Visual/Photoelectrochemical Off-On Sensor Based on Cu/Mn Double-Doped CeO2 and Branched Sheet Embedded Cu2O/CuO Nanocubes
Abstract
:1. Introduction
2. Experimental Section
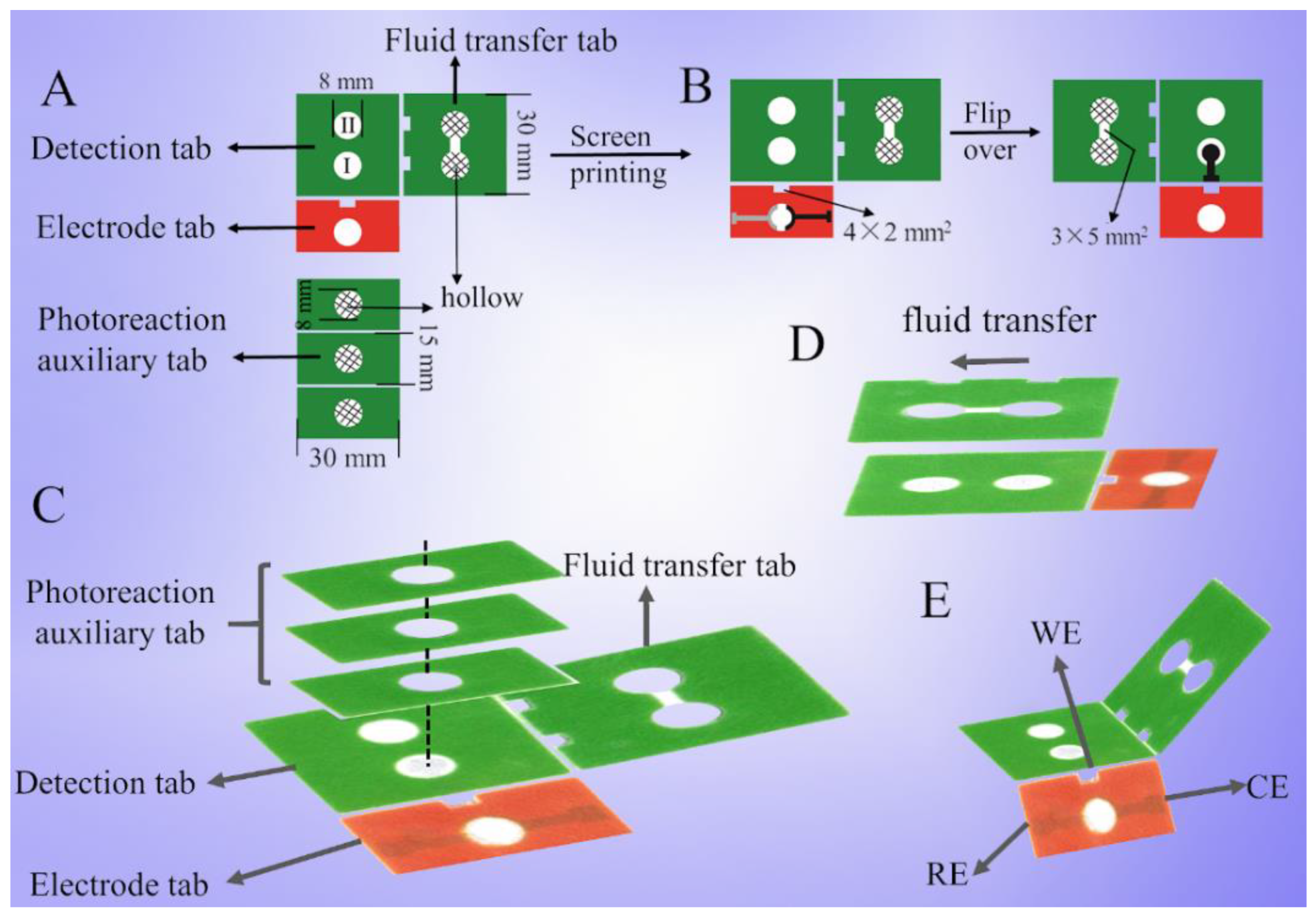
2.1. Design of Paper-Based Device
2.2. Preparation of CuMn@CeO2-DNA2
2.3. Fabrication of Dual-Signal Sensing Platform
2.4. Analysis Process of TB
3. Results and Discussion
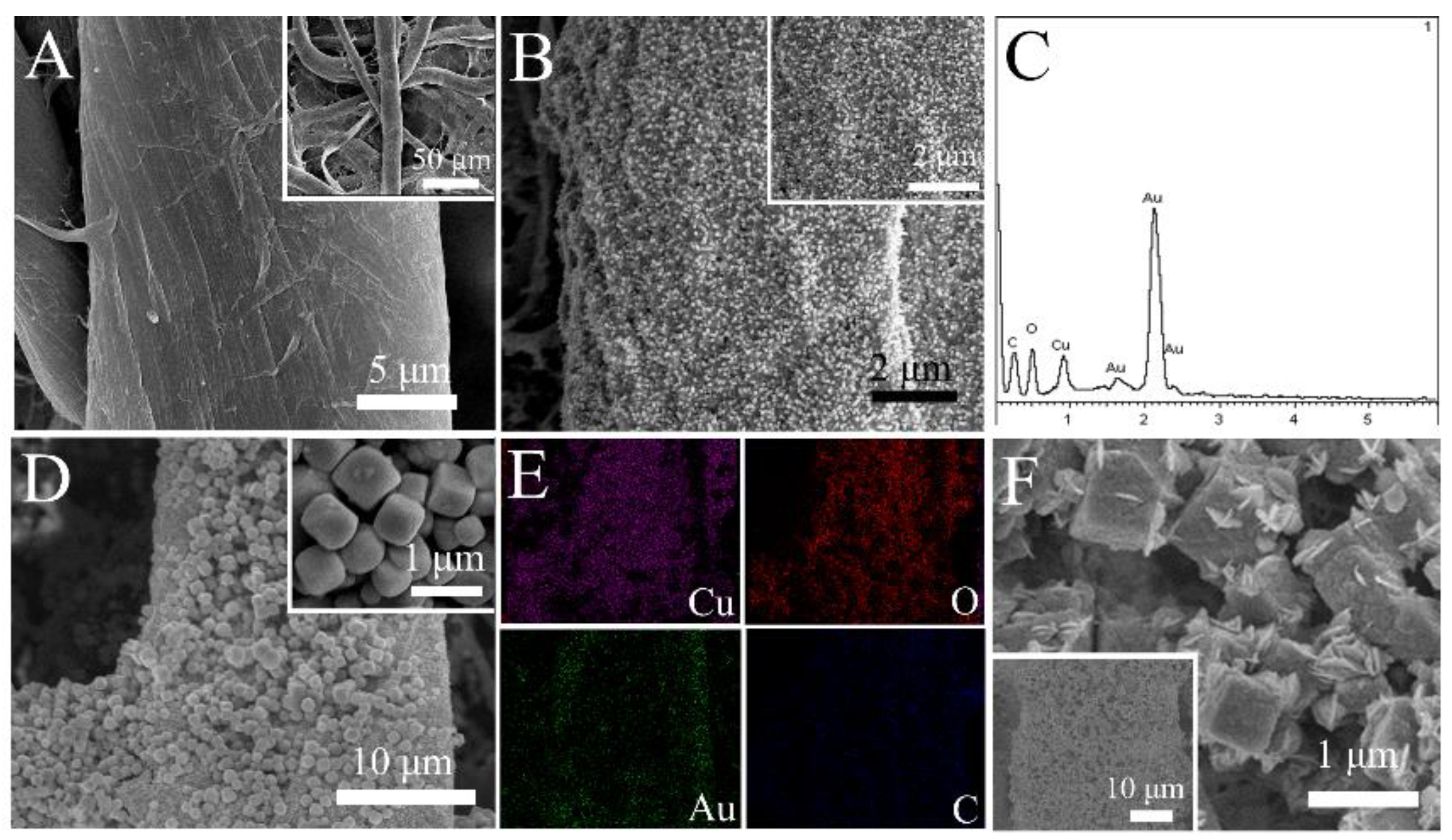
3.1. Structure Characterization
3.2. Ce-Based Materials Characterization
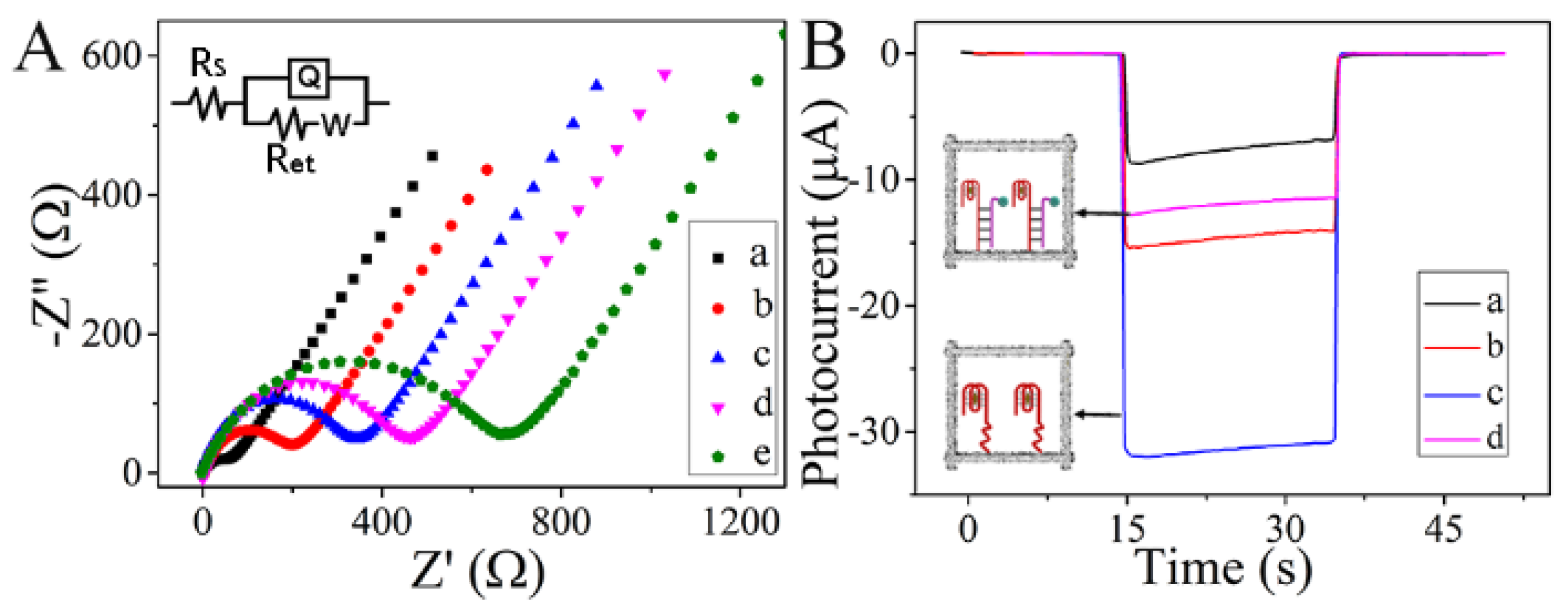
3.3. EIS and PEC Behavior
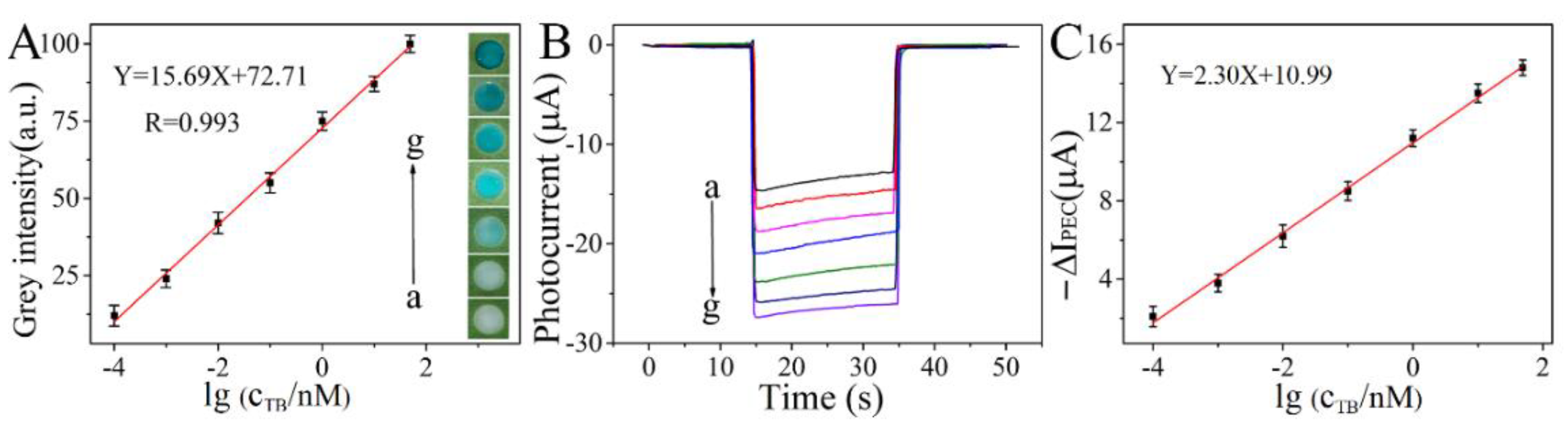
3.4. Analytical Performance
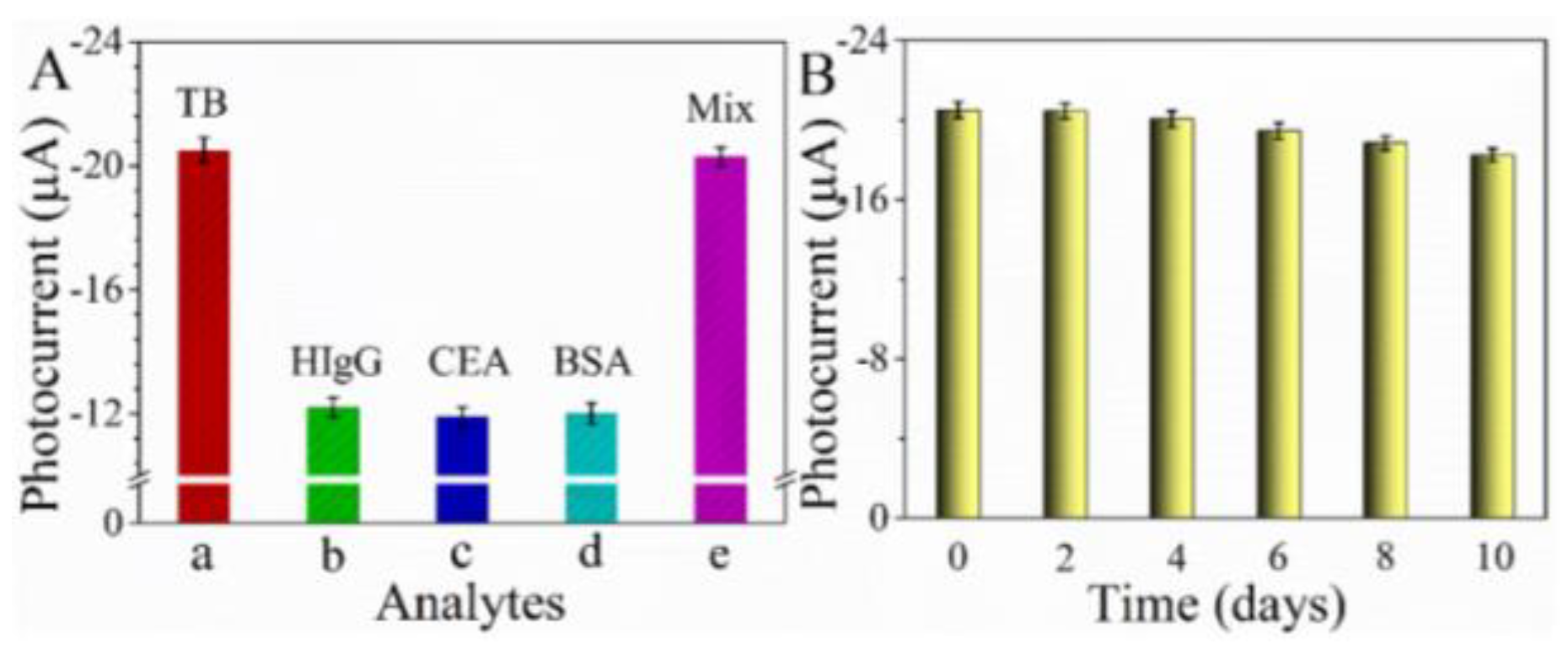
3.5. Specificity, Stability, and Reproducibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Zheng, Y.; Liang, W.; Yuan, Y.; Chai, Y.; Yuan, R. An ultrasensitive “on–off–on” photoelectrochemical aptasensor based on signal amplification of a fullerene/CdTe quantum dots sensitized structure and efficient quenching by manganese porphyrin. Chem. Commun. 2016, 52, 8138–8141. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Chi, J.; Chen, Z.; Cai, L.; Zhao, Y. Superwettable colloidal crystal micropatterns on butterfly wing surface for ultrasensitive detection. J. Colloid Interf. Sci. 2019, 546, 122–129. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, X.; Liu, H.; Dai, Y.; Han, R.; Gao, D.; Luo, C.; Wang, X.; Wei, Q. Novel chemiluminescence sensor for thrombin detection based on dual-aptamer biorecognition and mesoporous silica encapsulated with iron porphyrin. ACS Appl. Mater. Interfaces 2020, 12, 5569–5577. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, H.M.; Gu, Y.X.; Yu, L.; Wang, A.J.; Yuan, P.X.; Feng, J.J. Highly enhanced electrochemiluminescence luminophore generated by zeolitic imidazole framework-8-linked porphyrin and its application for thrombin detection. Anal. Chem. 2020, 92, 3206–3212. [Google Scholar] [CrossRef] [PubMed]
- Shao, K.; Wang, B.; Ye, S.; Zuo, Y.; Wu, L.; Li, Q.; Lu, Z.; Tan, X.; Han, H. Signal-amplified near-infrared ratiometric electrochemiluminescence aptasensor based on multiple quenching and enhancement effect of graphene/gold nanorods/G-quadruplex. Anal. Chem. 2016, 88, 8179–8187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, S.; Xu, L.; Ma, W.; Wu, X.; Kuang, H.; Wang, L.; Xu, C. Up-conversion fluorescence “off-on” switch based on heterogeneous core-satellite assembly for thrombin detection. Biosens. Bioelectron. 2015, 70, 372–375. [Google Scholar] [CrossRef]
- Yang, Z.H.; Zhuo, Y.; Yuan, R.; Chai, Y.Q. Amplified thrombin aptasensor based on alkaline phosphatase and hemin/G-quadruplex-catalyzed oxidation of 1-naphthol. ACS Appl. Mater. Interfaces 2015, 7, 10308–10315. [Google Scholar] [CrossRef] [PubMed]
- Heydari-Bafrooei, E.; Amini, M.; Ardakani, M.H. An electrochemical aptasensor based on TiO2/MWCNT and a novel synthesized Schiff base nanocomposite for the ultrasensitive detection of thrombin. Biosens. Bioelectron. 2016, 85, 828–836. [Google Scholar] [CrossRef]
- Wang, G.L.; Shu, J.X.; Dong, Y.M.; Wu, X.M.; Zhao, W.W.; Xu, J.J.; Chen, H.Y. Using G-quadruplex/hemin to “switch-on” the cathodic photocurrent of p-type PbS quantum dots: Toward a versatile platform for photoelectrochemical aptasensing. Anal. Chem. 2015, 87, 2892–2900. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhu, Y.C.; Ma, Z.Y.; Zhao, W.W.; Xu, J.J.; Chen, H.Y. An ultrasensitive energy-transfer based photoelectrochemical protein biosensor. Chem. Commun. 2016, 52, 3034–3037. [Google Scholar] [CrossRef]
- Wang, Z.J.; Li, Q.; Tan, L.L.; Liu, C.G.; Shang, L. Metal-organic frameworks-mediated assembly of gold nanoclusters for sensing applications. J. Anal. Test. 2022, 6, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fan, Q.; Wang, Y.; Wang, Y.; Ge, S.; Yan, M.; Yu, J. A paper-supported photoelectrochemical sensing platform based on surface plasmon resonance enhancement for real-time H2S determination. J. Anal. Test. 2019, 3, 89–98. [Google Scholar] [CrossRef]
- Shi, X.M.; Fan, G.C.; Shen, Q.; Zhu, J.J. Photoelectrochemical DNA biosensor based on dual-signal amplification strategy integrating inorganic-organic nanocomposites sensitization with λ-exonuclease-assisted target recycling. ACS Appl. Mater. Interfaces 2016, 8, 35091–35098. [Google Scholar] [CrossRef] [PubMed]
- Pravin, S.; Xiaoniu, P.; Jue, W.; Yanxiao, M.; Louis, M.; Nathan, H.; Arunava, G.; Shanlin, P. Rapid screening of photoanode materials using scanning photoelectrochemical microscopy technique and formation of Z-scheme solar water splitting system by coupling p- and n-type heterojunction photoelectrodes. ACS Appl. Energy Mater. 2018, 1, 2283–2294. [Google Scholar]
- Wang, B.; Mei, L.P.; Ma, Y.; Xu, Y.T.; Ren, S.W.; Cao, J.T.; Liu, Y.M.; Zhao, W.W. Photoelectrochemical-chemical-chemical redox cycling for advanced signal amplification: Proof-of-concept toward ultrasensitive photoelectrochemical bioanalysis. Anal. Chem. 2018, 90, 12347–12351. [Google Scholar] [CrossRef] [PubMed]
- Jessica, C.B.; Charles, R.M. Scalable methods for device patterning as an outstanding challenge in translating paper-based microfluidics from the academic benchtop to the point-of-care. J. Anal. Test. 2019, 3, 50–60. [Google Scholar]
- Tang, L.; Li, J. Plasmon-based colorimetric nanosensors for ultrasensitive molecular diagnostics. ACS Sens. 2017, 2, 857–875. [Google Scholar] [CrossRef]
- Kong, Q.; Cui, K.; Zhang, L.; Wang, Y.; Sun, J.; Ge, S.; Zhang, Y.; Yu, J. “On−Off−On” Photoelectrochemical/Visual Lab-on-Paper Sensing via Signal Amplification of CdS Quantum Dots@Leaf-Shape ZnO and Quenching of Au-Modified Prism-Anchored Octahedral CeO2 Nanoparticles. Anal. Chem. 2018, 90, 11297–11304. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, L.; Yang, X.; Elzatahry, A.; Alghamdi, A.; Deng, Y. Rational design of a stable peroxidase mimic for colorimetric detection of H2O2 and glucose: A synergistic CeO2/Zeolite Y nanocomposite. J. Colloid Interf. Sci. 2019, 535, 425–435. [Google Scholar] [CrossRef]
- Liu, W.; Liu, X.; Feng, L.; Guo, J.; Xie, A.; Wang, S.; Zhang, J.; Yang, Y. The synthesis of CeO2 nanospheres with different hollowness and size induced by copper doping. Nanoscale 2014, 6, 10693–10700. [Google Scholar] [CrossRef]
- Yang, Z.; Kang, T.; Ji, Y.; Li, J.; Zhu, Y.; Liu, H.; Jiang, X.; Zhong, Z.; Su, F. Architectural Cu2O@ CuO mesocrystals as superior catalyst for trichlorosilane synthesis. J. Colloid Interf. Sci. 2021, 589, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Fu, G.; Lv, W.; Wei, J.; Chen, W.; Yi, H.; Gu, M.; Bai, X.; Zhu, L.; Tan, C.; et al. A single-step sydrothermal route to 3D hierarchical Cu2O/CuO/rGO nanosheets as high-performance anode of lithium-ion batteries. Small 2018, 14, 1702667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jing, D.; Guo, L.; Yao, X. In situ photochemical synthesis of Zn-doped Cu2O hollow microcubes for high efficient photocatalytic H2 production. ACS Sustain. Chem. Eng. 2014, 2, 1446–1452. [Google Scholar] [CrossRef]
- Jiang, D.; Xue, J.; Wu, L.; Zhou, W.; Zhang, Y.; Li, X. Photocatalytic performance enhancement of CuO/Cu2O heterostructures for photodegradation of organic dyes: Effects of CuO morphology. Appl. Catal. B Environ. 2017, 211, 199–204. [Google Scholar] [CrossRef]
- Huang, Q.; Kang, F.; Liu, H.; Li, Q.; Xiao, X. Highly aligned Cu2O/CuO/TiO2 core/shell nanowire arrays as photocathodes for water photoelectrolysis. J. Mater. Chem. A 2013, 1, 2418–2425. [Google Scholar] [CrossRef]
- Zhao, W.W.; Xu, J.J.; Chen, H.Y. Photoelectrochemical bioanalysis: The state of the art. Chem. Soc. Rev. 2015, 44, 729–741. [Google Scholar] [CrossRef]
- Shu, J.; Tang, D.P. Recent advances in photoelectrochemical sensing: From engineered photoactive materials to sensing devices and detection modes. Anal. Chem. 2020, 92, 363–377. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Cui, K.; Ge, S.; Cheng, X.; Yan, M.; Yu, J.; Liu, H. Flexible electronics based on micro/nanostructured paper. Adv. Mater. 2018, 30, 1801588. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Lv, C.C.; Guo, Y.L.; Ma, X.H.; Liu, W.; Jin, Y.; Yao, S.Y. Recent advances and applications in paper-based devices for point-of-care testing. J. Anal. Test. 2022, 6, 247–273. [Google Scholar] [CrossRef]
- Zhu, L.; Lv, X.; Yu, H.; Tan, X.; Rong, Y.; Feng, W.; Zhang, L.; Yu, J.; Zhang, Y. Paper-Based Bipolar Electrode Electrochemiluminescence Platform Combined with Pencil-Drawing Trace for the Detection of M.SssI Methyltransferase. Anal. Chem. 2022, 94, 8327–8334. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.L.; Cui, S.H.; Wen, C.Y.; Li, P.; Yu, J.F.; Zeng, J.B. Distance-based detection of Ag+ with gold nanoparticles coated microfluidic paper. J. Anal. Test. 2021, 5, 11–18. [Google Scholar] [CrossRef]
- Shyamal, S.; Maity, A.; Satpati, A.K.; Bhattacharya, C. Amplification of PEC hydrogen production through synergistic modification of Cu2O using cadmium as buffer layer and dopant. Appl. Catal. B Environ. 2019, 246, 111–119. [Google Scholar] [CrossRef]
- Cheng, S.; DelaCruz, S.; Chen, C.; Tang, Z.; Shi, T.; Carraro, C.; Maboudian, R. Hierarchical Co3O4/CuO nanorod array supported on carbon cloth for highly sensitive non-enzymatic glucose biosensing. Sens. Actuators B 2019, 298, 126860. [Google Scholar] [CrossRef]
- Li, H.; Su, Z.; Hu, S.; Yan, Y. Free-standing and flexible Cu/Cu2O/CuO heterojunction net: A novel material as cost-effective and easily recycled visible-light photocatalyst. Appl. Catal. B: Environ. 2017, 207, 134–142. [Google Scholar] [CrossRef]
- Yao, W.; Li, F.L.; Li, H.X.; Lang, J.P. Fabrication of hollow Cu2O@CuO-supported Au-Pd alloy nanoparticles with high catalytic activity through the galvanic replacement reaction. J. Mater. Chem. A 2015, 3, 4578–4585. [Google Scholar] [CrossRef]
- Xue, S.; Li, Q.; Wang, L.; You, W.; Zhang, J.; Che, R. Copper- and cobalt-codoped CeO2 nanospheres with abundant oxygen vacancies as highly efficient electrocatalysts for dual-mode electrochemical sensing of MicroRNA. Anal. Chem. 2019, 91, 2659–2666. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zhou, S.; Zhu, L.; Lv, X.; Zhang, J.; Zhang, L.; Zhu, P.; Yu, J. DNAzyme-triggered visual and ratiometric electrochemiluminescence dual-readout assay for Pb(II) based on an assembled paper device. Anal. Chem. 2020, 92, 3874–3881. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xin, X.; Pang, X.; Pietraszkiewicz, M.; Hozyst, R.; Sun, X.; Wei, Q. Application of europium multiwalled carbon nanotubes as novel luminophores in an electrochemiluminescent aptasensor for thrombin using multiple amplification strategies. ACS Appl. Mater. Interfaces 2015, 7, 12663–12670. [Google Scholar] [CrossRef]
- Qiu, F.; Gan, X.; Jiang, B.; Yuan, R.; Xiang, Y. Electrode immobilization-free and sensitive electrochemical sensing of thrombin via magnetic nanoparticle-decorated DNA polymers. Sens. Actuators B Chem. 2021, 331, 129395. [Google Scholar] [CrossRef]
- Yang, J.; Dou, B.; Yuan, R.; Xiang, Y. Aptamer/protein proximity binding-triggered molecular machine for amplified electrochemical sensing of thrombin. Anal. Chem. 2017, 89, 5138–5143. [Google Scholar] [CrossRef]
- Tian, Y.; Xin, C.; Liu, S.; Liu, Y.; Liu, S. Affinity binding-induced Hg2+ release and quantum dot doping for general, label-free, and homogenous fluorescence protein assay. ACS Sens. 2018, 3, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jian, Y.; Kong, Q.; Liu, H.; Lan, F.; Liang, L.; Ge, S.; Yu, J. Ultrasensitive electrochemical paper-based biosensor for microRNA via strand displacement reaction and metal-organic frameworks. Sens. Actuators B Chem. 2018, 257, 561–569. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Tang, Y.; Wu, P.; Xu, S.; Zhangg, X. A both-end blocked peroxidase-mimicking DNAzyme for low-background chemiluminescent sensing of miRNA. ACS Sens. 2017, 2, 810–816. [Google Scholar] [CrossRef]



| Method | Materials | Liner Range (nM) | Detection Limit (pM) | References |
|---|---|---|---|---|
| Electrochemiluminescence | Graphene oxide and carbon nanotubes | 0.001–5 | 0.23 | [38] |
| Electrochemical | Au NPs | 0.005–50 | 1.1 | [39] |
| Electrochemical | Au electrode and methylene blue | 0.005–1 | 1.7 | [40] |
| Fluorescence | Zinc selenide quantum dots | 0.1–20 | 25 | [41] |
| PEC | Au-ZnO Nanoflowers | 0.001–30 | 0.37 | [7] |
| Visual/PEC | Cu2O/CuO and CuMn@CeO2 | 0.0001–50 | 0.035 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Che, Y.; Rong, Y.; Wang, J.; Wang, Y.; Yu, J.; Zhang, Y. Visual/Photoelectrochemical Off-On Sensor Based on Cu/Mn Double-Doped CeO2 and Branched Sheet Embedded Cu2O/CuO Nanocubes. Biosensors 2023, 13, 227. https://doi.org/10.3390/bios13020227
Shi H, Che Y, Rong Y, Wang J, Wang Y, Yu J, Zhang Y. Visual/Photoelectrochemical Off-On Sensor Based on Cu/Mn Double-Doped CeO2 and Branched Sheet Embedded Cu2O/CuO Nanocubes. Biosensors. 2023; 13(2):227. https://doi.org/10.3390/bios13020227
Chicago/Turabian StyleShi, Huihui, Yanfei Che, Yumeng Rong, Jiajun Wang, Yanhu Wang, Jinghua Yu, and Yan Zhang. 2023. "Visual/Photoelectrochemical Off-On Sensor Based on Cu/Mn Double-Doped CeO2 and Branched Sheet Embedded Cu2O/CuO Nanocubes" Biosensors 13, no. 2: 227. https://doi.org/10.3390/bios13020227
APA StyleShi, H., Che, Y., Rong, Y., Wang, J., Wang, Y., Yu, J., & Zhang, Y. (2023). Visual/Photoelectrochemical Off-On Sensor Based on Cu/Mn Double-Doped CeO2 and Branched Sheet Embedded Cu2O/CuO Nanocubes. Biosensors, 13(2), 227. https://doi.org/10.3390/bios13020227







