Microfluidic Electrochemical Glucose Biosensor with In Situ Enzyme Immobilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
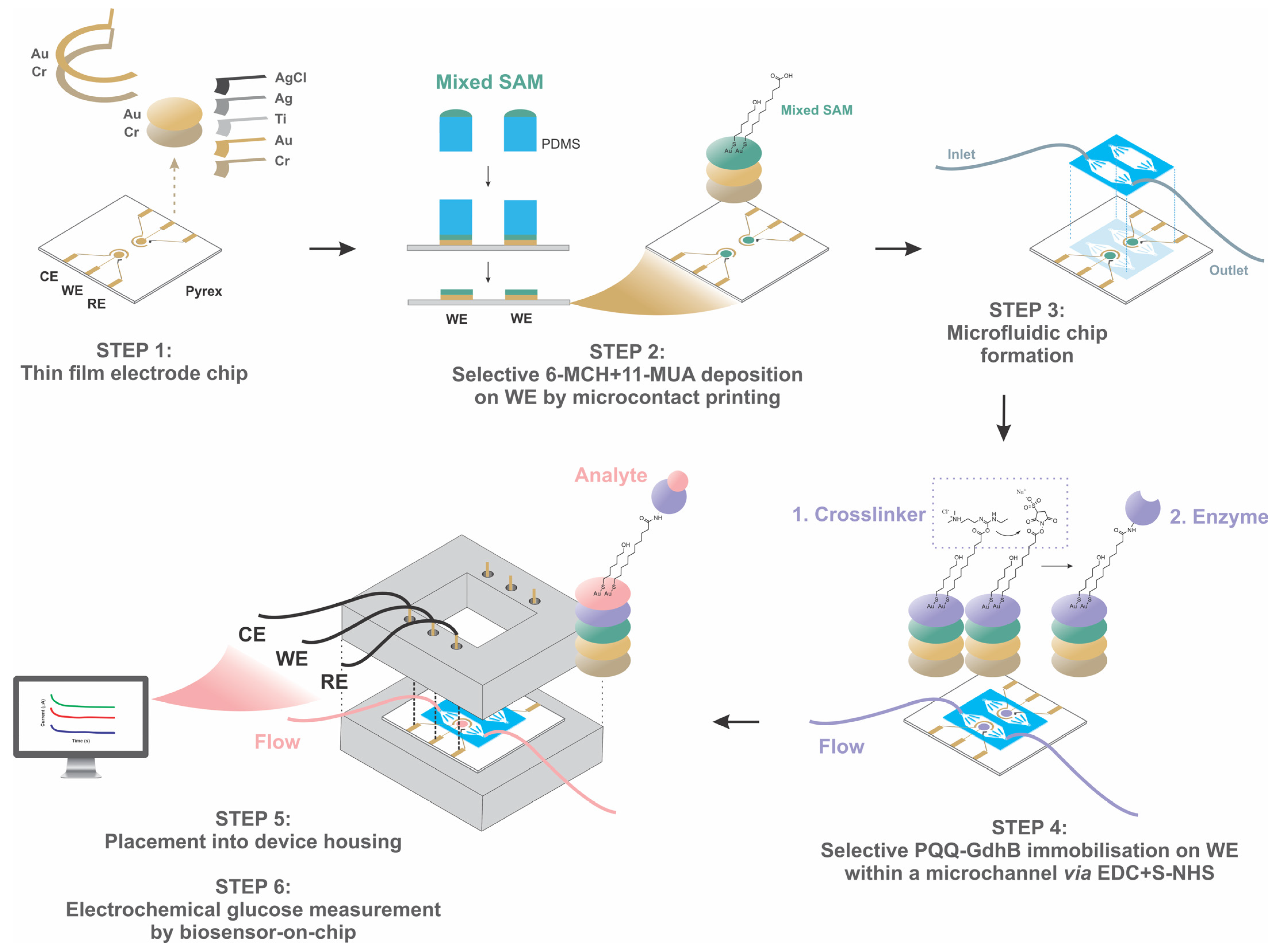
2.2. Design and Fabrication of Microfluidic Electrochemical PQQ-GdhB Biosensor
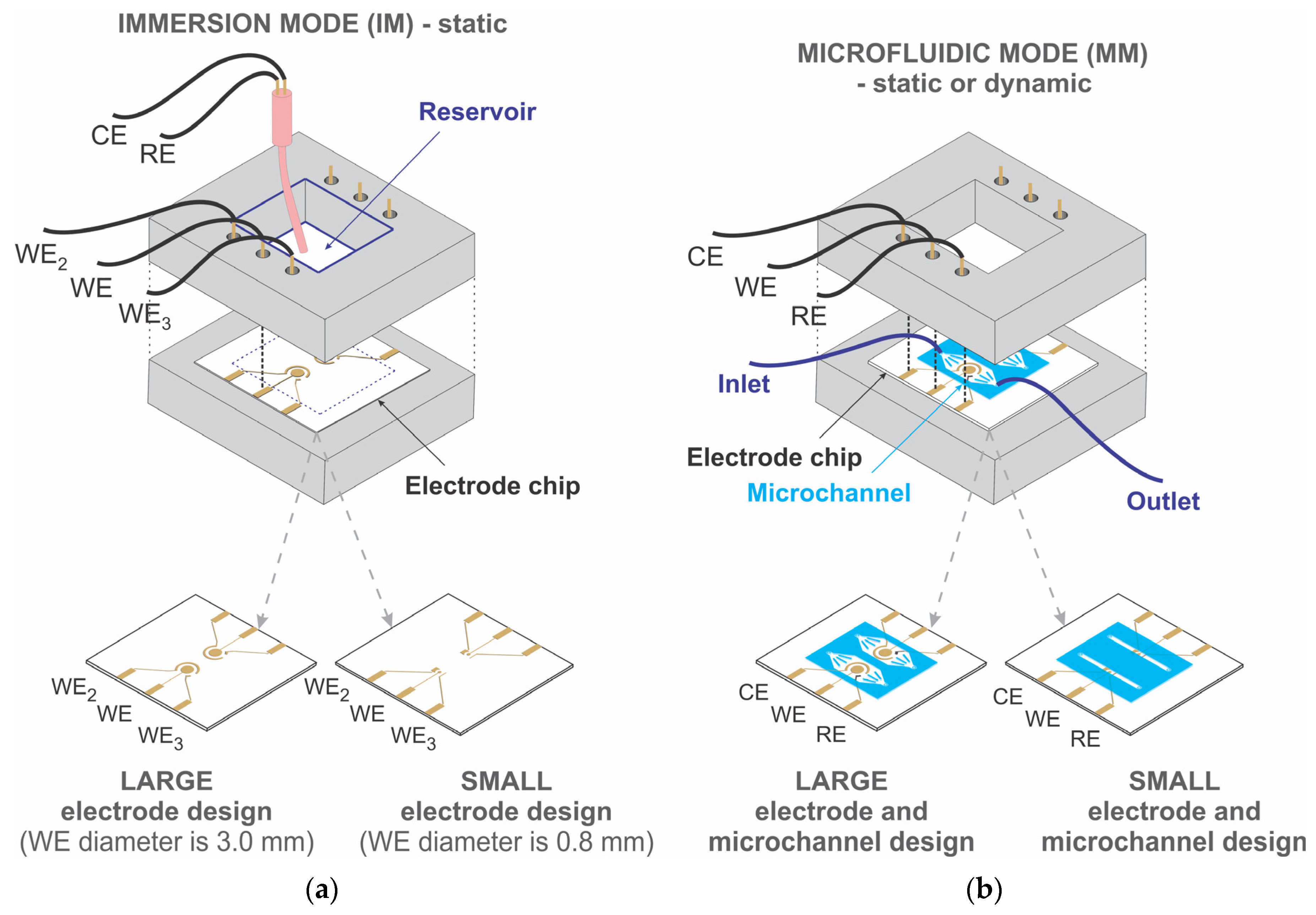
2.3. Experimental Setup
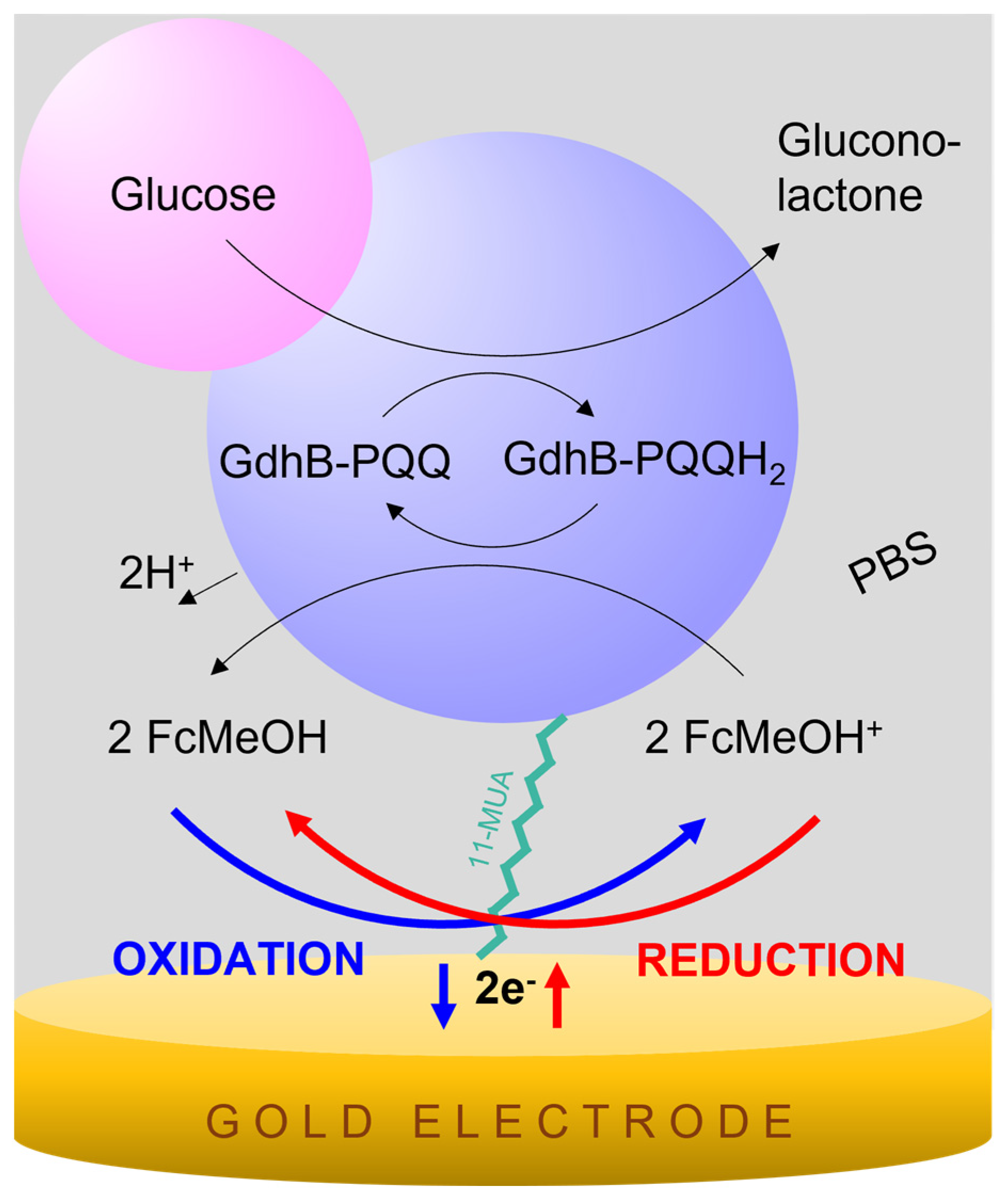
2.4. Biosensor Glucose Detection Principle
3. Results and Discussion
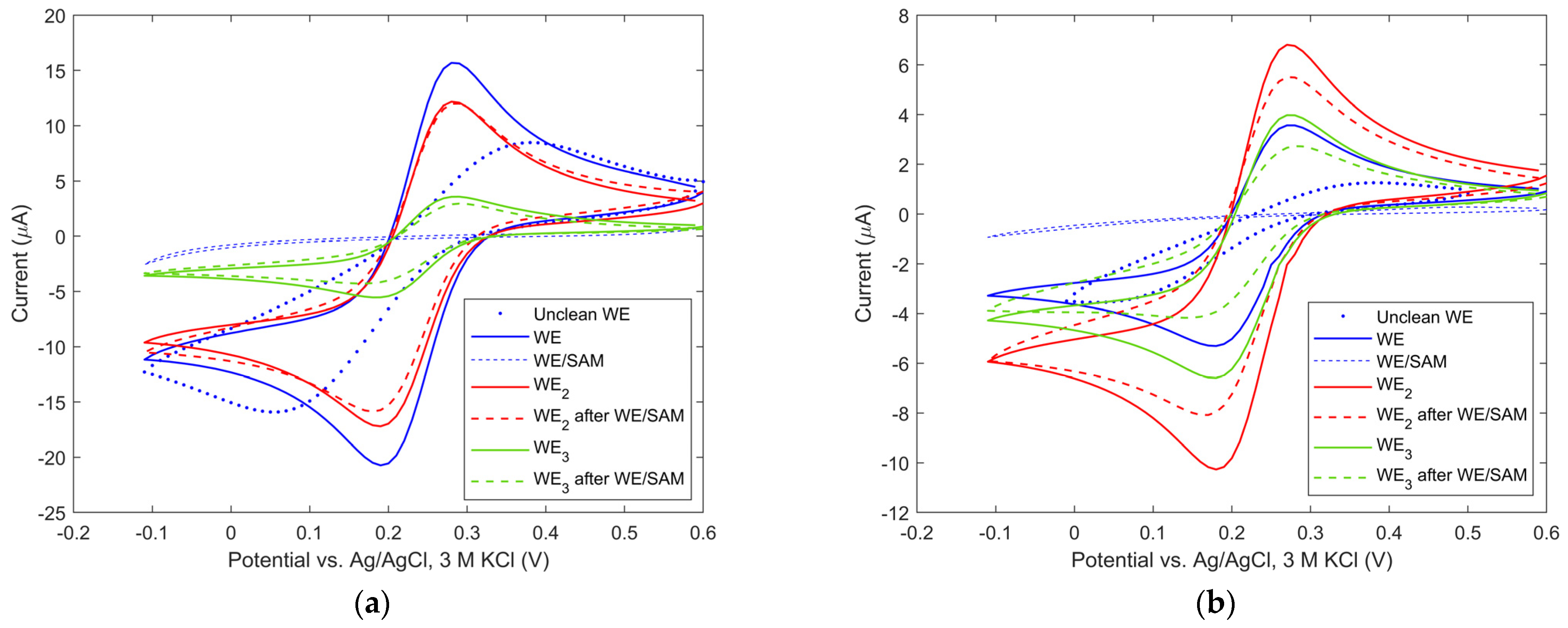
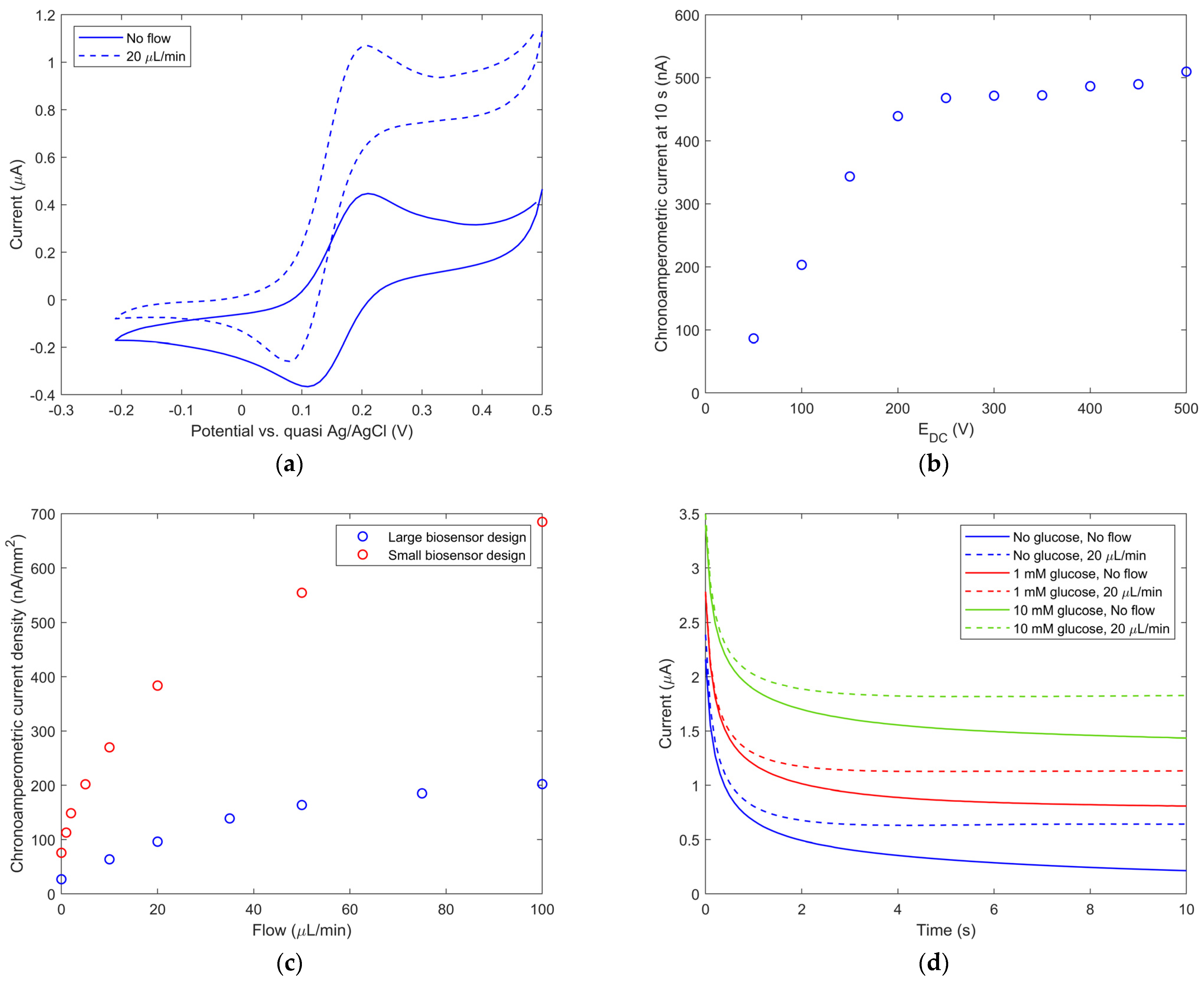
3.1. Immersion Mode Biosensor Characterization
3.2. Microfluidic Mode Biosensor Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rackus, D.G.; Shamsi, M.H.; Wheeler, A.R. Electrochemistry, biosensors and microfluidics: A convergence of fields. Chem. Soc. Rev. 2015, 44, 5320–5340. [Google Scholar] [CrossRef] [PubMed]
- Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M.; Andronescu, E. Biosensors-on-Chip: An Up-to-Date Review. Molecules 2020, 25, 6013. [Google Scholar] [CrossRef] [PubMed]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics Integrated Biosensors: A Leading Technology towards Lab-on-a-Chip and Sensing Applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yan, Z.; Liu, Q. Smartphone-Based Electrochemical Systems for Glucose Monitoring in Biofluids: A Review. Sensors 2022, 22, 5670. [Google Scholar] [CrossRef]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.; Brunetti, A.; Fiorillo, A.S. Glucose biosensors in clinical practice: Principles, limits and perspectives of currently used devices. Theranostics 2022, 12, 493–511. [Google Scholar] [CrossRef]
- Kap, Ö.; Kılıç, V.; Hardy, J.G.; Horzum, N. Smartphone-based colorimetric detection systems for glucose monitoring in the diagnosis and management of diabetes. Analyst 2021, 146, 2784–2806. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, Y.; Su, L.; Zhao, D.; Zhao, L.; Zhang, X. Microfluidic Chip-Based Wearable Colorimetric Sensor for Simple and Facile Detection of Sweat Glucose. Anal. Chem. 2019, 91, 14803–14807. [Google Scholar] [CrossRef]
- Kalinke, C.; Wosgrau, V.; Oliveira, P.R.; Oliveira, G.A.; Martins, G.; Mangrich, A.S.; Bergamini, M.F.; Marcolino-Junior, L.H. Green method for glucose determination using microfluidic device with a non-enzymatic sensor based on nickel oxyhydroxide supported at activated biochar. Talanta 2019, 200, 518–525. [Google Scholar] [CrossRef]
- Khor, S.M.; Choi, J.; Won, P.; Ko, S.H. Challenges and Strategies in Developing an Enzymatic Wearable Sweat Glucose Biosensor as a Practical Point-Of-Care Monitoring Tool for Type II Diabetes. Nanomaterials 2022, 12, 221. [Google Scholar] [CrossRef]
- Rodrigues, D.; Barbosa, A.I.; Rebelo, R.; Kwon, I.K.; Reis, R.L.; Correlo, V.M. Skin-Integrated Wearable Systems and Implantable Biosensors: A Comprehensive Review. Biosensors 2020, 10, 79. [Google Scholar] [CrossRef]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, J. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Lee, Y.-C.; Lai, Y.-H.; Lim, J.-C.; Huang, N.-T.; Lin, C.-T.; Huang, J.-J. Review of Integrated Optical Biosensors for Point-of-Care Applications. Biosensors 2020, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, X.; Kumar, S.; Singh, R.; Zhang, B.; Bai, C.; Pu, X. Development of Glucose Sensor Using Gold Nanoparticles and Glucose-Oxidase Functionalized Tapered Fiber Structure. Plasmonics 2020, 15, 841–848. [Google Scholar] [CrossRef]
- Singh, L.; Singh, R.; Zhang, B.; Cheng, S.; Kaushik, B.K.; Kumar, S. LSPR based uric acid sensor using graphene oxide and gold nanoparticles functionalized tapered fiber. Opt. Fiber Technol. 2019, 53, 102043. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Kaushik, B.K.; Chen, N.; Yang, Q.S.; Zhang, X. LSPR-Based Cholesterol Biosensor Using Hollow Core Fiber Structure. IEEE Sens. J. 2019, 19, 7399–7406. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Wang, Z.; Li, M.; Liu, X.; Zhang, W.; Zhang, B.; Li, G. (Invited) Advances in 2D nanomaterials-assisted plasmonics optical fiber sensors for biomolecules detection. Results Opt. 2023, 10, 100342. [Google Scholar] [CrossRef]
- Shinwari, M.W.; Zhitomirsky, D.; Deen, I.A.; Selvaganapathy, P.R.; Deen, M.J.; Landheer, D. Microfabricated Reference Electrodes and their Biosensing Applications. Sensors 2010, 10, 1679–1715. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Biosensors and Microfluidic Biosensors: From Fabrication to Application. Biosensors 2022, 12, 543. [Google Scholar] [CrossRef]
- Gharib, G.; Bütün, I.; Muganlı, Z.; Kozalak, G.; Namlı, I.; Sarraf, S.S.; Ahmadi, V.E.; Toyran, E.; van Wijnen, A.J.; Koşar, A. Biomedical Applications of Microfluidic Devices: A Review. Biosensors 2022, 12, 1023. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Huang, C.-H.; Pai, P.-C.; Seo, J.; Lei, K.F. A Review on Microfluidics-Based Impedance Biosensors. Biosensors 2023, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Zhang, H.; Wang, X. Microfluidic-Chip-Integrated Biosensors for Lung Disease Models. Biosensors 2021, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Campaña, A.L.; Florez, S.L.; Noguera, M.J.; Fuentes, O.P.; Puentes, P.R.; Cruz, J.C.; Osma, J.F. Enzyme-Based Electrochemical Biosensors for Microfluidic Platforms to Detect Pharmaceutical Residues in Wastewater. Biosensors 2019, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.O.; Singh, B. Electrochemical Biosensors for Detection of Pesticides and Heavy Metal Toxicants in Water: Recent Trends and Progress. ACS EST Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- Bhardwaj, T.; Ramana, L.N.; Sharma, T.K. Current Advancements and Future Road Map to Develop ASSURED Microfluidic Biosensors for Infectious and Non-Infectious Diseases. Biosensors 2022, 12, 357. [Google Scholar] [CrossRef]
- Manessis, G.; Gelasakis, A.I.; Bossis, I. Point-of-Care Diagnostics for Farm Animal Diseases: From Biosensors to Integrated Lab-on-Chip Devices. Biosensors 2022, 12, 455. [Google Scholar] [CrossRef]
- Mahari, S.; Gandhi, S. Recent Advances in Electrochemical Biosensors for the Detection of Salmonellosis: Current Prospective and Challenges. Biosensors 2022, 12, 365. [Google Scholar] [CrossRef]
- Kaur, G.; Tomar, M.; Gupta, V. Development of a microfluidic electrochemical biosensor: Prospect for point-of-care cholesterol monitoring. Sens. Actuators B Chem. 2018, 261, 460–466. [Google Scholar] [CrossRef]
- Wisitsoraat, A.; Sritongkham, P.; Karuwan, C.; Phokharatkul, D.; Maturos, T.; Tuantranont, A. Fast cholesterol detection using flow injection microfluidic device with functionalized carbon nanotubes based electrochemical sensor. Biosens. Bioelectron. 2010, 26, 1514–1520. [Google Scholar] [CrossRef]
- Kurbanoglu, S.; Mayorga-Martinez, C.C.; Medina-Sánchez, M.; Rivas, L.; Ozkan, S.A.; Merkoçi, A. Antithyroid drug detection using an enzyme cascade blocking in a nanoparticle-based lab-on-a-chip system. Biosens. Bioelectron. 2015, 67, 670–676. [Google Scholar] [CrossRef]
- Itoh, D.; Sassa, F.; Nishi, T.; Kani, Y.; Murata, M.; Suzuki, H. Droplet-based microfluidic sensing system for rapid fish freshness determination. Sens. Actuators B Chem. 2012, 171–172, 619–626. [Google Scholar] [CrossRef]
- Ferri, S.; Kojima, K.; Sode, K. Review of Glucose Oxidases and Glucose Dehydrogenases: A Bird’s Eye View of Glucose Sensing Enzymes. J. Diabetes Sci. Technol. 2011, 5, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Raitman, O.A.; Patolsky, F.; Katz, E.; Willner, I. Electrical contacting of glucose dehydrogenase by the reconstitution of a pyrroloquinoline quinone-functionalized polyaniline film associated with an Au-electrode: An in situ electrochemical SPR study. Chem. Commun. 2002, 17, 1936–1937. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.T.H. Efficient Direct Electron Transfer for a Highly Oriented PQQ-GDH Immobilized Electrode for Bioanode. J Biosens. Bioelectron. 2014, 5, 1. [Google Scholar] [CrossRef]
- Ivnitski, D.; Atanassov, P.; Apblett, C. Direct Bioelectrocatalysis of PQQ-Dependent Glucose Dehydrogenase. Electroanalysis 2007, 19, 1562–1568. [Google Scholar] [CrossRef]
- Vajdič, T.; Ošlaj, M.; Kopitar, G.; Mrak, P. Engineered, highly productive biosynthesis of artificial, lactonized statin side-chain building blocks: The hidden potential of Escherichia coli unleashed. Metab. Eng. 2014, 24, 160–172. [Google Scholar] [CrossRef]
- Kim, Y.-P.; Park, S.J.; Lee, D.; Kim, H.-S. Electrochemical glucose biosensor by electrostatic binding of PQQ-glucose dehydrogenase onto self-assembled monolayers on gold. J. Appl. Electrochem. 2012, 42, 383–390. [Google Scholar] [CrossRef]
- Parra-Cabrera, C.; Samitier, J.; Homs-Corbera, A. Multiple biomarkers biosensor with just-in-time functionalization: Application to prostate cancer detection. Biosens. Bioelectron. 2016, 77, 1192–1200. [Google Scholar] [CrossRef]
- Veselinovic, J.; Li, Z.; Daggumati, P.; Seker, E. Electrically Guided DNA Immobilization and Multiplexed DNA Detection with Nanoporous Gold Electrodes. Nanomaterials 2018, 8, 351. [Google Scholar] [CrossRef]
- Wang, Z.; Jinlong, L.; An, Z.; Kimura, M.T.O. Enzyme immobilization in completely packaged freestanding SU-8 microfluidic channel by electro click chemistry for compact thermal biosensor. Process Biochem. 2019, 79, 57–64. [Google Scholar] [CrossRef]
- Kaufmann, T.; Ravoo, B.J. Stamps, inks and substrates: Polymers in microcontact printing. Polym. Chem. 2010, 1, 371–387. [Google Scholar] [CrossRef]
- Prissanaroon-Ouajai, W.; Pigram, P.J.; Sirivat, A. Simple Solid-state Ag/AgCl Reference Electrode and Its Integration with Conducting Polypyrrole Electrode for the Production of All-solid-state pH Sensor. KMUTNB Int. J. Appl. Sci. Technol. 2016, 9, 225–233. [Google Scholar] [CrossRef]
- Rahman, T.; Ichiki, T. Fabrication and Characterization of a Stabilized Thin Film Ag/AgCl Reference Electrode Modified with Self-Assembled Monolayer of Alkane Thiol Chains for Rapid Biosensing Applications. Sensors 2017, 17, 2326. [Google Scholar] [CrossRef] [PubMed]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Ignat, T.; Miu, M.; Kleps, I.; Bragaru, A.; Simion, M.; Danila, M. Electrochemical characterization of BSA/11-mercaptoundecanoic acid on Au electrode. Mater. Sci. Eng. B 2010, 169, 55–61. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, 2nd edition; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Ahmad, R.; Tripathy, N.; Khan, M.Y.; Bhat, K.S.; Ahn, M.-S.; Khang, G.; Hahn, Y.-B. Hierarchically assembled ZnO nanosheets microspheres for enhanced glucose sensing performances. Ceram. Int. 2016, 42, 13464–13469. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Khan, M.Y.; Bhat, K.S.; Ahn, M.-S.; Khang, G.; Hahn, Y.-B. Skin-Attachable, Stretchable Electrochemical Sweat Sensor for Glucose and pH Detection. ACS Appl. Mater. Interfaces 2018, 10, 13729–13740. [Google Scholar] [CrossRef]
- Yan, L.; Miao, K.; Ma, P.; Ma, X.; Bi, R.; Chen, F. A feasible electrochemical biosensor for determination of glucose based on Prussian blue–Enzyme aggregates cascade catalytic system. Bioelectrochemistry 2021, 141, 107838. [Google Scholar] [CrossRef]
- Yoon, H.; Nah, J.; Kim, H.; Ko, S.; Sharifuzzaman; Barman, S.C.; Xuan, X.; Kim, J.; Park, J.Y. A chemically modified laser-induced porous graphene based flexible and ultrasensitive electrochemical biosensor for sweat glucose detection. Sens. Actuators B Chemical. 2020, 311, 127866. [Google Scholar] [CrossRef]
- Zhu, X.; Ju, Y.; Chen, J.; Liu, D.; Liu, H. Nonenzymatic Wearable Sensor for Electrochemical Analysis of Perspiration Glucose. ACS Sens. 2018, 3, 1135–1141. [Google Scholar] [CrossRef]
- Ma, L.; Yue, Z.; Huo, G.; Zhang, S.; Zhu, B.; Zhang, S.; Huang, W. 3D Hydrogen Titanate Nanotubes on Ti Foil: A Carrier for Enzymatic Glucose Biosensor. Sensors 2020, 20, 1024. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Minimally-invasive Microneedle-based Biosensor Array for Simultaneous Lactate and Glucose Monitoring in Artificial Interstitial Fluid. Electroanalysis 2019, 31, 374–382. [Google Scholar] [CrossRef]
- Nashruddin, S.; Abdullah, J.; Haniff, M.M.; Zaid, M.M.; Choon, O.; Wee, M.M.R. Label Free Glucose Electrochemical Biosensor Based on Poly(3,4-ethylenedioxy thiophene):Polystyrene Sulfonate/Titanium Carbide/Graphene Quantum Dots. Biosensors 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, G.; Xu, T.; Su, L.; Yan, D.; Zhang, X. Fully integrated flexible biosensor for wearable continuous glucose monitoring. Biosens. Bioelectron. 2022, 196, 113760. [Google Scholar] [CrossRef] [PubMed]
- Azimi, S.; Farahani, A.; Docoslis, A.; Vahdatifar, S. Developing an integrated microfluidic and miniaturized electrochemical biosensor for point of care determination of glucose in human plasma samples. Anal. Bioanal. Chem. 2021, 413, 1441–1452. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Nayak, P.; Xia, C.; Alshareef, H.N. Novel amperometric glucose biosensor based on MXene nanocomposite. Sci. Rep. 2016, 6, 36422. [Google Scholar] [CrossRef]
- Jędrzak, A.; Rębiś, T.; Klapiszewski, Ł.; Zdarta, J.; Milczarek, G.; Jesionowski, T. Carbon paste electrode based on functional GOx/silica-lignin system to prepare an amperometric glucose biosensor. Sens. Actuators B Chem. 2018, 256, 176–185. [Google Scholar] [CrossRef]


| Materials | Sensitivity (μA mM−1 cm−2) | Linear Range (mM) | LOD (μM) | Ref. |
|---|---|---|---|---|
| ZnO nanosheets microspheres | 210.8 | 0.05–23 | 50 | [47] |
| CoWO4/CNT | 10.89 | 0.05–0.3 | 1.3 | [48] |
| Prussian blue enzyme | 70.76 | 0.05–3.15 | 10 | [49] |
| Porous graphene | 65.6 | 0.0003–2.1 | 0.3 | [50] |
| Fluorocarbon | 114 | 0.03–1.1 | 15 | [51] |
| Three-dimensional hydrogen titanate nanotubes | 1.541 | 1–10 | 59 | [52] |
| Au-multiwalled carbon nanotubes | 405.2 | 0.05–5 | 3 | [53] |
| Graphene quantum dots | 21.64 | 0–0.5 | 65 | [54] |
| A biocompatible conjugated polymer | 12.69 | 1–30 | 4.7 | [55] |
| Vertically aligned carbon nanotube | 1462 | 1.2–7.8 | 23 | [56] |
| Nafion solubilized Au/MXene nanocomposite | 4.2 | 0.1–18 | 5.9 | [57] |
| Functional GOx/silica–lignin system | 0.78 | 0.5–9 | 145 | [58] |
| Thin film Au PQQ-GdhB | 79 | 0.03–0.2 | 30 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lokar, N.; Pečar, B.; Možek, M.; Vrtačnik, D. Microfluidic Electrochemical Glucose Biosensor with In Situ Enzyme Immobilization. Biosensors 2023, 13, 364. https://doi.org/10.3390/bios13030364
Lokar N, Pečar B, Možek M, Vrtačnik D. Microfluidic Electrochemical Glucose Biosensor with In Situ Enzyme Immobilization. Biosensors. 2023; 13(3):364. https://doi.org/10.3390/bios13030364
Chicago/Turabian StyleLokar, Nina, Borut Pečar, Matej Možek, and Danilo Vrtačnik. 2023. "Microfluidic Electrochemical Glucose Biosensor with In Situ Enzyme Immobilization" Biosensors 13, no. 3: 364. https://doi.org/10.3390/bios13030364
APA StyleLokar, N., Pečar, B., Možek, M., & Vrtačnik, D. (2023). Microfluidic Electrochemical Glucose Biosensor with In Situ Enzyme Immobilization. Biosensors, 13(3), 364. https://doi.org/10.3390/bios13030364




