Carrageenan from Gigartina skottsbergii: A Novel Molecular Probe to Detect SARS-CoV-2
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
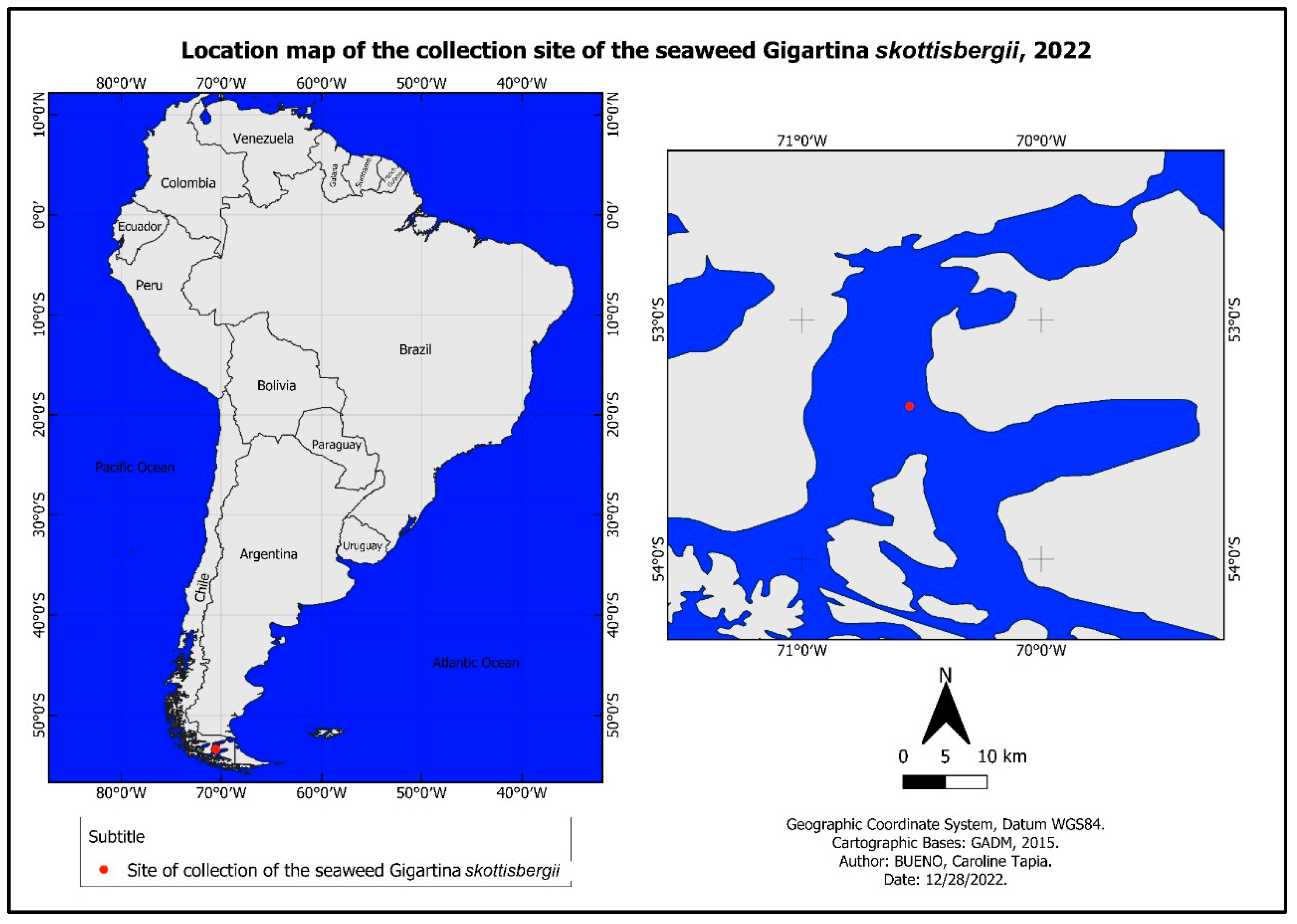
2.1.1. Sampling
2.1.2. Materials
2.2. Methods
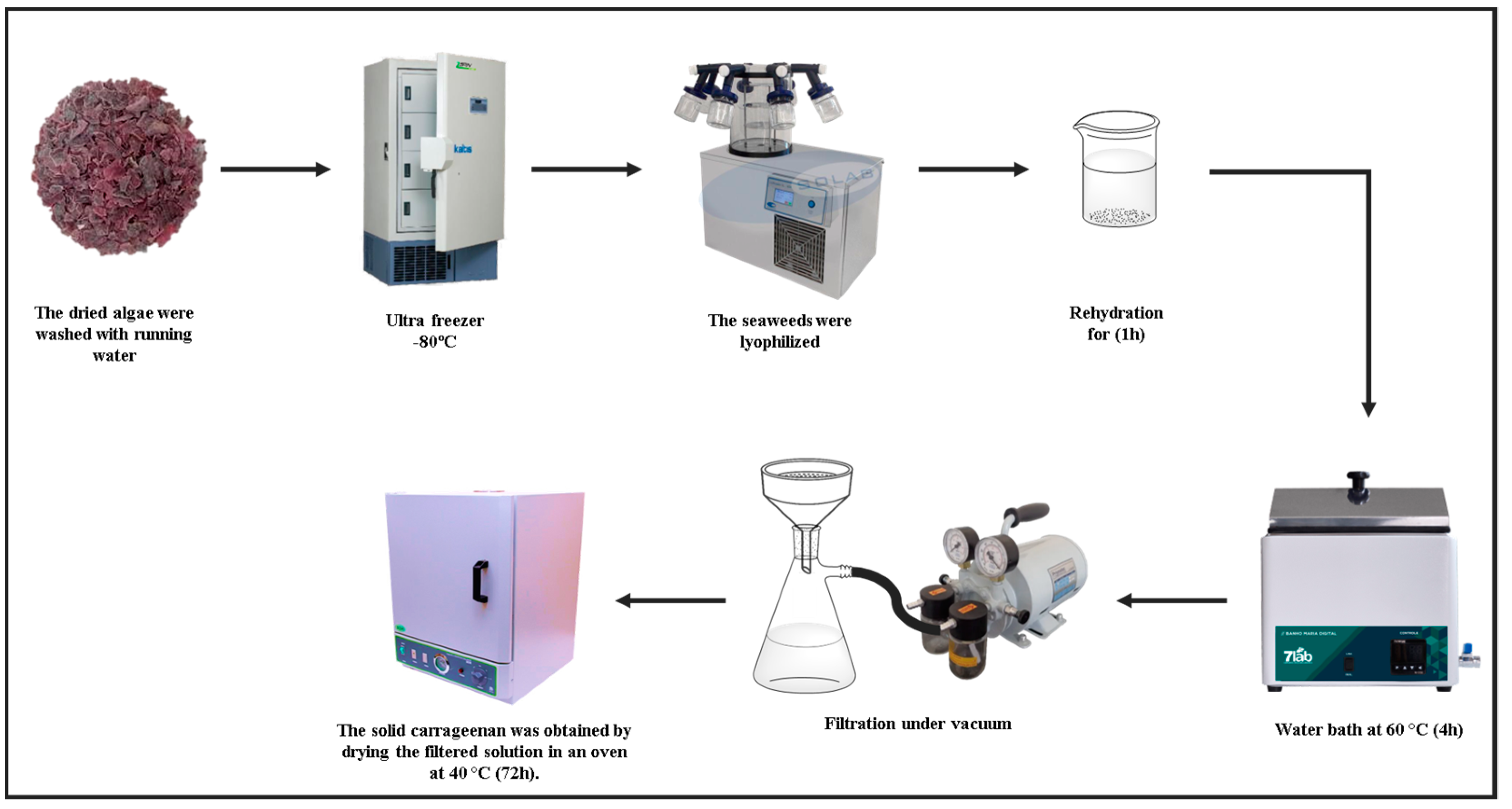
2.2.1. Carrageenan Extraction
2.2.2. UV-Vis Scanning Spectroscopy
2.2.3. (2,2′-Azino-bis(3-Ethylbenzothiazoline-6-Sulfonic Acid)) ABTS Assay
2.2.4. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
2.2.5. Attenuated Total Reflectance Fourier Transform (ATR-FTIR) Spectroscopy
2.2.6. Qualitative High-Performance Liquid Chromatography (HPLC)
2.2.7. Carrageenan As a Binding Probe for SARS-CoV-2
2.2.8. RNA Extraction
2.2.9. Real-Time Reverse Transcription PCR (qRT-PCR)
2.2.10. Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP)
2.3. Statistical Methodology
3. Results and Discussion
3.1. Carrageenan Extraction Process
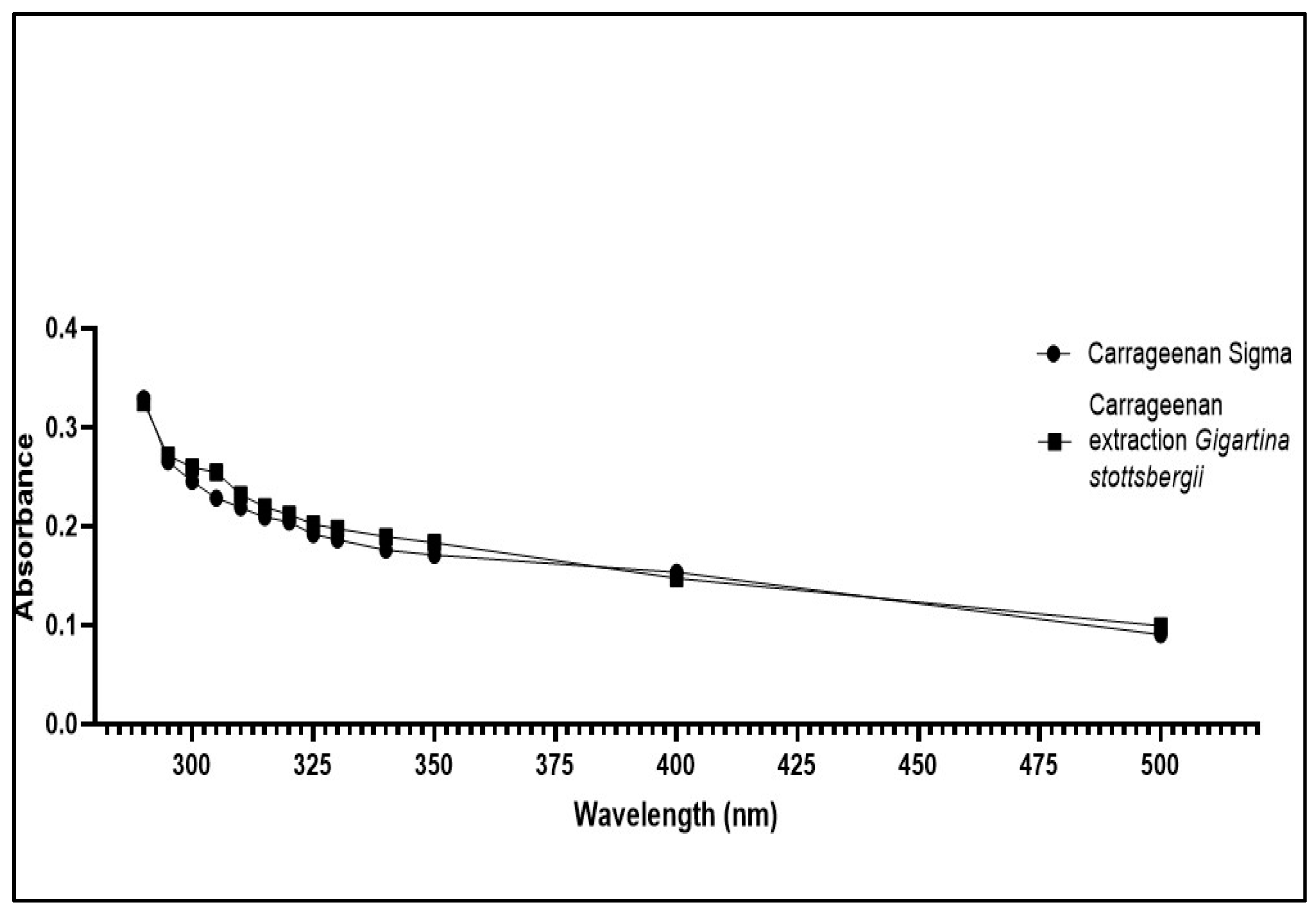
3.2. UV-Vis Scanning Spectroscopy
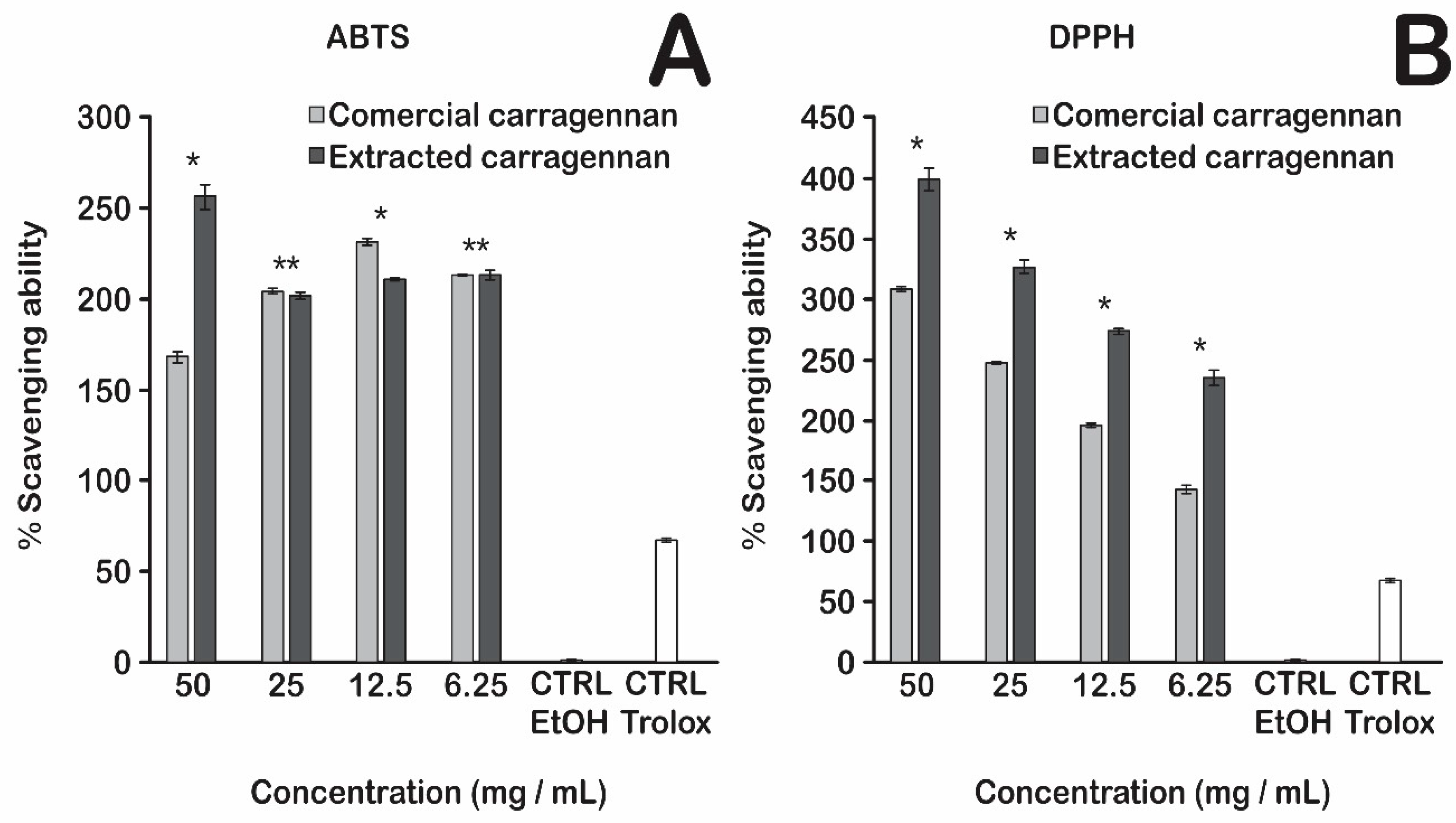
3.3. Radical Scavenging Capacity
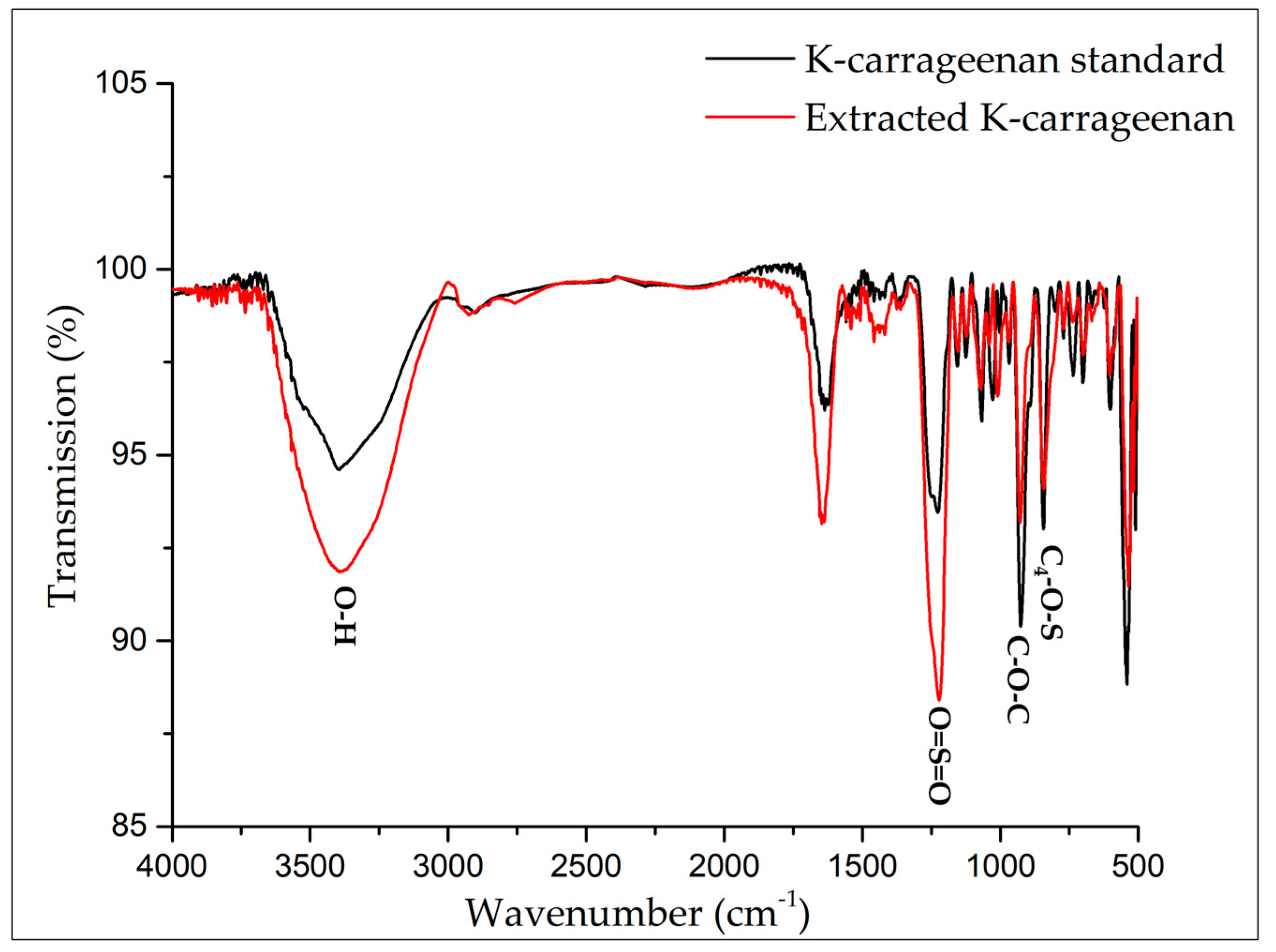
3.4. ATR-FTIR Spectroscopy
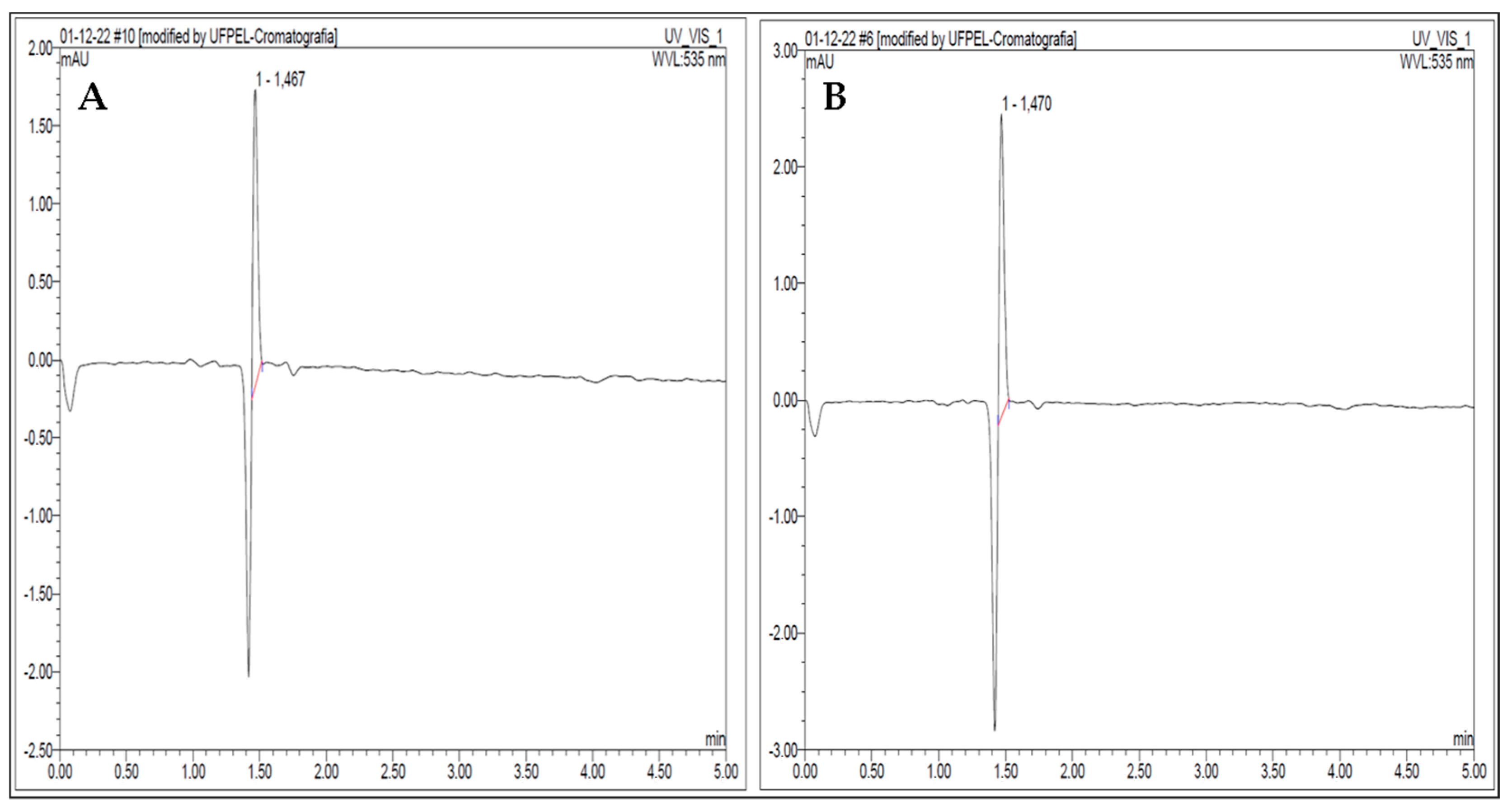
3.5. κ-Carrageenan Determination by UHPLC-UV-Vis
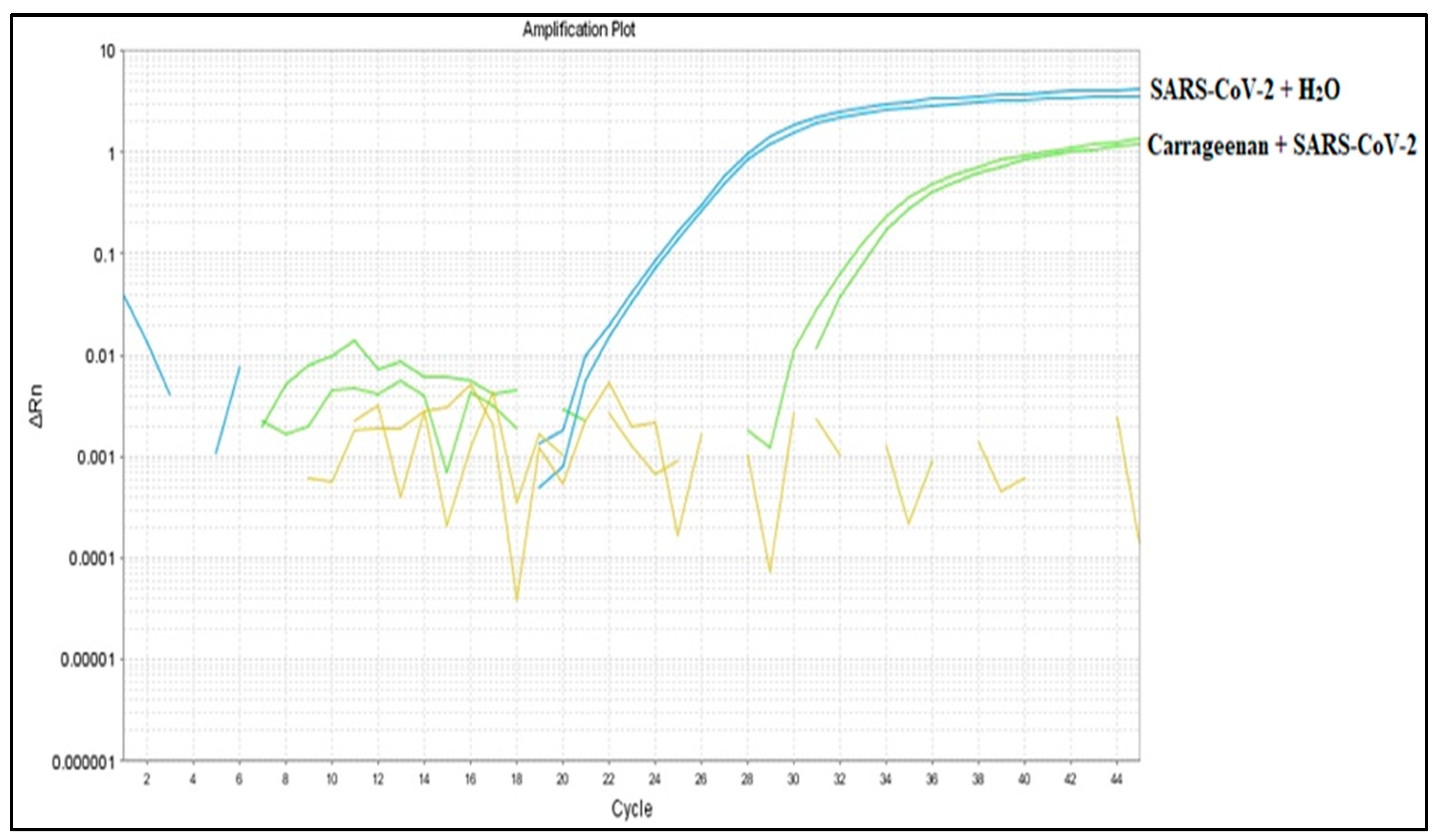
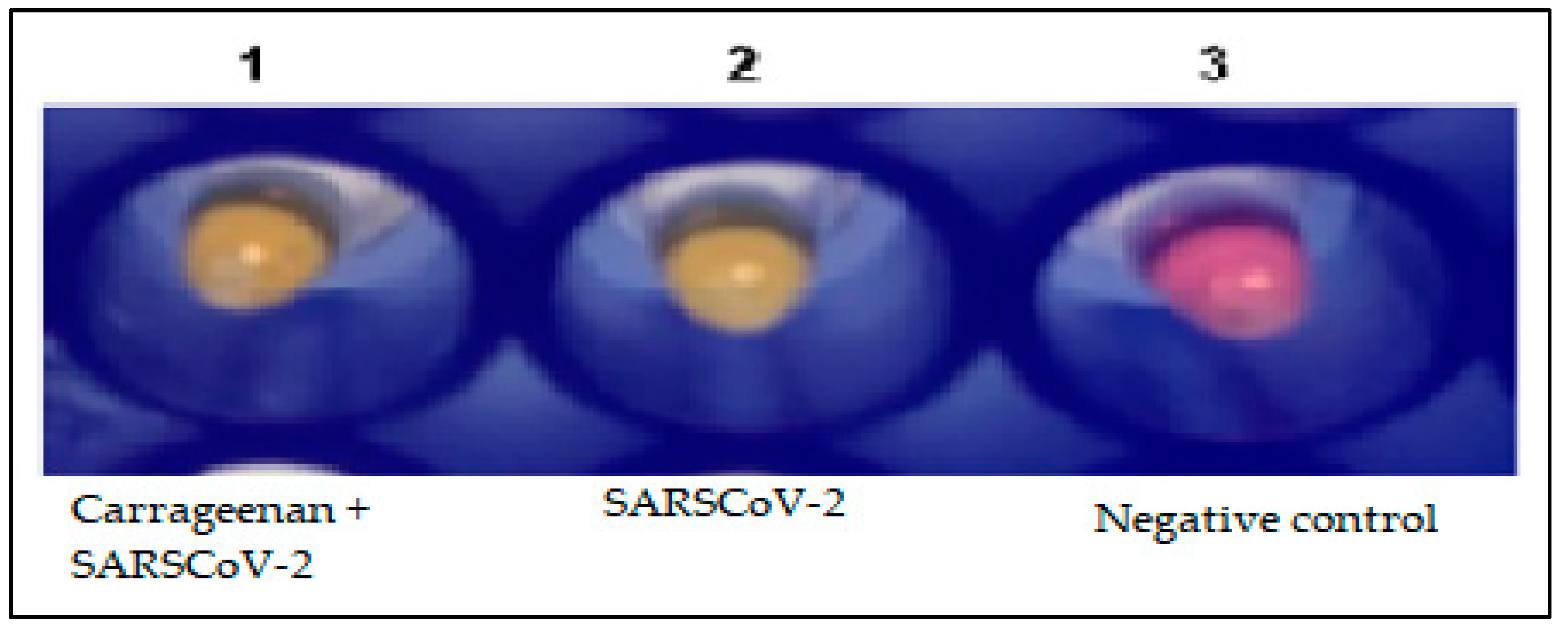
3.6. Binding Capacity Evaluation of G. Skottsbergii Carrageenan with SARS-CoV-2 Viral Particles
3.7. RT-LAMP Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 13 December 2022).
- Schoeler, G.P.; Afonso, T.F.; Demarco, C.F.; dos Santos Barboza, V.; Sant’anna Cadaval, T.R.; Igansi, A.V.; Gelesky, M.A.; Giongo, J.L.; de Almeida Vaucher, R.; de Avila Delucis, R.; et al. SARS-CoV-2 Removal with a Polyurethane Foam Composite. Environ. Sci. Pollut. Res. 2022, 30, 22024–22032. [Google Scholar] [CrossRef]
- WHO. WHO Coronavirus (COVID-19) Dashboard|WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/ (accessed on 13 December 2022).
- Pokhrel, P.; Hu, C.; Mao, H. Detecting the Coronavirus (COVID-19). ACS Sensors 2020, 5, 2283–2297. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Lei, L.R.; Zouzas, W.; April Si, X. Nasally Inhaled Therapeutics and Vaccination for COVID-19: Developments and Challenges. MedComm 2021, 2, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Trassante, C.M.; dos Santos Barboza, V.; dos S. Rocha, L.; Correa, P.M.; Luchese, C.; Wilhelm, E.A.; Pereira de Pereira, C.M.; Baldissera, M.D.; Rech, V.C.; Giongo, J.L.; et al. Detection of SARS-CoV-2 Virus Using an Alternative Molecular Method and Evaluation of Biochemical, Hematological, Inflammatory, and Oxidative Stress in Healthcare Professionals. Microb. Pathog. 2021, 158, 104975. [Google Scholar] [CrossRef]
- Maglaras, P.; Lilis, I.; Paliogianni, F.; Bravou, V.; Kalogianni, D.P. A Molecular Lateral Flow Assay for SARS-CoV-2 Quantitative Detection. Biosensors 2022, 12, 926. [Google Scholar] [CrossRef] [PubMed]
- Talwar, C.S.; Park, K.H.; Ahn, W.C.; Kim, Y.S.; Kwon, O.S.; Yong, D.; Kang, T.; Woo, E. Detection of Infectious Viruses Using Crispr-Cas12-Based Assay. Biosensors 2021, 11, 301. [Google Scholar] [CrossRef]
- Song, S.; Peng, H.; Wang, Q.; Liu, Z.; Dong, X.; Wen, C.; Ai, C.; Zhang, Y.; Wang, Z.; Zhu, B. Inhibitory Activities of Marine Sulfated Polysaccharides against SARS-CoV-2. Food Funct. 2020, 11, 7415–7420. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.M.; Ruiz-Caro, R.; Veiga, M.D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Martin, A.H.; Douglas Goff, H.; Smith, A.; Dalgleish, D.G. Immobilization of Casein Micelles for Probing Their Structure and Interactions with Polysaccharides Using Scanning Electron Microscopy (SEM). Food Hydrocoll. 2006, 20, 817–824. [Google Scholar] [CrossRef]
- Lobregas, M.O.S.; Bantang, J.P.O.; Camacho, D.H. Carrageenan-Stabilized Silver Nanoparticle Gel Probe Kit for Colorimetric Sensing of Mercury (II) Using Digital Image Analysis. Sens. Bio-Sens. Res. 2019, 26, 100303. [Google Scholar] [CrossRef]
- Mandal, N.; De, N.; Jana, P.; Sannigrahi, A.; Chattopadhyay, K. Correlation between CNS Tuberculosis and the COVID-19 Pandemic: The Neurological and Therapeutic Insights. ACS Chem. Neurosci. 2020, 11, 2789–2792. [Google Scholar] [CrossRef] [PubMed]
- Ana, P.; Nathalie, B.; Gilles, B.; Daniel, R.; Tomás, M.S.; Yolanda, F.P. Anti-Herpes Simplex Virus (HSV-1) Activity and Antioxidant Capacity of Carrageenan-Rich Enzymatic Extracts from Solieria Filiformis (Gigartinales, Rhodophyta). Int. J. Biol. Macromol. 2021, 168, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Kwon, P.S.; Oh, H.; Kwon, S.J.; Jin, W.; Zhang, F.; Fraser, K.; Hong, J.J.; Linhardt, R.J.; Dordick, J.S. Sulfated Polysaccharides Effectively Inhibit SARS-CoV-2 in Vitro. Cell Discov. 2020, 6, 50. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.X.; Guan, H.S. The Antiviral Activities and Mechanisms of Marine Polysaccharides: An Overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef] [PubMed]
- ANVISA. National Health Surveillance Agency. Brazilian Pharmacopoeia, 5th ed. Brasilia. 2010. Available online: bibliotecadigital.anvisa.ibict.br (accessed on 16 December 2022).
- D’Archino, R.; Nelson, W.A.; Sutherland, J.E. Unnamed for over 30 Years: Gigartina Falshawiae Sp. Nov. (Gigartinaceae, Rhodophyta) and Its Confusion with Iridaea Tuberculosa in New Zealand. Phycologia 2020, 59, 45–53. [Google Scholar] [CrossRef]
- Berneira, L.M.; de Santi, I.I.; da Silva, C.C.; Venzke, D.; Colepicolo, P.; Vaucher, R.D.A.; dos Santos, M.A.Z.; de Pereira, C.M.P. Bioactivity and Composition of Lipophilic Metabolites Extracted from Antarctic Macroalgae. Braz. J. Microbiol. 2021, 52, 1275–1285. [Google Scholar] [CrossRef]
- Westermeier, R.; González, C.; Murúa, P.; Morales, J.; Patiño, D.J.; Fabres, N.; Zamorano, J.; Müller, D.G. Seasonal Variation of Carrageenan Yield, Gel Strength and Viscosity in Sarcopeltis (Ex Gigartina) Skottsbergii from Southern Chile. Phycol. Res. 2022, 70, 42–49. [Google Scholar] [CrossRef]
- Martiny, T.R.; Pacheco, B.S.; Pereira, C.M.P.; Mansilla, A.; Astorga–España, M.S.; Dotto, G.L.; Moraes, C.C.; Rosa, G.S. A Novel Biodegradable Film Based on κ-Carrageenan Activated with Olive Leaves Extract. Food Sci. Nutr. 2020, 8, 3147–3156. [Google Scholar] [CrossRef]
- dos Santos, M.A.Z.; Berneira, L.M.; Goulart, N.L.; Mansilla, A.; Astorga-España, M.S.; de Pereira, C.M.P. Rhodophyta, Ochrophyta and Chlorophyta Macroalgae from Different Sub-Antarctic Regions (Chile) and Their Potential for Polyunsaturated Fatty Acids. Rev. Bras. Bot. 2021, 44, 429–438. [Google Scholar] [CrossRef]
- Demarco, C.F.; Afonso, T.F.; Schoeler, G.P.; dos Santos Barboza, V.; dos Santos Rocha, L.; Pieniz, S.; Giongo, J.L.; de A. Vaucher, R.; Igansi, A.V.; Cadaval, T.R.S.A.; et al. New Low-Cost Biofilters for SARS-CoV-2 Using Hymenachne Grumosa as a Precursor. J. Clean. Prod. 2022, 331, 130000. [Google Scholar] [CrossRef]
- dos Santos Barboza, V.; Domingues, W.B.; de Souza, T.T.; Collares, T.V.; Seixas, F.K.; Pacheco, B.S.; Sousa, F.S.S.; Oliveira, T.L.; de Lima, M.; de Pereira, C.M.P.; et al. Reverse Transcription-Loop-Mediated Isothermal Amplification (RT-LAMP) Assay as a Rapid Molecular Diagnostic Tool for COVID-19 in Healthcare Workers. J. Clin. Virol. Plus 2023, 3, 100134. [Google Scholar] [CrossRef]
- De Freitas, S.C.; Berneira, L.M.; dos Santos, M.A.Z.; Poletti, T.; Mansilla, A.; Astorga-España, M.S.; Garcia, M.O.; Hartwig, D.D.; Hübner, S.D.O.; de Pereira, C.M.P. Bioactivity Evaluation and Composition of Extracts from Sub-Antarctic Macroalgae Mazzaella Laminarioides at Distinct Development Phases. Rev. Bras. Bot. 2020, 43, 689–696. [Google Scholar] [CrossRef]
- Webber, V.; de Carvalho, S.M.; Ogliari, P.J.; Hayashi, L.; Barreto, P.L.M. Optimization of the Extraction of Carrageenan from Kappaphycus Alvarezii Using Response Surface Methodology. Food Sci. Technol. 2012, 32, 812–818. [Google Scholar] [CrossRef]
- Cerveira, M.M.; Vianna, H.S.; Ferrer, E.M.K.; da Rosa, B.N.; de Pereira, C.M.P.; Baldissera, M.D.; Lopes, L.Q.S.; Rech, V.C.; Giongo, J.L.; de Almeida Vaucher, R. Bioprospection of Novel Synthetic Monocurcuminoids: Antioxidant, Antimicrobial, and In Vitro Cytotoxic Activities. Biomed. Pharmacother. 2021, 133, 111052. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant Activity and Free Radical Scavenging Capacity between Korean Medicinal Plants and Flavonoids by Assay-Guided Comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- Navikaite, V.; Simanaviciute, D.; Klimaviciute, R.; Jakstas, V.; Ivanauskas, L. Interaction between κ- And ι-Carrageenan and Anthocyanins from Vaccinium Myrtillus. Carbohydr. Polym. 2016, 148, 36–44. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. CDC 2019-Novel Coronavirus (2019-NCoV) Real-Time RT-PCR Diagnostic Panel. 2020. Available online: https://www.fda.gov/media/134922/download (accessed on 13 December 2022).
- Park, G.-S.; Ku, K.; Baek, S.-H.; Kim, S.-J.; Kim, S.I.; Kim, B.-T.; Maeng, J.-S. Development of Reverse Transcription Loop-Mediated Isothermal Amplification Assays Targeting SARS-CoV-2. J. Mol. Diagn. 2020, 22, 729–735. [Google Scholar] [CrossRef]
- Maciel, O.M.C.; Tavares, R.S.N.; Caluz, D.R.E.; Gaspar, L.R.; Debonsi, H.M. Photoprotective Potential of Metabolites Isolated from Algae-Associated Fungi Annulohypoxylon Stygium. J. Photochem. Photobiol. B Biol. 2018, 178, 316–322. [Google Scholar] [CrossRef]
- Pandey, S.; Goswami, G.K.; Nanda, K.K. Green Synthesis of Biopolymer-Silver Nanoparticle Nanocomposite: An Optical Sensor for Ammonia Detection. Int. J. Biol. Macromol. 2012, 51, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W.; Wang, L.F. Preparation and Characterization of Carrageenan-Based Nanocomposite Films Reinforced with Clay Mineral and Silver Nanoparticles. Appl. Clay Sci. 2014, 97–98, 174–181. [Google Scholar] [CrossRef]
- Muthulakshmi, L.; Pavithra, U.; Sivaranjani, V.; Balasubramanian, N.; Sakthivel, K.M.; Pruncu, C.I. A Novel Ag/Carrageenan–Gelatin Hybrid Hydrogel Nanocomposite and Its Biological Applications: Preparation and Characterization. J. Mech. Behav. Biomed. Mater. 2021, 115, 104257. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, M.; Tiasto, V.; Kalitnik, A.; Begun, M.; Khotimchenko, R.; Leonteva, E.; Bryukhovetskiy, I.; Khotimchenko, Y. Antitumor Potential of Carrageenans from Marine Red Algae. Carbohydr. Polym. 2020, 246, 116568. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, W.; Li, X.; Lü, X.; Li, N.; Gao, X.; Song, J. Preparation and in Vitro Antioxidant Activity of κ-Carrageenan Oligosaccharides and Their Oversulfated, Acetylated, and Phosphorylated Derivatives. Carbohydr. Res. 2005, 340, 685–692. [Google Scholar] [CrossRef]
- Suganya, A.M.; Sanjivkumar, M.; Chandran, M.N.; Palavesam, A.; Immanuel, G. Pharmacological Importance of Sulphated Polysaccharide Carrageenan from Red Seaweed Kappaphycus Alvarezii in Comparison with Commercial Carrageenan. Biomed. Pharmacother. 2016, 84, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Sousa, A.; Coelho, H.; Amado, A.M.; Ribeiro-Claro, P.J.A. Use of FTIR, FT-Raman and 13C-NMR Spectroscopy for Identification of Some Seaweed Phycocolloids. Biomol. Eng. 2003, 20, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Amado, A.M.; Critchley, A.T.; van de Velde, F.; Ribeiro-Claro, P.J.A. Identification of Selected Seaweed Polysaccharides (Phycocolloids) by Vibrational Spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar] [CrossRef]
- Şen, M.; Erboz, E.N. Determination of Critical Gelation Conditions of κ-Carrageenan by Viscosimetric and FT-IR Analyses. Food Res. Int. 2010, 43, 1361–1364. [Google Scholar] [CrossRef]
- Morokutti-Kurz, M.; Fröba, M.; Graf, P.; Große, M.; Grassauer, A.; Auth, J.; Schubert, U.; Prieschl-Grassauer, E. Iota-Carrageenan Neutralizes SARS-CoV-2 and Inhibits Viral Replication In Vitro. PLoS ONE 2021, 16, e0237480. [Google Scholar] [CrossRef]
- Mutesa, L.; Ndishimye, P.; Butera, Y.; Souopgui, J.; Uwineza, A.; Rutayisire, R.; Ndoricimpaye, E.L.; Musoni, E.; Rujeni, N.; Nyatanyi, T.; et al. A Pooled Testing Strategy for Identifying SARS-CoV-2 at Low Prevalence. Nature 2021, 589, 276–280. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Nolan, T.; Pfaffl, M.W. Quantitative Real-Time RT-PCR—A Perspective. J. Mol. Endocrinol. 2005, 34, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 Novel Coronavirus (2019-NCoV) by Real-Time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef]
- Soares da Silva, M.; Gularte, J.S.; Demoliner, M.; Hansen, A.W.; Heldt, F.H.; Filippi, M.; Luckmann, C.B.; Malayhka de Abreu Góes Pereira, V.; de Almeida Vaucher, R.; dos Santos Barboza, V.; et al. Brief Dispersion of a Putative B.1.1.28-Derived SARS-CoV-2 Lineage Harboring Additional N234P and E471Q Spike Protein Mutations in Individuals Crossing the Argentina-Brazil Border. Travel Med. Infect. Dis. 2022, 49, 102390. [Google Scholar] [CrossRef]
- Schutz, D.; Conzelmann, C.; Fois, G.; Groß, R.; Weil, T.; Wettstein, L.; Stenger, S.; Zelikin, A.; Hoffmann, T.K.; Frick, M.; et al. Carrageenan-Containing over-the-Counter Nasal and Oral Sprays Inhibit SARS-CoV-2 Infection of Airway Epithelial Cultures. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2021, 320, L750–L756. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, M.J.; Pujol, C.A.; Ciancia, M.; Noseda, M.D.; Matulewicz, M.C.; Damonte, E.B.; Cerezo, A.S. Antiherpetic and Anticoagulant Properties of Carrageenans from the Red Seaweed Gigartina Skottsbergii and Their Cyclized Derivatives: Correlation between Structure and Biological Activity. Int. J. Biol. Macromol. 1997, 20, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Diogo, J.V.; Novo, S.G.; González, M.J.; Ciancia, M.; Bratanich, A.C. Antiviral Activity of Lambda-Carrageenan Prepared from Red Seaweed (Gigartina Skottsbergii) against BoHV-1 and SuHV-1. Res. Vet. Sci. 2015, 98, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Müller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan Is a Potent Inhibitor of Papillomavirus Infection. PLoS Pathog. 2006, 2, 671–680. [Google Scholar] [CrossRef]
- Minami, K.; Masutani, R.; Suzuki, Y.; Kubota, M.; Osaka, N.; Nakanishi, T.; Nakano, T.; Ukimura, A. Evaluation of SARS-CoV-2 RNA Quantification by RT-LAMP Compared to RT-QPCR. J. Infect. Chemother. 2021, 27, 1068–1071. [Google Scholar] [CrossRef]
- Aoki, M.N.; de Oliveira Coelho, B.; Góes, L.G.B.; Minoprio, P.; Durigon, E.L.; Morello, L.G.; Marchini, F.K.; Riediger, I.N.; do Carmo Debur, M.; Nakaya, H.I.; et al. Colorimetric RT-LAMP SARS-CoV-2 Diagnostic Sensitivity Relies on Color Interpretation and Viral Load. Sci. Rep. 2021, 11, 9026. [Google Scholar] [CrossRef]
- Kitajima, H.; Tamura, Y.; Yoshida, H.; Kinoshita, H.; Katsuta, H.; Matsui, C.; Matsushita, A.; Arai, T.; Hashimoto, S.; Iuchi, A.; et al. Clinical COVID-19 Diagnostic Methods: Comparison of Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) and Quantitative RT-PCR (QRT-PCR). J. Clin. Virol. 2021, 139, 104813. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, J.; Pang, B.; Zhang, H.; Le, X.C. CRISPR/Cas12a-Mediated Gold Nanoparticle Aggregation for Colorimetric Detection of SARS-CoV-2. Chem. Commun. 2021, 57, 6871–6874. [Google Scholar] [CrossRef] [PubMed]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Singh, J.; Streithorst, J.; Granados, A.; Sotomayor-Gonzalez, A.; Zorn, K.; Gopez, A.; et al. Rapid Detection of 2019 Novel Coronavirus SARS-CoV-2 Using a CRISPR-Based DETECTR Lateral Flow Assay. medRxiv 2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Liu, C.; Chen, J.; Luo, X.; Xue, Y.; Liang, Q.; Zhou, L.; Tao, Y.; Li, M.; et al. Sensitive and Rapid On-Site Detection of SARS-CoV-2 Using a Gold Nanoparticle-Based High-Throughput Platform Coupled with CRISPR/Cas12-Assisted RT-LAMP. Sens. Actuators B Chem. 2021, 345, 130411. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Fanton, A.; Chandrasekaran, S.S.; Prywes, N.; Lukarska, M.; Biering, S.B.; Smock, D.C.J.; Mok, A.; Knott, G.J.; Van, E.; et al. Rapid Detection of SARS-CoV-2 with Cas13. medRxiv 2020. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zank, P.D.; Cerveira, M.M.; Santos, V.B.d.; Klein, V.P.; Souza, T.T.d.; Bueno, D.T.; Poletti, T.; Leitzke, A.F.; Luehring Giongo, J.; Carreño, N.L.V.; et al. Carrageenan from Gigartina skottsbergii: A Novel Molecular Probe to Detect SARS-CoV-2. Biosensors 2023, 13, 378. https://doi.org/10.3390/bios13030378
Zank PD, Cerveira MM, Santos VBd, Klein VP, Souza TTd, Bueno DT, Poletti T, Leitzke AF, Luehring Giongo J, Carreño NLV, et al. Carrageenan from Gigartina skottsbergii: A Novel Molecular Probe to Detect SARS-CoV-2. Biosensors. 2023; 13(3):378. https://doi.org/10.3390/bios13030378
Chicago/Turabian StyleZank, Patrícia Daiane, Milena Mattes Cerveira, Victor Barboza dos Santos, Vitor Pereira Klein, Thobias Toniolo de Souza, Danielle Tapia Bueno, Tais Poletti, Amanda Fonseca Leitzke, Janice Luehring Giongo, Neftali Lenin Villarreal Carreño, and et al. 2023. "Carrageenan from Gigartina skottsbergii: A Novel Molecular Probe to Detect SARS-CoV-2" Biosensors 13, no. 3: 378. https://doi.org/10.3390/bios13030378
APA StyleZank, P. D., Cerveira, M. M., Santos, V. B. d., Klein, V. P., Souza, T. T. d., Bueno, D. T., Poletti, T., Leitzke, A. F., Luehring Giongo, J., Carreño, N. L. V., Mansilla, A., Astorga-España, M. S., Pereira, C. M. P. d., & Vaucher, R. d. A. (2023). Carrageenan from Gigartina skottsbergii: A Novel Molecular Probe to Detect SARS-CoV-2. Biosensors, 13(3), 378. https://doi.org/10.3390/bios13030378




