A Multiple-Array SPRi Biosensor as a Tool for Detection of Gynecological–Oncological Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Chip and Multiple Biosensor Architecture
2.3. Antibody Immobilization
2.4. Measurement of Analytical Signals
2.5. Determination of CA125, HE4, CEA, IL-6 and Aromatase by the Array SPRi Technique Using Single Biosensors
3. Results
3.1. Calibration of the Multiple-Array Biosensor
3.2. Comparison of Results from the Multiple Biosensor with Those Obtained Using Single Biosensors
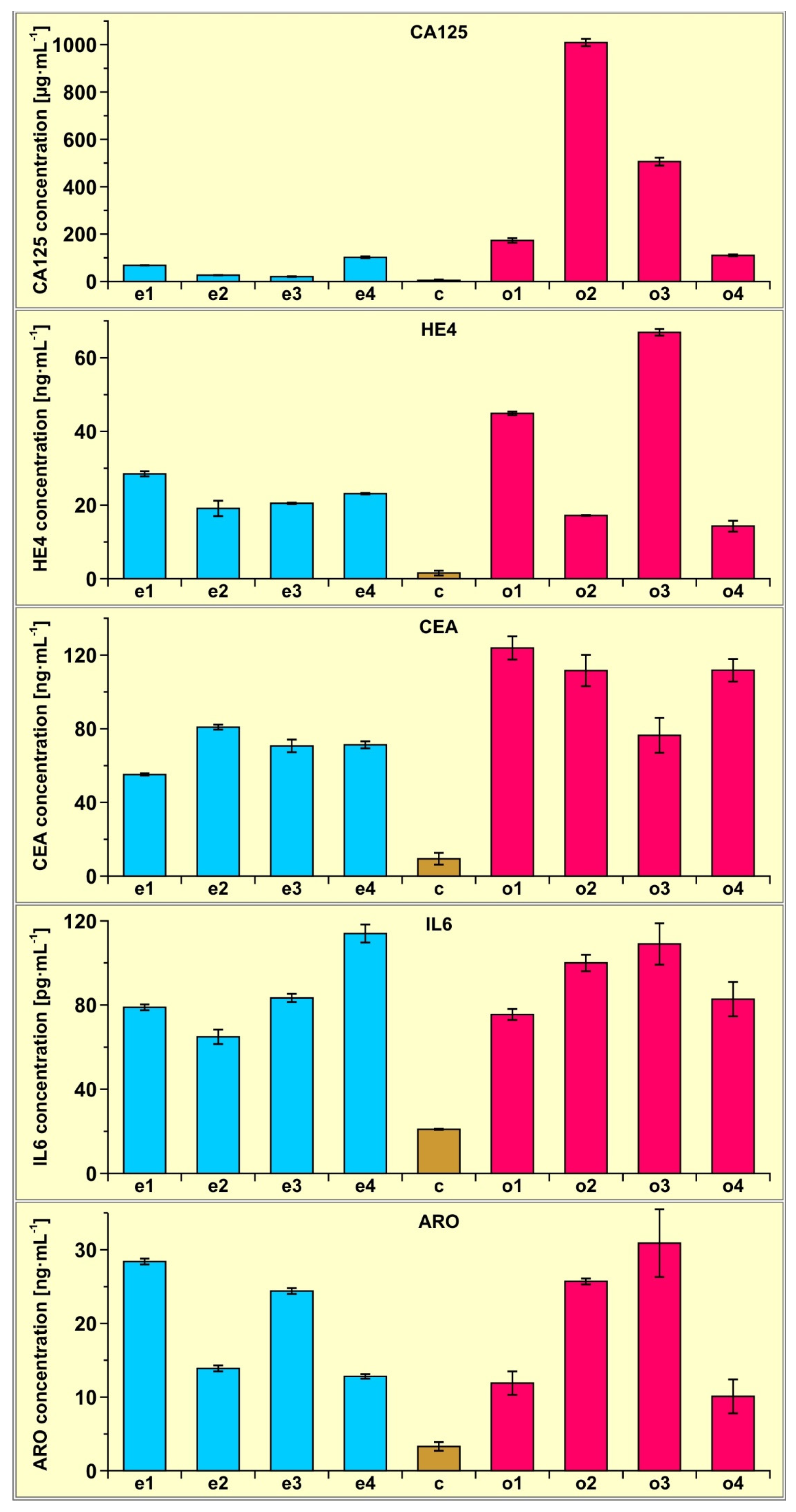
3.3. Examples of CA125, HE4, CEA, Il-6 and Aromatase Determination with the Multiple Biosensor in Real-Blood Serum Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lukaszewski, Z.; Gorodkiewicz, E. Biosensors for the determination of protein biomarkers (edtitorial to SI). Biosensors 2023, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, B.; Lukaszewski, Z.; Hermanowicz-Szamatowicz, K.; Gorodkiewicz, E. A biosensor for determination of the circulating biomarker CA125/MUC 16 by Surface Plasmon Resonance Imaging. Talanta 2020, 206, 120187. [Google Scholar] [CrossRef]
- Szymanska, B.; Lukaszewski, Z.; Zelazowska-Rutkowska, B.; Hermanowicz-Szamatowicz, K.; Gorodkiewicz, E. An SPRi Biosensor for Determination of the Ovarian Cancer Marker HE4 in Human Plasma. Sensors 2021, 21, 3567. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, B.; Lukaszewski, Z.; Hermanowicz-Szamatowicz, K.; Gorodkiewicz, E. An immunosemsor for the determination of carcinoembryonic antygen by Surface Plasmon Resonance imaging. Anal. Biochem. 2020, 609, 113964. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, B.; Lukaszewski, Z.; Oldak, L.; Zelazowska-Rutkowska, B.; Hermanowicz-Szamatowicz, K.; Gorodkiewicz, E. Two Biosensors for the Determination of Interleukin-6 in Blood Plasma by Array SPRi. Biosensors 2022, 12, 412. [Google Scholar] [CrossRef] [PubMed]
- Gorodkiewicz, E.; Sankiewicz, A.; Laudanski, P. Surface Plasmon Resonance Imaging biosensors for aromatase based on a potent inhibitor and a specific antibody: Sensor development and application for biological material. Cent. Eur. J. Chem. 2014, 12, 557–567. [Google Scholar] [CrossRef]
- Perez, B.H.; Gipson, I.K. Focus on molecules: Human mucin MUC16. Exp. Eye Res. 2008, 87, 400–401. [Google Scholar] [CrossRef]
- Bouanene, H.; Miled, A. Conflicting views on the molecular structure of the cancer antigen CA125/MUC16. Dis. Markers 2010, 28, 385–394. [Google Scholar] [CrossRef]
- Huy, N.V.Q.; Khoa, V.V.; Tam, L.M.; Vinh, T.Q.; Tung, N.S.; Thanh, C.N.; Chuang, L. Standard and optimal cut-off values of serum ca-125, HE4 and ROMA in preoperative prediction of ovarian cancer in Vietnam. Gynecol. Oncol. Rep. 2018, 25, 110–114. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Qu, W.; Wang, J.; Jiang, S.-W. Comparison of serum human epididymis protein 4 and CA125 on endometrial cancer detection: A meta-analysis. Clin. Chim. Acta 2019, 488, 215–220. [Google Scholar] [CrossRef]
- Chen, F.; Shen, J.; Cai, P.; Huang, Y. Clinical analysis of four serum tumor markers in 458 patients with ovarian tumors: Diagnostic value of the combined use of HE4, CA125, CA19-9, and CEA in ovarian tumors. Cancer Manag. Res. 2018, 10, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Bingle, L.; Singleton, V.; Bingle, C.D. The putative ovarian tumour marker gene HE4 (WFDC2), is expressed in normal tissues and undergoes complex alternative splicing to yield multiple protein isoforms. Oncogene 2002, 21, 2768–2773. [Google Scholar] [CrossRef] [PubMed]
- Granato, T.; Porpora, M.G.; Longo, F.; Angeloni, A.; Manganaro, L.; Anastasi, E. HE4 in the differential diagnosis of ovarian masses. Clin. Chim. Acta 2015, 446, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, E.; Marchei, G.G.; Viggiani, V.; Gennarini, G.; Frati, L.; Reale, M.G. HE4: A new potential early biomarker for the recurrence of ovarian cancer. Tumor Biol. 2010, 31, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Gasiorowska, E.; Kluz, T.; Lipski, D.; Warchoł, W.; Tykarski, A.; Nowak-Markwitz, E. Human Epididymis Protein 4 (HE4) Reference Limits in Polish Population of Healthy Women, Pregnant Women, and Women with Benign Ovarian Tumors. Dis. Markers 2019, 2019, 3890906. [Google Scholar] [CrossRef]
- Ferraro, S.; Borille, S.; Carnevale, A.; Frusciante, E.; Bassani, N.; Panteghini, M. Verification of the harmonization of human epididymis protein 4 assays. Clin. Chem. Lab. Med. 2016, 54, 1635–1643. [Google Scholar] [CrossRef]
- Ferraro, S.; Panteghini, M. Making new biomarkers a reality: The case of serum human epididymis protein 4. Clin. Chem. Lab. Med. 2018, 57, 1284–1294. [Google Scholar] [CrossRef]
- Cho, H.-I.; Kim, H.J.; Oh, S.-T.; Kim, T.-G. In vitro induction of carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes by dendritic cells transduced with recombinant adenoviruses. Vaccine 2003, 22, 224–236. [Google Scholar] [CrossRef]
- Thomas, P.; Toth, C.A.; Saini, K.S.; Jessup, J.M.; Steele, G., Jr. The structure, metabolism and function of the carcinoembryonic antigen gene family. Biochim. Biophys. Acta 1990, 1032, 177–189. [Google Scholar] [CrossRef]
- Duffy, M.J. Carcinoembryonic antigen as a marker for colorectal cancer: Is it clinically useful? Clin. Chem. 2001, 47, 624–630. [Google Scholar] [CrossRef]
- Thompson, J.A.; Grunert, F.; Zimmermann, W. Carcinoembryonic antigen gene family: Molecular biology and clinical perspectives. J. Clin. Lab. Anal. 1991, 5, 344–366. [Google Scholar] [CrossRef] [PubMed]
- Yamao, T.; Kai, S.; Kazami, A.; Koizumi, K.; Handa, T.; Takemoto, N.; Maruyama, M. Tumor markers CEA, CA19-9 and CA125 in monitoring of response to systemic chemotherapy in patients with advanced gastric cancer. Jpn. J. Clin. OncoI. 1999, 29, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Meropol, N.J.; Punt, C.J.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.A.; Mitchell, E. Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer. Ann. Oncol. 2013, 24, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Yang, S.-H.; Lin, J.-K.; Lin, T.-C.; Chen, W.-S.; Jiang, J.-K.; Wang, H.-S.; Chang, S.-C. Is it reasonable to add preoperative serum level of CEA and CA19-9 to staging for colorectal cancer? J. Surg. Res. 2005, 124, 169–174. [Google Scholar] [CrossRef]
- Canizares, F.; Sola, J.; Perez, M.; Tpvar, I.; De Las Heras, M.; Salinas, J.; Panafiel, R.; Martinez, P. Preoperative values of CA 15-3 and CEA as prognostic factors in breast cancer: A multivariate analysis. Tumour Biol. 2001, 22, 273–281. [Google Scholar] [CrossRef]
- Distler, M.; Pilarsky, E.; Kersting, S.; Grutzmann, R. Preoperative CEA and CA 19-9 are prognostic markers for survival after curative resection for ductal adenocarcinoma of the pancreas—A retrospective tumor marker prognostic study. Int. J. Surg. 2013, 11, 1067–1072. [Google Scholar] [CrossRef]
- Ozkan, H.; Kaya, M.; Cengiz, A. Comparison of tumor marker CA 242 with CA 19-9 and carcinoembryonic antigen (CEA) in pancreatic cancer. Hepatogastroenter 2003, 50, 1669–1674. [Google Scholar]
- Xie, Y.; Zhi, X.; Su, H.; Chen, D.; Cui, D. A novel electrochemical microfluidic chip combined with multiple biomarkers for early diagnosis of gastric cancer. Nanoscale Res. Lett. 2015, 10, 477. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Zhou, Y.; Sheng, S.; Qian, S.Y.; Huo, X. Evaluation of serum CEA, CA19-9, CA72-4, CA125 and ferritin as diagnostic markers and factors of clinical parameters for colorectal cancer. Sci. Rep. 2018, 8, 2732. [Google Scholar] [CrossRef]
- Trovato, M.; Sciacchitano, S.; Facciolà, A.; Valenti, A.; Visalli, G.; di Pietro, A. Interleukin-6 signalling as a valuable cornerstone for molecular medicine (review). Int. J. Mol. Med. 2021, 47, 107. [Google Scholar] [CrossRef]
- Brabek, J.; Jakubek, M.; Vellieux, F.; Novotný, J.; Kolá, M.; Lacina, J.; Szabo, P.; Strnadová, K.; Rösel, D.; Dvoránková, B.; et al. Interleukin-6: Molecule in the Intersection of Cancer, Ageing and COVID-19. Int. J. Mol. Sci. 2020, 21, 7937. [Google Scholar] [CrossRef] [PubMed]
- Amer, H.; Kartikasari, A.E.R.; Plebanski, M. Elevated Interleukin-6 Levels in the Circulation and Peritoneal Fluid of Patients with Ovarian Cancer as a Potential Diagnostic Biomarker: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 1335. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Liu, W.; Zhang, X.; Xue, L. The diagnostic value of interleukin 6 as a biomarker for gastric cancer: A meta-analysis and systematic review. Medicine 2021, 100, e27945. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ye, Y.; Zhang, H.; Szmitkowski, M.; Mäkinen, M.J.; Li, P. Diagnostic and prognostic value of serum interleukin-6 in colorectal cancer. Medicine 2016, 95, 2502. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Wang, X.L.; Li, S.B.; Yang, G.L.; Jin, S.; Gao, Z.Y.; Liu, X.B. Combined detection of IL-6 and IL-8 is beneficial to the diagnosis of early stage esophageal squamous cell cancer: A preliminary study based on the screening of serum markers using protein chips. OncoTargets Ther. 2018, 11, 5777–5787. [Google Scholar] [CrossRef]
- Kampan, N.C.; Madondo, M.T.; Reynolds, J.; Hallo, J.; McNally, O.M.; Jobling, T.W.; Stephens, A.N.; Quinn, M.A.; Plebanski, M. Pre-operative sera interleukin-6 in the diagnosis of high-grade serous ovarian cancer. Sci. Rep. 2020, 10, 221. [Google Scholar] [CrossRef]
- Montero, A.; Pascual, C.B.; Anaut, M.B.; López-Andrés, N.; Antona, G.; Martín-Calvo, N. Diagnostic performance of serum interleukin-6 in pediatric acute appendicitis: A systematic review. World J. Pediatr. 2022, 18, 91–99. [Google Scholar] [CrossRef]
- Hong, Y.; Li, H.; Yuan, Y.C.; Chen, S. Sequence-function correlation of aromatase and its interaction with reductase. J. Steroid Biochem. Mol. Biol. 2010, 118, 203–206. [Google Scholar] [CrossRef]
- Carreau, S.; Lambard, S.; Delaland, C.; Denis-Galeraud, I.; Bilinska, B.; Bourguiba, S. Aromatase expression and role of estrogens in male gonad: A review. Reprod. Biol. Endocrinol. 2003, 1, 352003. [Google Scholar] [CrossRef]
- Miedlich, S.U.; Karamooz, N.; Hammes, S.R. Aromatase deficiency in a male patient-case report and review of the literature. Bone 2016, 93, 181–186. [Google Scholar] [CrossRef]
- Manna, P.R.; Molehin, D.; Ahmed, A.U. Dysregulation of Aromatase in Breast, Endometrial, and Ovarian Cancers: An Overview of Therapeutic Strategies. Progr. Mol. Biol. Translat. Sci. 2016, 144, 487–537. [Google Scholar] [CrossRef]
- Guszcz, T.; Szymańska, B.; Kozlowski, R.; Lukaszewski, Z.; Laskowski, P.; Gorodkiewicz, E. Plasma aromatase as a sensitive and selective potential biomarker of bladder cancer and its role in tumorigenesis. Oncol. Lett. 2020, 19, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Ermini, M.L.; Song, X.C.; Springer, T.; Homola, J. Peptide Functionalization of Gold Nanoparticles for the Detection of Carcinoembryonic Antigen in Blood Plasma via SPR-Based Biosensor. Front. Chem. 2019, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Feng, F.; Chen, Z.-Z.; Bai, Y.-F.; Guo, F.-F.; Wu, F.Y.; Zhou, G. Sensitive detection of carcinoembryonic antigen using surface plasmon resonance biosensor with gold nanoparticles signal amplification. Talanta 2015, 140, 143–149. [Google Scholar] [CrossRef]
- Makaraviciute, A.; Ramanavicius, A.; Ramanaviciene, A. Development of a reusable protein G based SPR immunosensor for direct human growth hormone detection in real samples. Anal. Methods 2015, 7, 9875. [Google Scholar] [CrossRef]
- Kausaite-Minkstimiene, A.; Ramanavicius, A.; Ruksnaite, J.; Ramanaviciene, A. A surface plasmon resonance immunosensor for human growth hormone based on fragmented antibodies. Anal. Methods 2013, 5, 4757. [Google Scholar] [CrossRef]
- Moore, R.G.; McMeekin, D.S.; Brown, A.K.; Di Silvestro, P.; Miller, M.C.; Allard, W.J.; Gajewski, W.; Kurman, R.; Bast, R.C.; Skates, S.J. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2009, 112, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, N.; Zhong, A.; Xiao, K.; Lu, R.; Guo, L. A combined strategy of TK1, HE4 and CA125 shows better diagnostic performance than risk of ovarian malignancy algorithm (ROMA) in ovarian carcinoma. Clin. Chim. Acta 2022, 524, 43–50. [Google Scholar] [CrossRef]
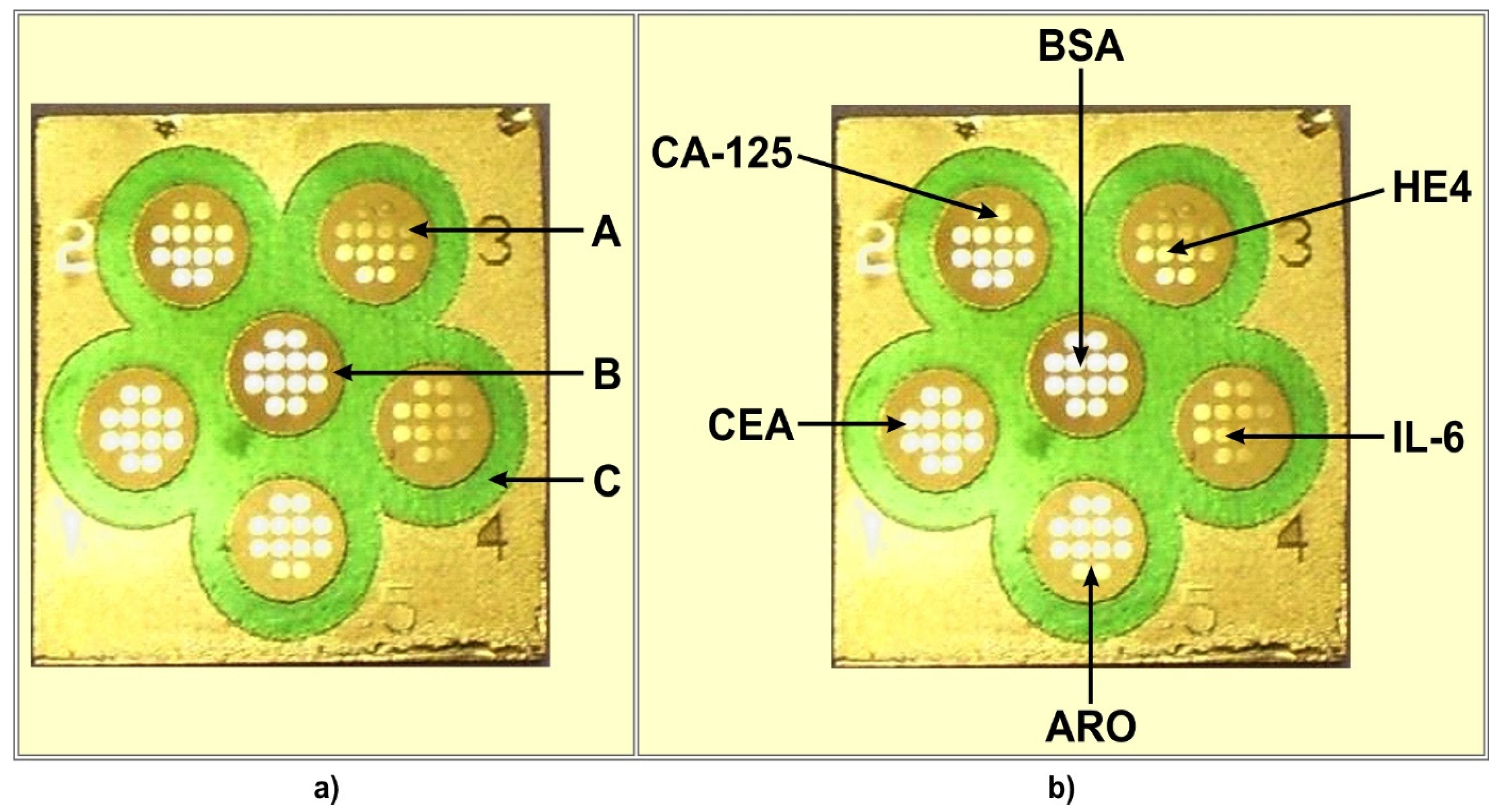

| Biomarker | Calibration Curve | Range/Units | R2 |
|---|---|---|---|
| CA125 | Y = 41.13X + 146 | 2.2–150/µg mL−1 | 0.995 |
| HE4 | Y = 557.3X + 227 | 0.088–5.28/ng mL−1 | 0.991 |
| CEA | Y = 151.5X + 85 | 0.40–20/ng mL−1 | 0.993 |
| IL-6 | Y = 126X + 7.4 | 3–20/pg mL−1 | 0.992 |
| Aromatase | Y = 1408X + 145 | 0.3–5/ng mL−1 | 0.994 |
| Biomarker | RSD [%] | Average [%] | Range [%] |
|---|---|---|---|
| CA125 | 0.73; 3.0; 5.8; 3.5; 5.5; 1.6; 3.3; 3.8 | 3.4 | 0.73–5.8 |
| HE4 | 2.5; 11; 1.0; 0.86; 1.1.; 0.58; 1.3; 10 | 3.5 | 0.58–11 |
| CEA | 1.1; 1.6; 4.8; 2.7; 5.1; 7.6; 12; 5.4 | 5.0 | 1.1–12 |
| IL-6 | 1.8; 5.2; 2.3; 3.8; 3.4; 3.9; 9.0; 11 | 5.0 | 1.8–11 |
| Aromatase | 1.4; 2.9; 1.6; 2.3; 13; 1.6; 15; 23 | 7.6 | 1.4–23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymanska, B.; Lukaszewski, Z.; Hermanowicz-Szamatowicz, K.; Gorodkiewicz, E. A Multiple-Array SPRi Biosensor as a Tool for Detection of Gynecological–Oncological Diseases. Biosensors 2023, 13, 279. https://doi.org/10.3390/bios13020279
Szymanska B, Lukaszewski Z, Hermanowicz-Szamatowicz K, Gorodkiewicz E. A Multiple-Array SPRi Biosensor as a Tool for Detection of Gynecological–Oncological Diseases. Biosensors. 2023; 13(2):279. https://doi.org/10.3390/bios13020279
Chicago/Turabian StyleSzymanska, Beata, Zenon Lukaszewski, Kinga Hermanowicz-Szamatowicz, and Ewa Gorodkiewicz. 2023. "A Multiple-Array SPRi Biosensor as a Tool for Detection of Gynecological–Oncological Diseases" Biosensors 13, no. 2: 279. https://doi.org/10.3390/bios13020279
APA StyleSzymanska, B., Lukaszewski, Z., Hermanowicz-Szamatowicz, K., & Gorodkiewicz, E. (2023). A Multiple-Array SPRi Biosensor as a Tool for Detection of Gynecological–Oncological Diseases. Biosensors, 13(2), 279. https://doi.org/10.3390/bios13020279






