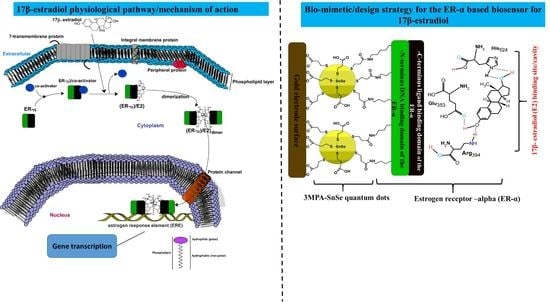
Quantum Dot-Sensitised Estrogen Receptor-α-Based Biosensor for 17β-Estradiol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Characterization Techniques
2.2.2. Preparation of the Tin Selenide Quantum Dots Capped with the 3-Mercaptopropionic Acid
2.2.3. Immobilisation of the 3-Mercaptopropionic Acid-Capped Tin Selenide Quantum Dots on the Gold Electrode Surface
2.2.4. Preparation of the Biosensor ER-α/SnSe-3MPA/AuE
2.2.5. Calibration of the Biosensor
2.2.6. Preparation of Milk Samples
3. Results and Discussion
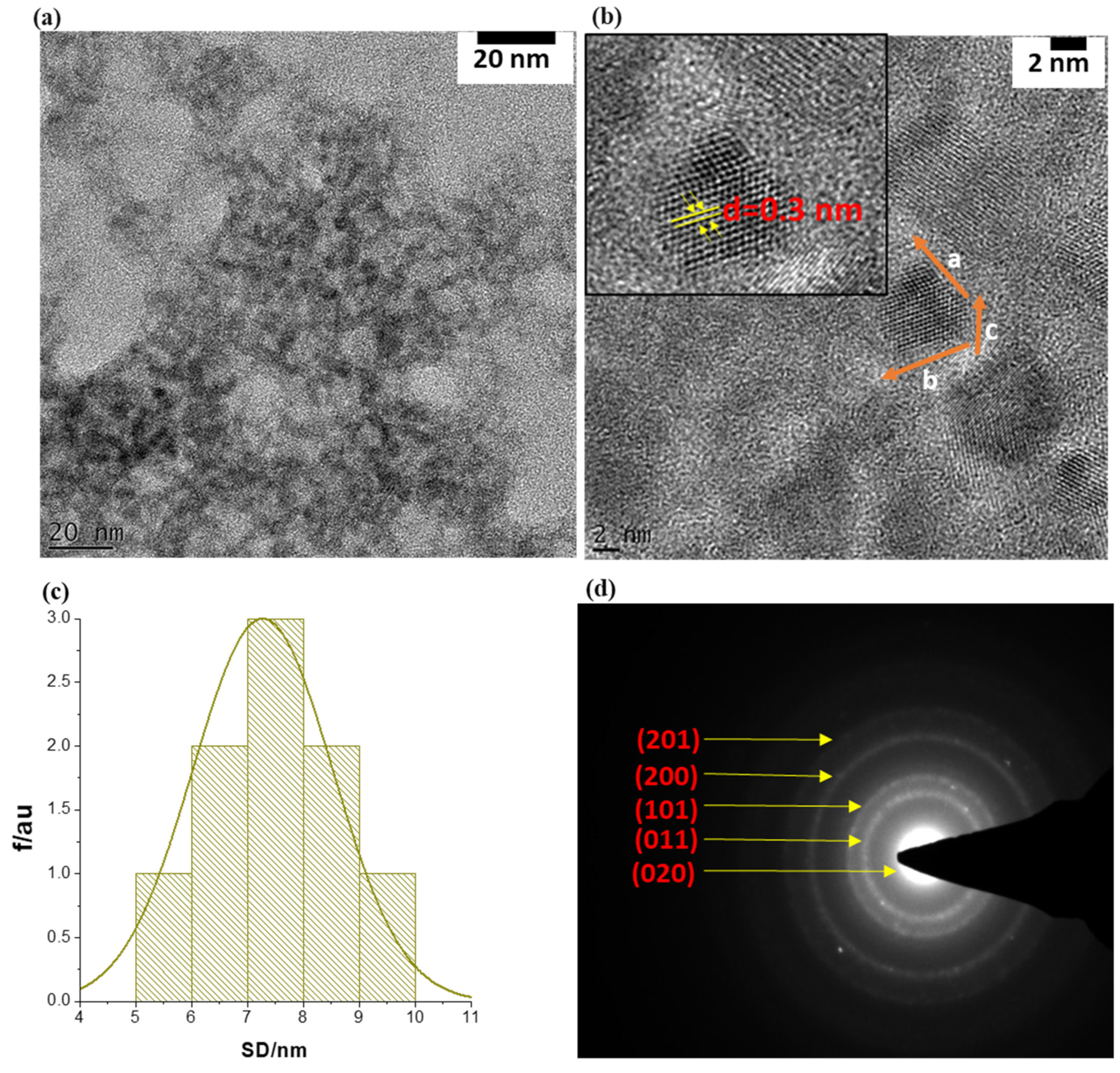
3.1. Size and Morphological Analysis of the SnSe-3MPA Quantum Dots
3.2. Surface Topography Analysis of the SnSe-3MPA Quantum Dots
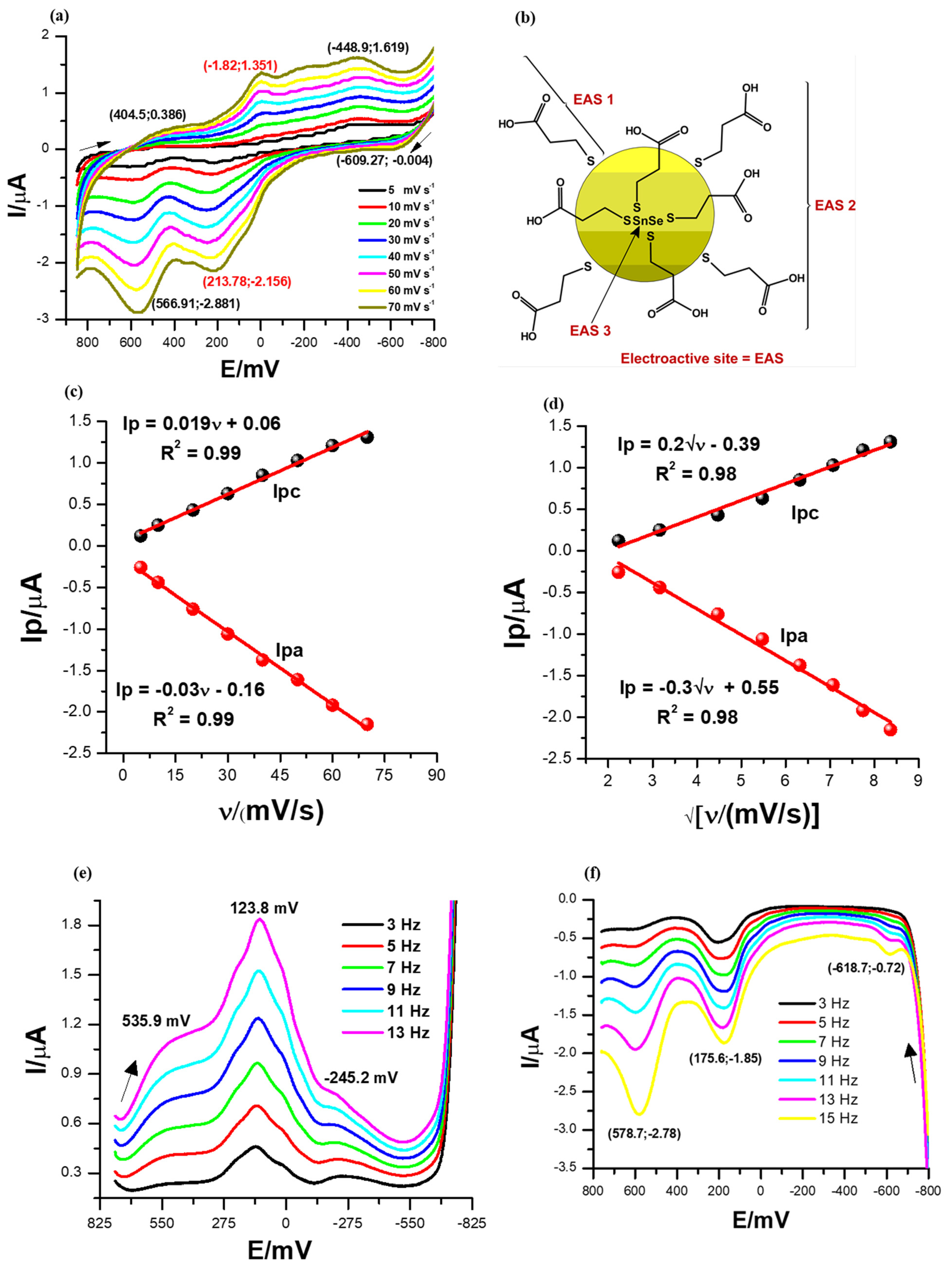
3.3. Electrochemical Properties of the SnSe-3MPA Quantum Dots
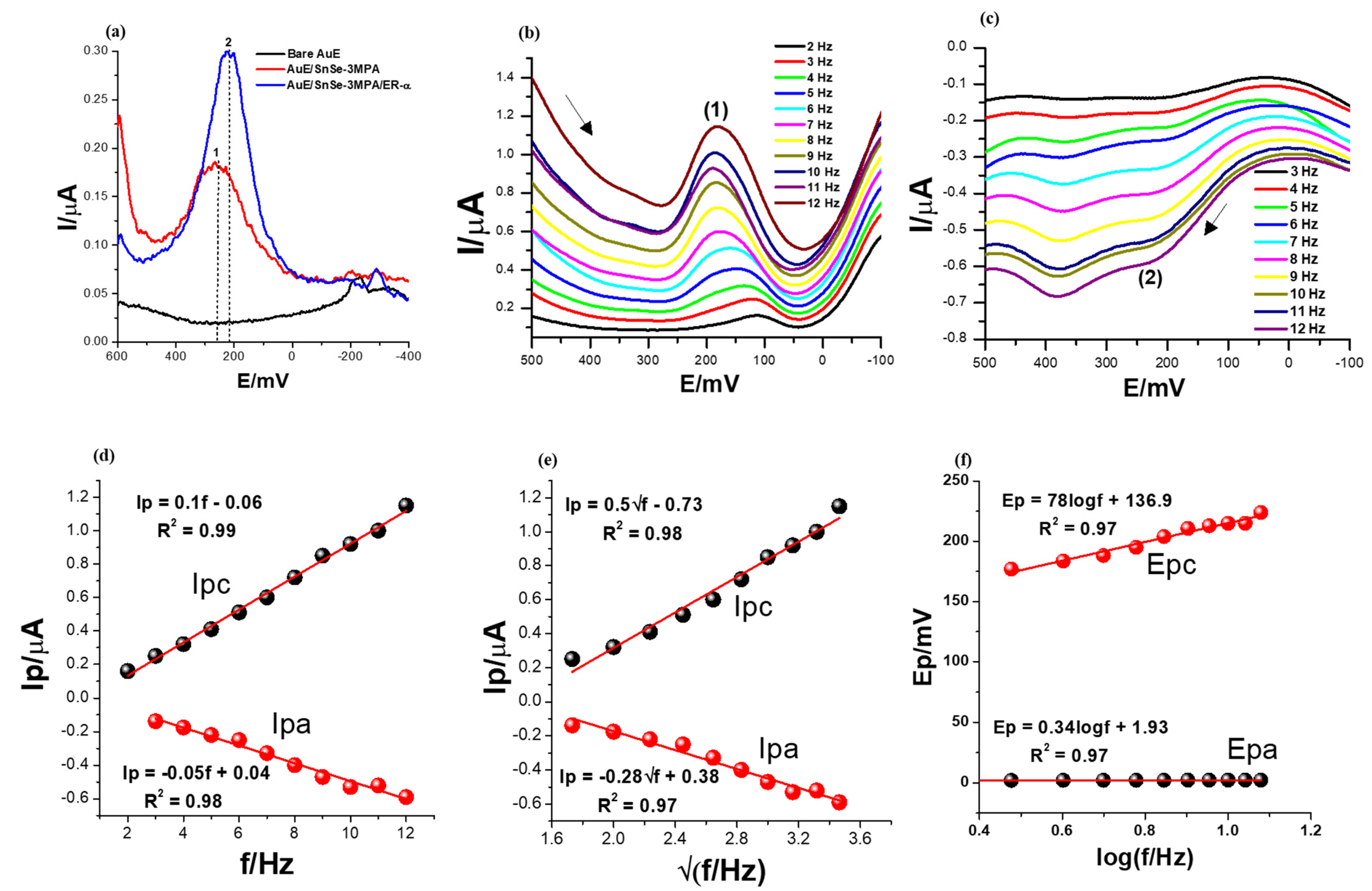
3.4. Optimisation of the Electroanalytical Signal of the ER-α/SnSe-3MPA/AuE Biosensor during the Detection of 17β-Estradiol
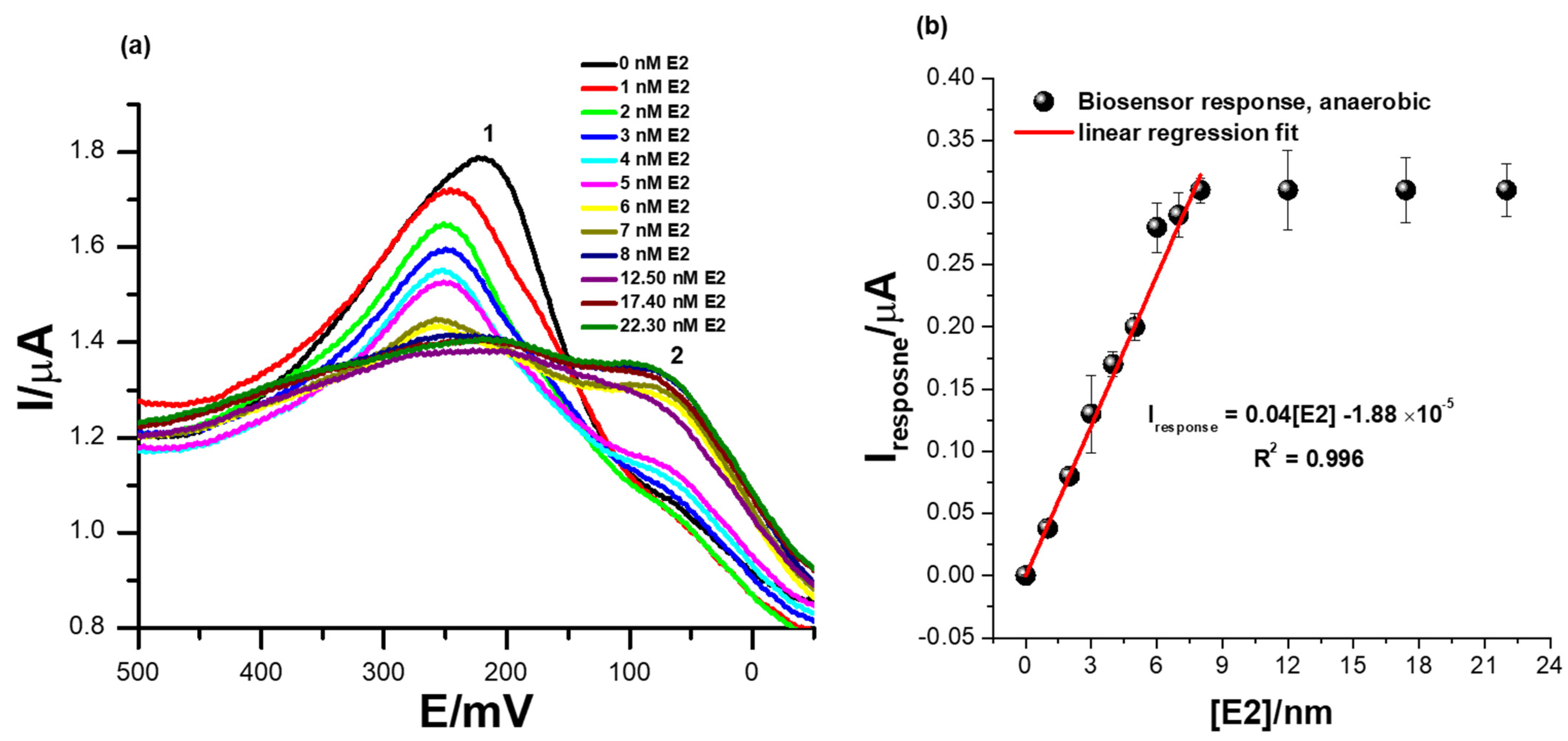
3.5. The Detection of Different Concentrations of 17β-Estradiol Using the Receptor Sensor ER-α/SnSe-3MPA/AuE (Anaerobic Conditions)
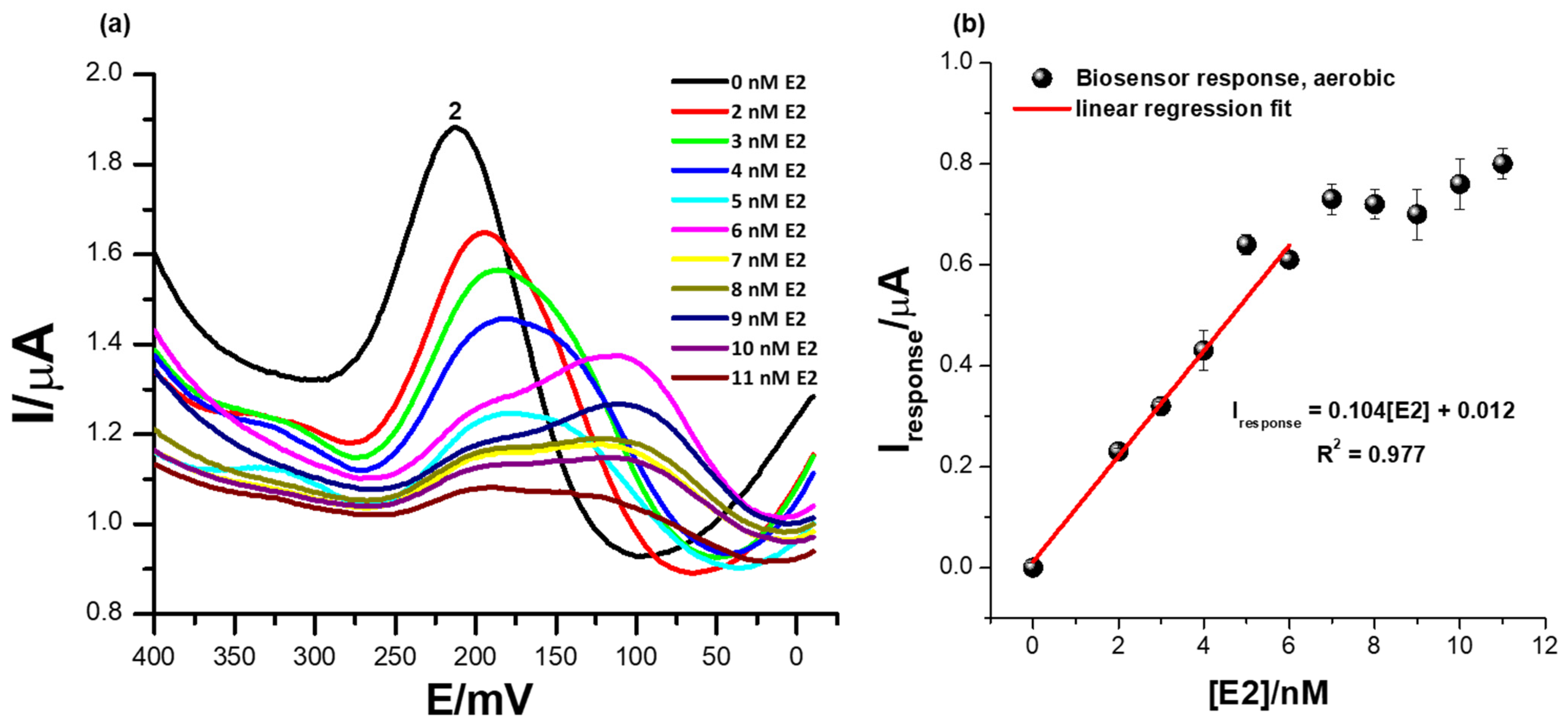
3.6. The Detection of Different Concentrations of 17β-Estradiol Using the ER-α/SnSe-3MPA/AuE Biosensor Platform in the Presence of Oxygen Molecules
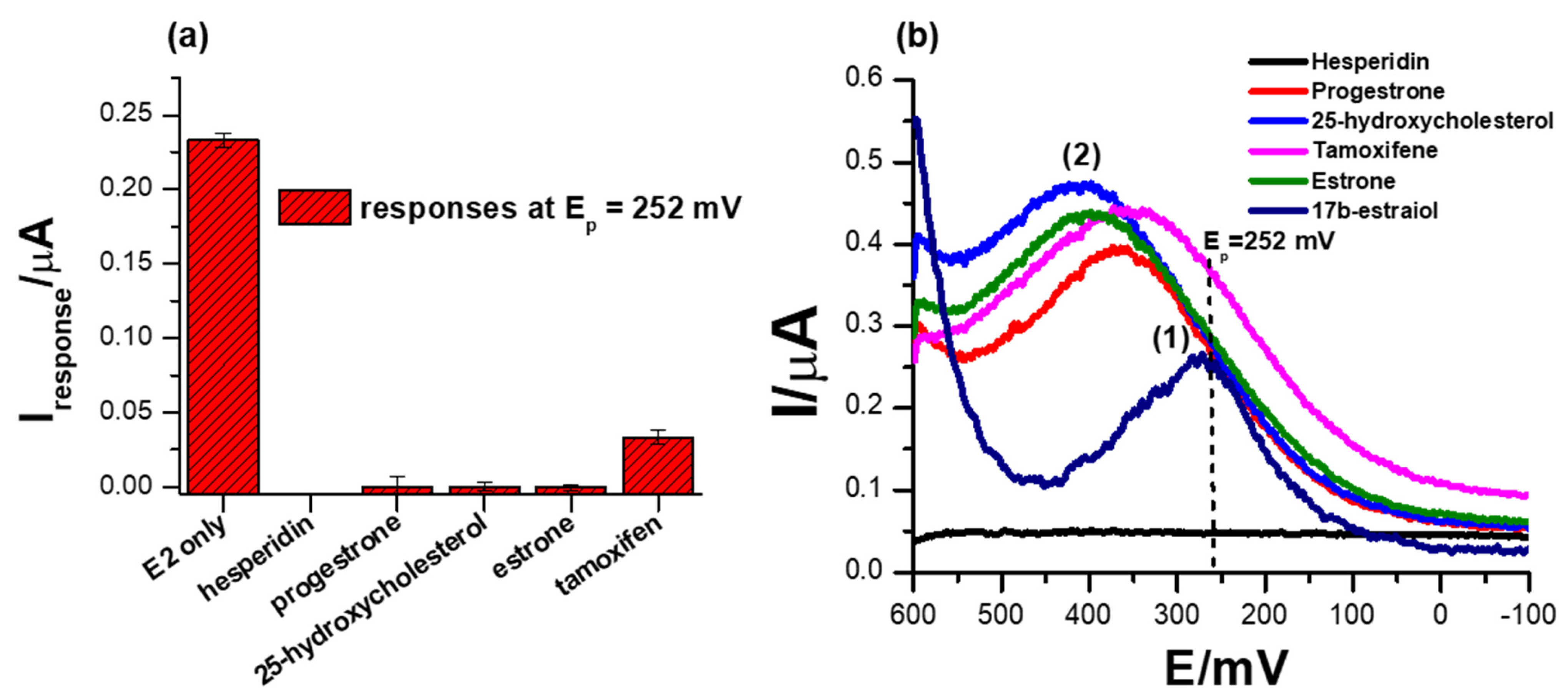
3.7. Selectivity Studies
3.8. Detection of 17β-Estradiol in Dairy Milk Samples
3.9. The Mechanism of ER-α/SnSe-3MPA/AuE Biosensor toward 17β-Estradiol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, A.; Ding, Y.; Li, L.; Duan, D.; Mei, Q.; Zhuang, Q.; Cui, S.; He, X. A novel electrochemical enzyme biosensor for detection of 17β-estradiol by mediated electron-transfer system. Talanta 2018, 192, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Geraci, A.; Calvani, R.; Ferri, E.; Marzetti, E.; Arosio, B.; Cesari, M. Sarcopenia and Menopause: The Role of Estradiol. Front. Endocrinol. 2021, 12, 682012. [Google Scholar] [CrossRef] [PubMed]
- Nazari, E.; Suja, F. Effects of 17β-estradiol (E2) on aqueous organisms and its treatment problem: A review. Rev. Environ. Heal. 2016, 31, 465–491. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Sun, J.; Sun, X. Recent advances in biosensors for the detection of estrogens in the environment and food. TrAC Trends Anal. Chem. 2020, 127, 115882. [Google Scholar] [CrossRef]
- Nili-Ahmadabadi, A.; Rezaei, F.; Heshmati, A.; Ranjbar, A.; Larki-Harchegani, A. Steroid Hormone Exposure as a Potential Hazard in Milk Consumers: A Significant Health Challenge in Iran. J. Food Qual. 2021, 2021, 5595555. [Google Scholar] [CrossRef]
- Serrano, A.; Domènech, A.; Pich, S.; Arís, A.; Plasencia, C.; Bach, A. Heat identification by 17β-estradiol and progesterone quantification in individual raw milk samples by enzyme immunoassay. Electron. J. Biotechnol. 2011, 14, 10. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, C.C. Detection of 17 β-Estradiol in Environmental Samples and for Health Care Using a Single-Use, Cost-Effective Biosensor Based on Differential Pulse Voltammetry (DPV). Biosensors 2017, 7, 15. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, C.; Niu, L.; Cai, W. Occurrence of endocrine disrupting compounds in aqueous environment and their bacterial degradation: A review. Crit. Rev. Environ. Sci. Technol. 2015, 46, 1–59. [Google Scholar] [CrossRef]
- Das, A.; Sangaranarayanan, M. A sensitive electrochemical detection of progesterone using tin-nanorods modified glassy carbon electrodes: Voltammetric and computational studies. Sensors Actuators B Chem. 2018, 256, 775–789. [Google Scholar] [CrossRef]
- Sifakis, S.; Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Human exposure to endocrine disrupting chemicals: Effects on the male and female reproductive systems. Environ. Toxicol. Pharmacol. 2017, 51, 56–70. [Google Scholar] [CrossRef]
- Díaz, N.; Piferrer, F. Estrogen exposure overrides the masculinizing effect of elevated temperature by a downregulation of the key genes implicated in sexual differentiation in a fish with mixed genetic and environmental sex determination. BMC Genom. 2017, 18, 973. [Google Scholar] [CrossRef]
- Kradolfer, D.; Flöter, V.L.; Bick, J.T.; Fürst, R.W.; Rode, K.; Brehm, R.; Henning, H.; Waberski, D.; Bauersachs, S.; Ulbrich, S.E. Epigenetic effects of prenatal estradiol-17β exposure on the reproductive system of pigs. Mol. Cell. Endocrinol. 2016, 430, 125–137. [Google Scholar] [CrossRef]
- Lecomte, S.; Habauzit, D.; Charlier, T.D.; Pakdel, F. Emerging Estrogenic Pollutants in the Aquatic Environment and Breast Cancer. Genes 2017, 8, 229. [Google Scholar] [CrossRef]
- Mahmoodzadeh, S.; Dworatzek, E. The Role of 17β-Estradiol and Estrogen Receptors in Regulation of Ca2+ Channels and Mitochondrial Function in Cardiomyocytes. Front. Endocrinol. 2019, 10, 310. [Google Scholar] [CrossRef]
- Yilmaz, B.; Kadioglu, Y. Determination of 17 b -estradiol in pharmaceutical preparation by UV spectrophotometry and high performance liquid chromatography methods. Arab. J. Chem. 2017, 10, 1422–1428. [Google Scholar] [CrossRef]
- Manickum, T.; John, W. The current preference for the immuno-analytical ELISA method for quantitation of steroid hormones (endocrine disruptor compounds) in wastewater in South Africa. Anal. Bioanal. Chem. 2015, 407, 4949–4970. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Y.; Gatcombe, M.; Farris, J.; Botelho, J.C.; Caudill, S.P.; Vesper, H.W. Simultaneous measurement of total estradiol and testosterone in human serum by isotope dilution liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 5943–5954. [Google Scholar] [CrossRef]
- Povedano, E.; Cincotto, F.H.; Parrado, C.; Díez, P.; Sánchez, A.; Canevari, T.C.; Machado, S.A.; Pingarrón, J.M.; Villalonga, R. Decoration of reduced graphene oxide with rhodium nanoparticles for the design of a sensitive electrochemical enzyme biosensor for 17β-estradiol. Biosens. Bioelectron. 2017, 89, 343–351. [Google Scholar] [CrossRef]
- Nameghi, M.A.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. An ultrasensitive electrochemical sensor for 17β-estradiol using split aptamers. Anal. Chim. Acta 2019, 1065, 107–112. [Google Scholar] [CrossRef]
- Singh, A.C.; Asif, M.; Bacher, G.; Danielsson, B.; Willander, M.; Bhand, S. Nanoimmunosensor based on ZnO nanorods for ultrasensitive detection of 17β-Estradiol. Biosens. Bioelectron. 2018, 126, 15–22. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Liu, J.; Li, X.; Kong, Z.; Jin, H.; Cai, X. Electrochemical integrated paper-based immunosensor modified with multi-walled carbon nanotubes nanocomposites for point-of-care testing of 17β-estradiol. Biosens. Bioelectron. 2018, 107, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Mollarasouli, F.; Kurbanoglu, S.; Ozkan, S.A. The Role of Electrochemical Immunosensors in Clinical Analysis. Biosensors 2019, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Alyamani, B.J.; Alsager, O.A.; Zourob, M. Label-Free Fluorescent Aptasensor for Small Targets via Displacement of Groove Bound Curcumin Molecules. Sensors 2019, 19, 4181. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, D.J.; Okerlund, A.; Brown, E.; Ramaswamy, S. One enzyme, many reactions: Structural basis for the various reactions catalyzed by naphthalene 1,2-dioxygenase. Iucrj 2017, 4, 648–656. [Google Scholar] [CrossRef]
- Temoçin, Z. Designing of a stable and selective glucose biosensor by glucose oxidase immobilization on glassy carbon electrode sensitive to H2O2 via nanofiber interface. J. Appl. Electrochem. 2020, 51, 283–293. [Google Scholar] [CrossRef]
- Li, R.; Zhang, R.; Zhang, B.; Fang, W.; Qiao, Y.; Wang, W.; Cui, Z.; Zhang, D. Effect of synthesis conditions on the bifunctional electrocatalytic properties of Co3O4/N-rGO for ORR and OER. J. Appl. Electrochem. 2020, 51, 155–171. [Google Scholar] [CrossRef]
- Mazurenko, S.; Prokop, Z.; Damborsky, J. Machine Learning in Enzyme Engineering. ACS Catal. 2019, 10, 1210–1223. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, X.; Hua, Y.; Li, Z.; Liu, Y.; Lin, Y. An impedance sensor based on chitosan-carbon quantum dots for the detection sialic acid in humuan serum. Microchem. J. 2021, 169, 106520. [Google Scholar] [CrossRef]
- Fan, H.; Liu, Y.; Dong, J.; Luo, Z. Screening Aptamers that Are Specific for Beclomethasone and the Development of Quantum Dot-Based Assay. Appl. Biochem. Biotechnol. 2021, 193, 3139–3150. [Google Scholar] [CrossRef]
- Mabrouk, S.; Rinnert, H.; Balan, L.; Blanchard, S.; Jasniewski, J.; Medjahdi, G.; Ben Chaabane, R.; Schneider, R. Aqueous synthesis of highly luminescent ternary alloyed Mn-doped ZnSeS quantum dots capped with 2-mercaptopropionic acid. J. Alloy. Compd. 2020, 858, 158315. [Google Scholar] [CrossRef]
- Venkatachalam, V.; Ganapathy, S.; Subramani, T.; Perumal, I. Aqueous CdTe colloidal quantum dots for bio-imaging of Artemia sp. Inorg. Chem. Commun. 2021, 128, 108510. [Google Scholar] [CrossRef]
- Koyappayil, A.; Lee, M.-H. Ultrasensitive Materials for Electrochemical Biosensor Labels. Sensors 2020, 21, 89. [Google Scholar] [CrossRef]
- Klayman, D.L.; Griffin, T.S. Reaction of selenium with sodium borohydride in protic solvents. A Facile Method for the introduction of selenium into organic molecules. J. Am. Chem. Soc. 1973, 95, 197–199. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Chen, C.; Zhao, X.; Qiu, Z.; Xu, H.; Sheng, F.; Hu, Q.; Zheng, Y.; Lin, M.; et al. High yield electrochemical exfoliation synthesis of tin selenide quantum dots for high-performance lithium-ion batteries. J. Mater. Chem. A 2019, 7, 23958–23963. [Google Scholar] [CrossRef]
- Jagani, H.S.; Gupta, S.U.; Bhoraniya, K.; Navapariya, M.; Pathak, V.M.; Solanki, G.K.; Patel, H. Photosensitive Schottky barrier diodes based on Cu/p-SnSe thin films fabricated by thermal evaporation. Mater. Adv. 2022, 3, 2425–2433. [Google Scholar] [CrossRef]
- Karmakar, G.; Halankar, K.K.; Tyagi, A.; Mandal, B.P.; Wadawale, A.P.; Kedarnath, G.; Srivastava, A.P.; Singh, V. Dimethyltin(iv)-4,6-dimethyl-2-pyridylselenolate: An efficient single source precursor for the preparation of SnSe nanosheets as anode material for lithium ion batteries. Dalton Trans. 2021, 50, 15730–15742. [Google Scholar] [CrossRef]
- Musa, I.; Qamhieh, N.; Said, K. Germanium antimony quantum dots morphology and Raman spectroscopy fabricated by inert gas condensation. Results Phys. 2019, 13, 102311. [Google Scholar] [CrossRef]
- Choi, H.; Lee, J.-G.; Mai, X.D.; Beard, M.C.; Yoon, S.S.; Jeong, S. Supersonically Spray-Coated Colloidal Quantum Dot Ink Solar Cells. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Zhang, N.; Cao, M.; Cui, W.-Z.; Hu, T.-C. Effect of rough surface morphology on secondary electron emission from metal surface. Jpn. J. Appl. Phys. 2017, 56, 75802. [Google Scholar] [CrossRef]
- Boulanouar, O.; Fromm, M.; Bass, A.D.; Cloutier, P.; Sanche, L. Absolute cross section for loss of supercoiled topology induced by 10 eV electrons in highly uniform /DNA/1,3-diaminopropane films deposited on highly ordered pyrolitic graphite. J. Chem. Phys. 2013, 139, 055104. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.; McCarthy, B.D.; Rountree, E.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2017, 95, 197–206. [Google Scholar] [CrossRef]
- Wang, C.W.; Xia, Y.Y.Y.; Tian, Z.; Jiang, J.; Li, B.H.; Cui, S.T.; Yang, H.F.; Liang, A.J.; Zhan, X.Y.; Hong, G.H.; et al. Photoemission study of the electronic structure of valence band convergent SnSe. Phys. Rev. B 2017, 96, 165118. [Google Scholar] [CrossRef]
- Mabbott, G.A. An introduction to cyclic voltammetry. J. Chem. Educ. 1983, 60, 697. [Google Scholar] [CrossRef]
- Ferrari, A.G.-M.; Foster, C.W.; Kelly, P.J.; Brownson, D.A.C.; Banks, C.E. Determination of the Electrochemical Area of Screen-Printed Electrochemical Sensing Platforms. Biosensors 2018, 8, 53. [Google Scholar] [CrossRef]
- Costigliola, L.; Heyes, D.M.; Schrøder, T.B.; Dyre, J.C. Revisiting the Stokes-Einstein relation without a hydrodynamic diameter. J. Chem. Phys. 2019, 150, 021101. [Google Scholar] [CrossRef]
- Abeykoon, S.W.; White, R.J. Continuous Square Wave Voltammetry for High Information Content Interrogation of Conformation Switching Sensors. ACS Meas. Sci. Au 2022. [Google Scholar] [CrossRef]
- Mirceski, V.; Skrzypek, S.; Stojanov, L. Square-wave voltammetry. Chemtexts 2018, 4, 17. [Google Scholar] [CrossRef]
- Mirceski, V.; Gulaboski, R.; Lovric, M.; Bogeski, I.; Kappl, R.; Hoth, M. Square-Wave Voltammetry: A Review on the Recent Progress. Electroanalysis 2013, 25, 2411–2422. [Google Scholar] [CrossRef]
- Bonazzola, C.; Gordillo, G. Advanced analysis for electrode kinetic studies of surface reactions by applying square-wave voltammetry. Electrochimica Acta 2016, 213, 613–619. [Google Scholar] [CrossRef]
- Guziejewski, D.; Stojanov, L.; Gulaboski, R.; Mirceski, V. Reversible and Quasireversible Electron Transfer under Conditions of Differential Square-Wave Voltammetry. J. Phys. Chem. C 2022, 126, 5584–5591. [Google Scholar] [CrossRef]
- Mirceski, V.; Guziejewski, D.; Lisichkov, K. Electrode kinetic measurements with square-wave voltammetry at a constant scan rate. Electrochimica Acta 2013, 114, 667–673. [Google Scholar] [CrossRef]
- Guziejewski, D. Electrode mechanisms with coupled chemical reaction—Amplitude effect in square-wave voltammetry. J. Electroanal. Chem. 2020, 870, 114186. [Google Scholar] [CrossRef]
- Song, J.; Yang, J.; Hu, X. Electrochemical determination of estradiol using a poly(l-serine) film-modified electrode. J. Appl. Electrochem. 2008, 38, 833–836. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Auten, R.L.; Davis, J.M. Oxygen Toxicity and Reactive Oxygen Species: The Devil Is in the Details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front. Cell Dev. Biol. 2021, 9, 181. [Google Scholar] [CrossRef]
- Chang, Z.; Zhu, B.; Liu, J.; Zhu, X.; Xu, M.; Travas-Sejdic, J. Electrochemical aptasensor for 17β-estradiol using disposable laser scribed graphene electrodes. Biosens. Bioelectron. 2021, 185, 113247. [Google Scholar] [CrossRef]
- Rozi, N.; Abu Hanifah, S.; Karim, N.H.A.; Heng, L.Y.; Higashi, S.L.; Ikeda, M. Enhancing Electrochemical Biosensor Performance for 17β-Estradiol Determination with Short Split—Aptamers. Biosensors 2022, 12, 1077. [Google Scholar] [CrossRef]
- da Silva, D.N.; Pereira, A.C. An electrochemical sensor modified with a molecularly imprinted polymer and carbon black for 17-β-estradiol detection. Anal. Methods 2022, 14, 1208–1213. [Google Scholar] [CrossRef]
- Bacchu, M.S.; Ali, M.R.; Hasan, M.N.; Mamun, M.R.A.; Hossain, M.I.; Khan, M.Z.H. Graphitic carbon nitride and APTES modified advanced electrochemical biosensor for detection of 17β-estradiol in spiked food samples. RSC Adv. 2022, 12, 16581–16588. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; Cheng, Y.; Huang, Z.; Wei, X.; Feng, J.; Cheng, J.; Mugo, S.M.; Jaffrezic-Renault, N.; Guo, Z. Electrochemical aptasensor based on electrodeposited poly(3,4-ethylenedioxythiophene)-graphene oxide coupled with Au@Pt nanocrystals for the detection of 17β-estradiol. Microchim. Acta 2022, 189, 178. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Liu, Z.; Huang, S.; Chen, J.; Yan, Y.; Li, N.; Zhang, M.; Jin, M.; Shui, L. A sensitive electrochemical sensor based on wrinkled mesoporous carbon nanomaterials for rapid and reliable assay of 17β-estradiol. Electrochimica Acta 2022, 408, 139960. [Google Scholar] [CrossRef]
- Girault, I.; Bièche, I.; Lidereau, R. Role of estrogen receptor α transcriptional coregulators in tamoxifen resistance in breast cancer. Maturitas 2006, 54, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Busayapongchai, P.; Siri, S. Sensitive detection of estradiol based on ligand binding domain of estrogen receptor and gold nanoparticles. Anal. Biochem. 2017, 518, 60–68. [Google Scholar] [CrossRef]
- Wörner, H.J.; Arrell, C.A.; Banerji, N.; Cannizzo, A.; Chergui, M.; Das, A.K.; Hamm, P.; Keller, U.; Kraus, P.; Liberatore, E.; et al. Charge migration and charge transfer in molecular systems. Struct. Dyn. 2017, 4, 061508. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Lin, H.; Shao, M. Determining Locations of Conduction Bands and Valence Bands of Semiconductor Nanoparticles Based on Their Band Gaps. ACS Omega 2020, 5, 10297–10300. [Google Scholar] [CrossRef]
- Zanatta, A.R. Revisiting the optical bandgap of semiconductors and the proposal of a unified methodology to its determination. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- R, C.H.; Schiffman, J.D.; Balakrishna, R.G. Quantum dots as fluorescent probes: Synthesis, surface chemistry, energy transfer mechanisms, and applications. Sensors Actuators B: Chem. 2018, 258, 1191–1214. [Google Scholar] [CrossRef]
- Amoli, B.M.; Gumfekar, S.; Hu, A.; Zhou, Y.N.; Zhao, B. Thiocarboxylate functionalization of silver nanoparticles: Effect of chain length on the electrical conductivity of nanoparticles and their polymer composites. J. Mater. Chem. 2012, 22, 20048–20056. [Google Scholar] [CrossRef]
- Lesiak, A.; Banski, M.; Halicka, K.; Cabaj, J.; Żak, A.; Podhorodecki, A. pH-dependent fluorescence of thiol-coated CdSe/CdS quantum dots in an aqueous phase. Nanotechnology 2020, 32, 075705. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Venkataraghavan, R.; Kasturi, T.R. Contribution to the infrared spectra of organosulphur compounds. Can. J. Chem. 1964, 42, 36–42. [Google Scholar] [CrossRef]




| Biosensor Material | Mode of Detection | Limit of Detection (* = Dynamic Linear Range) | References |
|---|---|---|---|
| Poly(β-CD)/AF1-ADA/ON1/AF2-Au | Electrochemical (DPV) | 63.1 fM (*1 × 10−13–1 × 10−9 M) | [57] |
| PPY/PMAA-nBA/Aptamer | Electrochemical (DPV) | 0.48 pM (*1 × 10−4–1 × 10−12 M) | [58] |
| Molecular imprinted polymer/CB | Electrochemical | (*0.10–23.0 × 10−6 M) | [59] |
| SPCE/g-C3N4/APTES | Electrochemical (DPV) | 9.9 × 10−19 (*1 × 10−6 to 1 × 10−18) | [60] |
| Au@Pt/PEDOT-GO | Electrochemical (DPV) | 0.08 × 10−12 M (*0.1 × 10−12–1 × 10−9 M) | [61] |
| W/MC0.67/GCE | Electrochemical (DPV) | 8.3 × 10−9 (*0.05 × 10−6–10 × 10−6) | [62] |
| AuE/SnSe-3MPA QDs/ER-α (receptor) | Electrochemical (SWV) | 1.69 × 10−8 M (*1–8 × 10−8 M) | This work |
| Sample | Spiked Concentration (nM) | Detected Concentration (nM) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|
| Milk | 0 | not detected | - | - |
| 2 | 2.65 | 132.5 | 1.03 | |
| 4 | 4.45 | 111.25 | 0.05 | |
| 6 | 5.2 | 86.7 | 2.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jijana, A.N.; Feleni, U.; Ndangili, P.M.; Bilibana, M.; Ajayi, R.F.; Iwuoha, E.I. Quantum Dot-Sensitised Estrogen Receptor-α-Based Biosensor for 17β-Estradiol. Biosensors 2023, 13, 242. https://doi.org/10.3390/bios13020242
Jijana AN, Feleni U, Ndangili PM, Bilibana M, Ajayi RF, Iwuoha EI. Quantum Dot-Sensitised Estrogen Receptor-α-Based Biosensor for 17β-Estradiol. Biosensors. 2023; 13(2):242. https://doi.org/10.3390/bios13020242
Chicago/Turabian StyleJijana, Abongile N., Usisipho Feleni, Peter M. Ndangili, Mawethu Bilibana, Rachel F. Ajayi, and Emmanuel I. Iwuoha. 2023. "Quantum Dot-Sensitised Estrogen Receptor-α-Based Biosensor for 17β-Estradiol" Biosensors 13, no. 2: 242. https://doi.org/10.3390/bios13020242
APA StyleJijana, A. N., Feleni, U., Ndangili, P. M., Bilibana, M., Ajayi, R. F., & Iwuoha, E. I. (2023). Quantum Dot-Sensitised Estrogen Receptor-α-Based Biosensor for 17β-Estradiol. Biosensors, 13(2), 242. https://doi.org/10.3390/bios13020242