 114505. [Google Scholar] [CrossRef]
- Nag, P.; Sadani, K.; Mukherji, S.; Mukherji, S. Beta-lactam antibiotics induced bacteriolysis on LSPR sensors for assessment of antimicrobial resistance and quantification of antibiotics. Sens. Actuators B-Chem. 2020, 311, 127945. [Google Scholar] [CrossRef]
- Ding, M.; Huang, Y.; Guo, T.; Sun, L.-P.; Guan, B.-O. Mesoporous nanospheres functionalized optical microfiber biosensor for low concentration neurotransmitter detection. Opt. Express 2016, 24, 27152–27159. [Google Scholar] [CrossRef]
- Yuan, H.; Ji, W.; Chu, S.; Liu, Q.; Guang, J.; Sun, G.; Zhang, Y.; Han, X.; Masson, J.-F.; Peng, W. Au nanoparticles as label-free competitive reporters for sensitivity enhanced fiber-optic SPR heparin sensor. Biosens. Bioelectron. 2020, 154, 112039. [Google Scholar] [CrossRef]
- Sung, H.K.; Oh, S.Y.; Park, C.; Kim, Y. Colorimetric Detection of Co2+ Ion Using Silver Nanoparticles with Spherical, Plate, and Rod Shapes. Langmuir 2013, 29, 8978–8982. [Google Scholar] [CrossRef]
- Zhao, S.; Cheng, Z.; Wang, S.; Hao, H.; Fang, Y. Maintaining the localized surface plasmon resonance of copper nanoparticles by defective TiO2 thin films. Appl. Phys. A-Mater. Sci. Process. 2021, 127, 930. [Google Scholar] [CrossRef]
- Liu, R.; Jiang, L.; Yu, Z.; Jing, X.; Liang, X.; Wang, D.; Yang, B.; Lu, C.; Zhou, W.; Jin, S.J.S.; et al. MXene (Ti3C2Tx)-Ag nanocomplex as efficient and quantitative SERS biosensor platform by in-situ PDDA electrostatic self-assembly synthesis strategy. Sens. Actuators B Chem. 2021, 333, 129581. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, S.; Zheng, X.; Wang, Y.; Xu, W. Optical Fiber LSPR Biosensor Prepared by Gold Nanoparticle Assembly on Polyelectrolyte Multilayer. Sensors 2010, 10, 3585–3596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xue, C.; Yuan, Y.; Lee, J.; Sun, D.; Xiong, J. Fiber Surface Modification Technology for Fiber-Optic Localized Surface Plasmon Resonance Biosensors. Sensors 2012, 12, 2729–2741. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A.; Punjabi, N.; Ghosh, D.; Maji, S.; Mukherji, S. Detection of α-Synuclein, Marker for Parkinson’s disease using Localized Surface Plasmon Resonance Fiber Optic Sensor. In Proceedings of the International Conference on Fibre Optics and Photonics, Kharagpur, India, 13–16 December 2014. M4A. 45. [Google Scholar]
- Tseng, Y.-T.; Li, W.-Y.; Yu, Y.-W.; Chiang, C.-Y.; Liu, S.-Q.; Chau, L.-K.; Lai, N.-S.; Chou, C.-C. Fiber Optic Particle Plasmon Resonance Biosensor for Label-Free Detection of Nucleic Acids and Its Application to HLA-B27 mRNA Detection in Patients with Ankylosing Spondylitis. Sensors 2020, 20, 3137. [Google Scholar] [CrossRef] [PubMed]
- Vu Thi, H.; Nguyen Tran Truc, P.; Nguyen Tien, T.; Nguyen Thuy, A.; Vu Dinh, L.; Do Hung, M.; Tran Thi Kim, C.; Ngoc Xuan Dat, M.; Viet-Duc, P.; Nhu Hoa Thi, T. Gold Nanoparticles Modified a Multimode Clad-Free Fiber for Ultrasensitive Detection of Bovine Serum Albumin. J. Nanomater. 2021, 2021, 5530709. [Google Scholar]
- Dhara, P.; Kumar, R.; Binetti, L.; Nguyen, H.T.; Alwis, L.S.; Sun, T.; Grattan, K.T.V. Optical Fiber-Based Heavy Metal Detection Using the Localized Surface Plasmon Resonance Technique. IEEE Sens. J. 2019, 19, 8720–8726. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, X.; Kumar, S.; Singh, R.; Zhang, B.; Bal, C.; Pu, X. Development of Glucose Sensor Using Gold Nanoparticles and Glucose-Oxidase Functionalized Tapered Fiber Structure. Plasmonics 2020, 15, 841–848. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, B.K.; Singh, R.; Chen, N.-K.; Yang, Q.S.; Zhang, X.; Wang, W.; Zhang, B. LSPR-based cholesterol biosensor using a tapered optical fiber structure. Biomed. Opt. Express 2019, 10, 2150–2160. [Google Scholar] [CrossRef]
- Hu, B.; Kong, F.; Gao, X.; Jiang, L.; Li, X.; Gao, W.; Xu, K.; Tang, B. Avoiding Thiol Compound Interference: A Nanoplatform Based on High-Fidelity Au-Se Bonds for Biological Applications. Angew. Chem.-Int. Ed. 2018, 57, 5306–5309. [Google Scholar] [CrossRef]
- Piwonski, I.; Grobelny, J.; Cichomski, M.; Celichowski, G.; Rogowski, J. Investigation of 3-mercaptopropyltrimethoxysilane self-assembled monolayers on Au(111) surface. Appl. Surf. Sci. 2005, 242, 147–153. [Google Scholar] [CrossRef]
- Jia, S.; Bian, C.; Sun, J.; Xia, S. Gold nanospheres-coated lspr fiber sensor with high ri sensitivity by a rapid fabricating method. In Proceedings of the 2018 IEEE 13th Annual International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Singapore, 22–26 April 2018; pp. 523–526. [Google Scholar]
- Goicoechea, J.; Rivero, P.J.; Sada, S.; Arregui, F.J. Self-Referenced Optical Fiber Sensor for Hydrogen Peroxide Detection Based on LSPR of Metallic Nanoparticles in Layer-by-Layer Films. Sensors 2019, 19, 3872. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Peng, W.; Lin, M.; Wang, F.; Zhang, Y. Gold Nanoparticle-Enhanced Detection of DNA Hybridization by a Block Copolymer-Templating Fiber-Optic Localized Surface Plasmon Resonance Biosensor. Nanomaterials 2021, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-M.; Jeong, D.H.; Lee, H.-Y.; Park, J.-H.; Lee, S.-K. Improved stability of gold nanoparticles on the optical fiber and their application to refractive index sensor based on localized surface plasmon resonance. Opt. Laser Technol. 2019, 114, 171–178. [Google Scholar] [CrossRef]
- Lu, M.; Zhu, H.; Lin, M.; Wang, F.; Hong, L.; Masson, J.-F.; Peng, W.J.S.; Chemical, A.B. Comparative study of block copolymer-templated localized surface plasmon resonance optical fiber biosensors: CTAB or citrate-stabilized gold nanorods. Sens. Actuators B Chem. 2021, 329, 129094. [Google Scholar] [CrossRef]
- Kim, H.-M.; Park, J.-H.; Jeong, D.H.; Lee, H.-Y.; Lee, S.-K. Real-time detection of prostate-specific antigens using a highly reliable fiber-optic localized surface plasmon resonance sensor combined with micro fluidic channel. Sens. Actuators B-Chem. 2018, 273, 891–898. [Google Scholar] [CrossRef]
- Kim, H.-M.; Uh, M.; Jeong, D.H.; Lee, H.-Y.; Park, J.-H.; Lee, S.-K. Localized surface plasmon resonance biosensor using nanopatterned gold particles on the surface of an optical fiber. Sens. Actuators B-Chem. 2019, 280, 183–191. [Google Scholar] [CrossRef]
- Dhawan, A.; Gerhold, M.; Madison, A.; Fowlkes, J.; Russell, P.E.; Vo-Dinh, T.; Leonard, D.N. Fabrication of Nanodot Plasmonic Waveguide Structures Using FIB Milling and Electron Beam-Induced Deposition. Scanning 2009, 31, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zhao, D.; Wang, J.; Lu, J.; Yao, G.; Liu, D.; Luo, H.; Li, Q.; Qiu, M. Solvent-free nanofabrication based on ice-assisted electron-beam lithography. Nano Lett. 2020, 20, 8841–8846. [Google Scholar] [CrossRef]
- Kim, H.-M.; Nam, K.-T.; Lee, S.-K.; Park, J.-H. Fabrication and measurement of microtip-array-based LSPR sensor using bundle fiber. Sens. Actuators A-Phys. 2018, 271, 146–152. [Google Scholar] [CrossRef]
- Bresin, M.; Nehru, N.; Hastings, J.T. Focused electron-beam induced deposition of plasmonic nanostructures from aqueous solutions. In Proceedings of the Advanced Fabrication Technologies for Micro/Nano Optics and Photonics VI, San Francisco, CA, USA, 5–6 February 2013; pp. 27–32. [Google Scholar]
- Dhawan, A.; Gerhold, M.; Vo-Dinh, T.J.N. Theoretical simulation and focused ion beam fabrication of gold nanostructures for surface-enhanced Raman scattering (SERS). Nanobiotechnology 2007, 3, 164–171. [Google Scholar] [CrossRef]
- Hoeflich, K.; Jurczyk, J.; Zhang, Y.; Puydinger dos Santos, M.V.; Goetz, M.; Guerra-Nunez, C.; Best, J.P.; Kapusta, C.; Utke, I. Direct Electron Beam Writing of Silver-Based Nanostructures. ACS Appl. Mater. Interfaces 2017, 9, 24071–24077. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, Y.M.; Wang, Q.; White, J.M. Electron-beam induced initial growth of platinum films using Pt(PF3)(4). J. Vac. Sci. Technol. B 2004, 22, 1803–1806. [Google Scholar] [CrossRef]
- Chien, M.-H.; Shawrav, M.M.; Hingerl, K.; Taus, P.; Schinnerl, M.; Wanzenboeck, H.D.; Schmid, S. Analysis of carbon content in direct-write plasmonic Au structures by nanomechanical scanning absorption microscopy. J. Appl. Phys. 2021, 129, 063105. [Google Scholar] [CrossRef]
- Flatae, A.M.; Tantussi, F.; Messina, G.C.; Mohammadi, A.; De Angelis, F.; Agio, M. Plasmonic Gold Nanocones in the Near-Infrared for Quantum Nano-Optics. Adv. Opt. Mater. 2017, 5, 1700586. [Google Scholar] [CrossRef]
- Vaiano, P.; Quero, G.; Fienga, F.; Di Meo, V.; Casolaro, P.; Campajola, L.; Breglio, G.; Crescitelli, A.; Esposito, E.; Cutolo, A.J.O.; et al. Characterization of Lab-on-Fiber-based dosimeters in ultra-high dose radiation fields. Opt. Laser Technol. 2023, 161, 109177. [Google Scholar] [CrossRef]
- Keiser, G.; Xiong, F.; Cui, Y.; Shum, P.P. Review of diverse optical fibers used in biomedical research and clinical practice. J. Biomed. Opt. 2014, 19, 080902. [Google Scholar] [CrossRef]
- Khanikar, T.; De, M.; Singh, V.K.J.P. A review on infiltrated or liquid core fiber optic SPR sensors. Photonics Nanostruct. Fundam. Appl. 2021, 46, 100945. [Google Scholar] [CrossRef]
- Agrawal, N.; Saha, C.; Kumar, C.; Singh, R.; Zhang, B.; Kumar, S. Development of Uric Acid Sensor Using Copper Oxide and Silver Nanoparticles Immobilized SMSMS Fiber Structure-Based Probe. IEEE Trans. Instrum. Meas. 2020, 69, 9097–9104. [Google Scholar] [CrossRef]
- Sanders, M.; Lin, Y.; Wei, J.; Bono, T.; Lindquist, R.G. An enhanced LSPR fiber-optic nanoprobe for ultrasensitive detection of protein biomarkers. Biosens. Bioelectron. 2014, 61, 95–101. [Google Scholar] [CrossRef]
- Malara, P.; Crescitelli, A.; Di Meo, V.; Giorgini, A.; Avino, S.; Esposito, E.; Ricciardi, A.; Cusano, A.; Rendina, I.; De Natale, P.J.S.; et al. Resonant enhancement of plasmonic nanostructured fiber optic sensors. Sens. Actuators B Chem. 2018, 273, 1587–1592. [Google Scholar] [CrossRef]
- Kim, H.-M.; Park, J.-H.; Lee, S.-K. Fiber optic sensor based on ZnO nanowires decorated by Au nanoparticles for improved plasmonic biosensor. Sci. Rep. 2019, 9, 15605. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-M.; Park, J.-H.; Lee, S.-K. Fabrication and measurement of fiber optic localized surface plasmon resonance sensor based on gold nanoparticle dimer. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2021, 261, 120034. [Google Scholar] [CrossRef]
- Flauraud, V.; Regmi, R.; Winkler, P.M.; Alexander, D.T.L.; Rigneault, H.; van Hulst, N.F.; Garcia-Parajo, M.F.; Wenger, J.; Brugger, J. In-Plane Plasmonic Antenna Arrays with Surface Nanogaps for Giant Fluorescence Enhancement. Nano Lett. 2017, 17, 1703–1710. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, G.; Singh, L.; Wang, Y.; Singh, R.; Zhang, B.; Zhang, X.; Kumar, S. Highly sensitive and selective sensor probe using glucose oxidase/gold nanoparticles/graphene oxide functionalized tapered optical fiber structure for detection of glucose. Optik 2020, 208, 164536. [Google Scholar] [CrossRef]
- Dalila, N.R.; Arshad, M.K.M.; Gopinath, S.C.B.; Norhaimi, W.M.W.; Fathil, M.F.M. Current and future envision on developing biosensors aided by 2D molybdenum disulfide (MoS2) productions. Biosens. Bioelectron. 2019, 132, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Esfahani Monfared, Y. Overview of Recent Advances in the Design of Plasmonic Fiber-Optic Biosensors. Biosensors 2020, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Semwal, V.; Gupta, B.D. Experimental studies on the sensitivity of the propagating and localized surface plasmon resonance-based tapered fiber optic refractive index sensors. Appl. Opt. 2019, 58, 4149–4156. [Google Scholar] [CrossRef]
- Wang, J.; Luo, Z.; Zhou, M.; Ye, C.; Fu, H.; Cai, Z.; Cheng, H.; Xu, H.; Qi, W. Evanescent-Light Deposition of Graphene Onto Tapered Fibers for Passive Q-Switch and Mode-Locker. IEEE Photonics J. 2012, 4, 1295–1305. [Google Scholar] [CrossRef]
- Wang, Z.; Singh, R.; Marques, C.; Jha, R.; Zhang, B.; Kumar, S. Taper-in-taper fiber structure-based LSPR sensor for alanine aminotransferase detection. Opt. Express 2021, 29, 43793–43810. [Google Scholar] [CrossRef]
- Zhu, G.; Agrawal, N.; Singh, R.; Kumar, S.; Zhang, B.; Saha, C.; Kumar, C. A novel periodically tapered structure-based gold nanoparticles and graphene oxide—Immobilized optical fiber sensor to detect ascorbic acid. Opt. Laser Technol. 2020, 127, 106156. [Google Scholar] [CrossRef]
- Wen, H.-Y.; Hsu, H.-C.; Tsai, Y.-T.; Feng, W.-K.; Lin, C.-L.; Chiang, C.-C.J.P. U-shaped optical fiber probes coated with electrically doped GQDs for humidity measurements. Polymers 2021, 13, 2696. [Google Scholar] [CrossRef] [PubMed]
- Sadani, K.; Nag, P.; Mukherji, S. LSPR based optical fiber sensor with chitosan capped gold nanoparticles on BSA for trace detection of Hg (II) in water, soil and food samples. Biosens. Bioelectron. 2019, 134, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Halkare, P.; Punjabi, N.; Wangchuk, J.; Nair, A.; Kondabagil, K.; Mukherji, S. Bacteria functionalized gold nanoparticle matrix based fiber-optic sensor for monitoring heavy metal pollution in water. Sens. Actuators B-Chem. 2019, 281, 643–651. [Google Scholar] [CrossRef]
- Arcas, A.d.S.; Dutra, F.d.S.; Allil, R.C.S.B.; Werneck, M.M. Surface Plasmon Resonance and Bending Loss-Based U-Shaped Plastic Optical Fiber Biosensors. Sensors 2018, 18, 648. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Xu, Y.; He, L.; He, F.; Wu, J.; Huang, Z.; Tian, Y.; Li, Y.; Duan, Y. Development of a rapid and ultra-sensitive cytosensor: Ω-shaped fiber optic LSPR integrated with suitable AuNPs coverage. Sens. Actuators B Chem. 2021, 336, 129706. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, Z.; Chen, J.; Huang, Z.; Wang, X.; An, H.; Duan, Y. Omega-Shaped Fiber-Optic Probe-Based Localized Surface Plasmon Resonance Biosensor for Real-Time Detection of Salmonella Typhimurium. Anal. Chem. 2018, 90, 13640–13646. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Ning, W.; Yang, E.; Li, Y.; Luo, Z.; Duan, Y. Sandwich method-based sensitivity enhancement of Ω-shaped fiber optic LSPR for time-flexible bacterial detection. Biosens. Bioelectron. 2022, 201, 113911. [Google Scholar] [CrossRef]
- He, L.; He, F.; Feng, Y.; Wang, X.; Li, Y.; Tian, Y.; Gao, A.; Zhang, P.; Qi, X.; Luo, Z.; et al. Hybridized nanolayer modified Omega-shaped fiber-optic synergistically enhances localized surface plasma resonance for ultrasensitive cytosensor and efficient photothermal therapy. Biosens. Bioelectron. 2021, 194, 113599. [Google Scholar] [CrossRef]
- Chauhan, S.K.; Tharion, J.; Punjabi, N.; Sharma, D.K.; Mukherji, S. A comparison of S-shaped and U-shaped optical fiber sensors. In Proceedings of the Optical Sensors, Barcelona, Spain, 27–31 July 2014; p. SeTh3B. 5. [Google Scholar]
- Chauhan, S.; Punjabi, N.; Sharma, D.; Mukherji, S. Evanescent wave absorption based S-shaped fiber-optic biosensor for immunosensing applications. Procedia Eng. 2016, 168, 117–120. [Google Scholar] [CrossRef]
- Dimpi, P.; Biswas, R. Clad modified varying geometries of fiber optic LSPR sensors towards detection of hazardous volatile liquids and their comparative analysis. Environ. Technol. Innov. 2022, 25, 102112. [Google Scholar]
- Gong, W.; Jiang, S.; Li, Z.; Li, C.; Xu, J.; Pan, J.; Huo, Y.; Man, B.; Liu, A.; Zhang, C. Experimental and theoretical investigation for surface plasmon resonance biosensor based on graphene/Au film/D-POF. Opt. Express 2019, 27, 3483–3495. [Google Scholar] [CrossRef] [PubMed]
- Virk, J.K.; Das, S.; Kaler, R.S.; Singh, H.; Kundu, T. D-shape optical fiber probe dimension optimization for LSPR based bio-sensor. Opt. Fiber Technol. 2022, 71, 102930. [Google Scholar] [CrossRef]
- Liu, G.; Feng, D. Evanescent wave analysis and experimental realization of refractive index sensor based on D-shaped plastic optical fiber. Optik 2016, 127, 690–693. [Google Scholar] [CrossRef]
- Cennamo, N.; Dona, A.; Pallavicini, P.; D’Agostino, G.; Dacarro, G.; Zeni, L.; Pesavento, M. Sensitive detection of 2,4,6-trinitrotoluene by tridimensional monitoring of molecularly imprinted polymer with optical fiber and five-branched gold nanostars. Sens. Actuators B-Chem. 2015, 208, 291–298. [Google Scholar] [CrossRef]
- Pathak, A.K.; Rahman, B.M.A.; Singh, V.K.; Kumari, S. Sensitivity Enhancement of a Concave Shaped Optical Fiber Refractive Index Sensor Covered with Multiple Au Nanowires. Sensors 2019, 19, 4210. [Google Scholar] [CrossRef]
- Feng, J.; Gao, J.; Yang, W.; Liu, R.; Shafi, M.; Zha, Z.; Liu, C.; Xu, S.; Ning, T.; Jiang, S. LSPR optical fiber sensor based on 3D gold nanoparticles with monolayer graphene as a spacer. Opt. Express 2022, 30, 10187–10198. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, Y.; Xu, Y.; Wang, X.; Huang, Z.; Chen, J.; Li, Y.; Duan, Y. Ultrasensitive U-shaped fiber optic LSPR cytosensing for label-free and in situ evaluation of cell surface N-glycan expression. Sens. Actuators B-Chem. 2019, 284, 582–588. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Donà, A.; Dacarro, G.; Pallavicini, P.; Pesavento, M.; Zeni, L.J.S. Localized surface plasmon resonance with five-branched gold nanostars in a plastic optical fiber for bio-chemical sensor implementation. Sensors 2013, 13, 14676–14686. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, N.; Li, P.; Yu, L.; Chen, S.; Zhang, Y.; Jing, Z.; Peng, W.J.N. Low-cost localized surface plasmon resonance biosensing platform with a response enhancement for protein detection. Nanomaterials 2019, 9, 1019. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Yu, Q.; Peng, W. Microcapillary-based integrated LSPR device for refractive index detection and biosensing. J. Light. Technol. 2020, 38, 2485–2492. [Google Scholar] [CrossRef]
- Li, M.; Singh, R.; Marques, C.; Zhang, B.; Kumar, S. 2D material assisted SMF-MCF-MMF-SMF based LSPR sensor for creatinine detection. Opt. Express 2021, 29, 38150–38167. [Google Scholar] [CrossRef]
- Kumar, S.; Guo, Z.; Singh, R.; Wang, Q.; Zhang, B.; Cheng, S.; Liu, F.-Z.; Marques, C.; Kaushik, B.K.; Jha, R.J. MoS2 functionalized multicore fiber probes for selective detection of shigella bacteria based on localized plasmon. J. Light. Technol. 2021, 39, 4069–4081. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, S.; Liu, F.-Z.; Shuang, C.; Zhang, B.; Jha, R.; Kaushik, B.K. Etched multicore fiber sensor using copper oxide and gold nanoparticles decorated graphene oxide structure for cancer cells detection. Biosens. Bioelectron. 2020, 168, 112557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, Y.; Wang, Z.; Singh, R.; Marques, C.; Wu, Q.; Kaushik, B.K.; Jha, R.; Zhang, B.; Kumar, S. Localized Plasmon-Based Multicore Fiber Biosensor for Acetylcholine Detection. IEEE Trans. Instrum. Meas. 2021, 71, 1–9. [Google Scholar] [CrossRef]
- Li, X.; Chen, N.; Zhou, X.; Gong, P.; Wang, S.; Zhang, Y.; Zhao, Y. A review of specialty fiber biosensors based on interferometer configuration. J. Biophotonics 2021, 14, e202100068. [Google Scholar] [CrossRef] [PubMed]
- Mollah, M.A.; Islam, M.S. Novel Single Hole Exposed-Suspended Core Localized Surface Plasmon Resonance Sensor. IEEE Sens. J. 2021, 21, 2813–2820. [Google Scholar] [CrossRef]
- Csaki, A.; Jahn, F.; Latka, I.; Henkel, T.; Malsch, D.; Schneider, T.; Schroeder, K.; Schuster, K.; Schwuchow, A.; Spittel, R.; et al. Nanoparticle Layer Deposition for Plasmonic Tuning of Microstructured Optical Fibers. Small 2010, 6, 2584–2589. [Google Scholar] [CrossRef]
- Wang, B.-T.; Wang, Q. Sensitivity-Enhanced Optical Fiber Biosensor Based on Coupling Effect Between SPR and LSPR. IEEE Sens. J. 2018, 18, 8303–8310. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.-Z.; Song, H.; Zhao, W.-M.; Jing, J.-Y. A dual channel self-compensation optical fiber biosensor based on coupling of surface plasmon polariton. Opt. Laser Technol. 2020, 124, 106002. [Google Scholar] [CrossRef]
- Kuriki, K.; Koike, Y.; Okamoto, Y. Plastic optical fiber lasers and amplifiers containing lanthanide complexes. Chem. Rev. 2002, 102, 2347–2356. [Google Scholar] [CrossRef]
- Boruah, B.S.; Biswas, R. Localized surface plasmon resonance based U-shaped optical fiber probe for the detection of Pb2+ in aqueous medium. Sens. Actuators B-Chem. 2018, 276, 89–94. [Google Scholar] [CrossRef]
- Halkare, P.; Punjabi, N.; Wangchuk, J.; Kondabagil, K.; Mukherji, S. LSPR based fiber optic sensor for detection of E. coli using bacteriophage T4. In Proceedings of the 2015 Workshop on Recent Advances in Photonics (WRAP), Bangalore, India, 16–17 December 2015; pp. 1–4. [Google Scholar]
- Gowri, A.; Sai, V. U-bent plastic optical fiber based plasmonic biosensor for nucleic acid detection. In Proceedings of the Optical Sensors 2017, Prague, Czech Republic, 24–27 April 2017; pp. 170–175. [Google Scholar]
- Kumar, S.; Singh, R.; Yang, Q.; Cheng, S.; Zhang, B.; Kaushik, B.K. Highly Sensitive, Selective and Portable Sensor Probe Using Germanium-Doped Photosensitive Optical Fiber for Ascorbic Acid Detection. IEEE Sens. J. 2021, 21, 62–70. [Google Scholar] [CrossRef]
- Wang, Y.; Singh, R.; Chaudhary, S.; Zhang, B.; Kumar, S. 2-D Nanomaterials Assisted LSPR MPM Optical Fiber Sensor Probe for Cardiac Troponin I Detection. IEEE Trans. Instrum. Meas. 2022, 71, 1–9. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Kaushik, B.K.; Chen, N.-K.; Yang, Q.S.; Zhang, X. LSPR-Based Cholesterol Biosensor Using Hollow Core Fiber Structure. IEEE Sens. J. 2019, 19, 7399–7406. [Google Scholar] [CrossRef]
- Vaiano, P.; Carotenuto, B.; Pisco, M.; Ricciardi, A.; Quero, G.; Consales, M.; Crescitelli, A.; Esposito, E.; Cusano, A.J.L.; Reviews, P. Lab on Fiber Technology for biological sensing applications. Laser Photonics Rev. 2016, 10, 922–961. [Google Scholar] [CrossRef]
- Ricciardi, A.; Crescitelli, A.; Vaiano, P.; Quero, G.; Consales, M.; Pisco, M.; Esposito, E.; Cusano, A.J.A. Lab-on-fiber technology: A new vision for chemical and biological sensing. Analyst 2015, 140, 8068–8079. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Yuan, H.; Wang, J.; Guang, J.; Peng, W. Smartphone based LSPR biosensor. In Proceedings of the Asia Communications and Photonics Conference, Hangzhou, China, 26–29 October 2018. Su2A. 148. [Google Scholar]













| Method | Advantage | Disadvantage | Ref | |
|---|---|---|---|---|
| specific recognition | Antigen-antibody binding | High specificity sensitivity, good stability, low interference from the natural environment, etc. | Combining with the fiber surface complicated | [25] |
| Aptamer | Strong selectivity, high affinity | Aptamers target only one target | [26] | |
| Molecular imprinting | Stable against harsh environments, materials used can be reused, predeterminability, high recognition, and practicability | Templates are not readily available and expensive | [27] | |
| Microbial detection method | Rapid sensitivity test | Narrow detection range | [28] | |
| Physical adsorption of Mesoporous nanospheres | High repeatability and stability | The complicated preparation process, no selectivity | [29] | |
| Polyelectrolyte method | Easy to operate, No complex functionalization process | Difficult requirements on the charge of the measured substance, no selectivity | [30] | |
| Shape | Target | Sensor Performance | Advantage | Disadvantage | Ref |
|---|---|---|---|---|---|
| Tapered | Glucose | One tapered, sensitivity: 1.06 nm/mM | Ultrahigh sensitivity, superfine diameter. | Fragile structure, Complex optical fiber manufacturing method | [69] |
| Alanine aminotransferase | Taper-in-taper, Sensitivity: 4.1 pm/(U/L) | [74] | |||
| Ascorbic acid Ascorbic acid Ascorbic acid | Four tapered, Sensitivity: 1.1 nm/mM Five tapered, Sensitivity: 8.3 nm/mM Eight tapered, Sensitivity: 0.5 nm/mM | [75] [75] [75] | |||
| U-type | Cancer cell detection | LOD: 30 cells/mL | Small size, stable structure, Simple preparation process | Only transmissive structure | [93] |
| Ω-type | MCF-7 cancer cells | LOD: 12 cells/ml | Small size, 2.5 times higher sensitivity than U-type | Only transmissive structure | [80] |
| Salmonella typhimurium | LOD: 7.4 CFU/mL | [82] | |||
| MCF-7 cancer cells | LOD: 2.6 cells/ml | [83] | |||
| S-type | 2,4,6- Trinitrotoluene; 2,4-Dinitrotoluene; | LOD: 10 parts per billion (ppb) | 1.5 times higher sensitivity than U-type | Fragile structure | [85] |
| D-type | Goat human IgG 2,4,6-trinitrotoluene Water-glycerin solutions | LOD: 0.6 μg/mL nm/M RI sensitivity: 84 nm/RIU | Flexible structure and larger sensing platform | Grinding and polishing surface rough | [88] [90] |
| [94] |
| Type | Target | Sensor Performance | Advantage | Disadvantage | Ref |
|---|---|---|---|---|---|
| HCF | Cholesterol | LOD: 25.5 nM | Providing liquid flow channels, saving solution | Complex detection process | [112] |
| Human IgG | LOD: 3 nM | [96] | |||
| Transferrin Immunoglobulin G | Range: 0.01–0.15 mg/L Range: 0.01–0.15 mg/L | [95] [95] | |||
| MCF | Shigella bacterial HepG2, Hepa1 6, A549, MCF-7, LO2, NCF cell Acetylcholine Creatinine | LOD: 1.56 CFU/mL LOD: 3, 2, 2, 2, 4, 10 cells/mL Sensitivity: 0.062 nm/uM LOD: 14.28 uM Sensitivity: 0.0025 nm/μM LOD: 128.4 μM | High sensitivity to small RI changes, low connection loss, simultaneous measurements on each core | Complex introducing and detecting light from the individual cores | [98] |
| [99] [100] [97] | |||||
| MOF | Refractive Index Solution | RI sensitivity:78 nm/RIU | Providing liquid flow channels, saving solution, temperature not cross interference | The complex optical fiber manufacturing method | [103] |
| POF | Pb2+ DNA E. coli | Sensitivity: 0.19 nm/μM LOD: 1 pg/mL Qualitative detection | Low manufacturing cost, good toughness, lightweight, easy processing, | High attenuation, poor heat resistance; | [107] [109] [108] |
| PSF | Ascorbic acid Cardiac Troponin I | LOD: 15.12 μM Sensitivity: 3.4 pm/(ng/mL), LOD: 96.2638 ng/mL | High sensitivity and low loss | The complex optical fiber manufacturing method | [110] |
| [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhou, X.; Li, X.; Gong, P.; Zhang, Y.; Zhao, Y. Recent Advancements of LSPR Fiber-Optic Biosensing: Combination Methods, Structure, and Prospects. Biosensors 2023, 13, 405. https://doi.org/10.3390/bios13030405
Zhang H, Zhou X, Li X, Gong P, Zhang Y, Zhao Y. Recent Advancements of LSPR Fiber-Optic Biosensing: Combination Methods, Structure, and Prospects. Biosensors. 2023; 13(3):405. https://doi.org/10.3390/bios13030405
Chicago/Turabian StyleZhang, Hongxin, Xue Zhou, Xuegang Li, Pengqi Gong, Yanan Zhang, and Yong Zhao. 2023. "Recent Advancements of LSPR Fiber-Optic Biosensing: Combination Methods, Structure, and Prospects" Biosensors 13, no. 3: 405. https://doi.org/10.3390/bios13030405
APA StyleZhang, H., Zhou, X., Li, X., Gong, P., Zhang, Y., & Zhao, Y. (2023). Recent Advancements of LSPR Fiber-Optic Biosensing: Combination Methods, Structure, and Prospects. Biosensors, 13(3), 405. https://doi.org/10.3390/bios13030405







