Abstract
This review focuses on the development of microbial biofuel cells to demonstrate how similar principles apply to the development of bioelectronic devices. The low specificity of microorganism-based amperometric biosensors can be exploited in designing microbial biofuel cells, enabling them to consume a broader range of chemical fuels. Charge transfer efficiency is among the most challenging and critical issues while developing biofuel cells. Nanomaterials and particular redox mediators are exploited to facilitate charge transfer between biomaterials and biofuel cell electrodes. The application of conductive polymers (CPs) can improve the efficiency of biofuel cells while CPs are well-suitable for the immobilization of enzymes, and in some specific circumstances, CPs can facilitate charge transfer. Moreover, biocompatibility is an important issue during the development of implantable biofuel cells. Therefore, biocompatibility-related aspects of conducting polymers with microorganisms are discussed in this review. Ways to modify cell-wall/membrane and to improve charge transfer efficiency and suitability for biofuel cell design are outlined.
1. Introduction
Green energy production has recently attracted significant interest from the scientific community. One of the up-and-coming technologies is biofuel cells. Biofuel cells (BFCs) are bioelectrochemical systems or devices that generate electric power by exploiting naturally occurring catalytic or metabolic processes of enzymes, nano enzymes, or even whole cells. Biofuel that can be used can vary from simple high-energy substrates such as glucose, fructose, and saccharose to complex organic molecules. On another hand, BFCs can be applied not only to generate electric power from pure substrates, but also to help treat wastewater by simultaneously producing power and reducing organic waste. Typically, BFCs are classified by their driving force. Enzymatic biofuel cells (EBFCs) [1] use enzymes for energy conversion from substrate stored to electric power. EBFCs show great selectivity towards the substrate and can be implemented for self-powered sensors. However, before use, enzymes need to be purified and efficiently immobilized onto the electrode, thus adding several lengthy, complicated, and expensive steps. Nonenzymatic biofuel cells are a novel idea, where nanomaterials with catalytic properties which mimic natural enzymes are used to produce energy, even though this approach has several advantages compared to EBFCs: high stability, and extended lifetime. However, it suffers from low catalytic efficiency and poor selectivity. Microbial biofuel cells (MFCs) are driven by microorganisms. Such a system allows us to employ the whole metabolic process to be used for energy generation. Hence, multiple substrates or mixed substrates can be used as a fuel source. Additionally, constructed MFCs can renew themselves and prolong the BFCs’ useful life. One of the most novel articles about the use and challenges of using MFCs describes the recent “explosion” in the popularity of these devices [2]. Figure 1 depicts the increasing interest in MFC research.
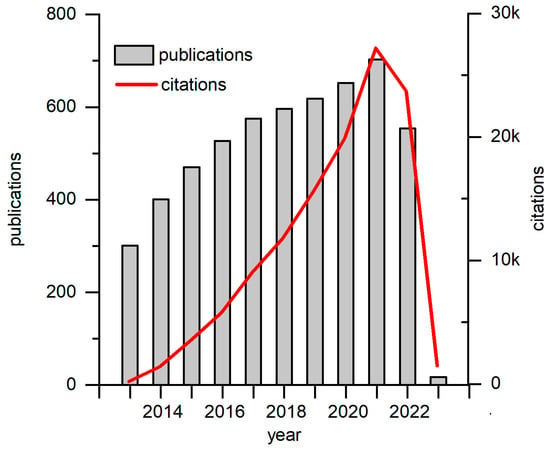
Figure 1.
Graphs of recent decade publications and citations on MFC research. Data has been generated on and taken from Web of Science webpage.
Furthermore, MFCs compared to other types are less expensive because they can be constructed using microorganisms that are already present in sludge, soil, and other natural habitats. While it has many advantages, the main drawback of MFCs is the inferior capability to transfer charge via cell walls and membranes, which limits widespread adoption. Many efforts have been made to improve MFCs efficiency, primary focus amplifies the charge transfer (CT) from living cells toward the anode. Some of the solutions are the introduction of membrane-bound electron-transferable compounds as intra-/extracellular electron transfer mediators and electrode modifications that improve CT or direct electron transfer from the living cell [1]. The most common microorganisms used in direct electron transfer-based fuel cells are Shewanella putrefaciens [3,4], Geobacter sulfurreducens [5,6], Rhodoferax ferrireducens [7], and Aeromonas hydrophila [8]. Metabolic processes and electron transfer mechanisms of these microorganisms are extensively studied, and it is found that physical interaction between the electrode and cytochromes located in the outer membrane or/and conductive layer of bacteria can deliver direct “wiring”. Greater BFCs efficiency is achieved when utilizing microbes capable of producing compounds with redox mediating capabilities rather than using bacteria incapable of producing such compounds [1,9].
Eucaryotic cells used for MFCs are not yet common. However, eukaryotic microorganisms such as Saccharomyces cerevisiae are highly investigated for use as MFCs biocatalysts [10,11,12]. S. cerevisiae has many desirable characteristics such as a broad substrate range, well-known metabolic pathways, simple and rapid mass cultivation, and affordable prices, which simplifies MFCs construction. Even so, S. cerevisiae naturally does not produce compounds with redox mediating capabilities, so the system requires the addition of redox mediators to perform efficiently [11,12,13,14,15]. The redox mediator, a molecule positioned within the cell membrane, is easily accessible to NADH and can join the anaerobic glycolysis NADH/NAD+ redox cycle [10]. The addition of a redox mediator does not hinder the normal metabolism of the cell and energy can be extracted when NADH is re-oxidized into NAD+ while a redox mediator gets reduced. Additionally, to enhance S. cerevisiae-based MFCs, different modifications of electrodes, cell walls, and membranes can be applied [1,16,17,18].
This paper overviews some recent developments in the design of microbial biofuel cells.
2. MFC Working Principles
MFCs have two compartments: (1) anode and (2) cathode (Figure 2). Compartments are separated by a membrane through which protons are transferred from the anode to the cathode compartment. Charges (protons and electrons) in the anode compartment are released from the metabolic activity of microorganisms during the microbial oxidation reaction of a substrate that is the fuel of MFCs. Electrons are transported via the anode to the cathode through an external load. At the cathode, an oxygen reduction process takes place in which electrons react with protons and oxygen to form water. MFCs typically use glucose as a fuel, which has a high energetic value and can generate 0.3–0.5 volts [19].
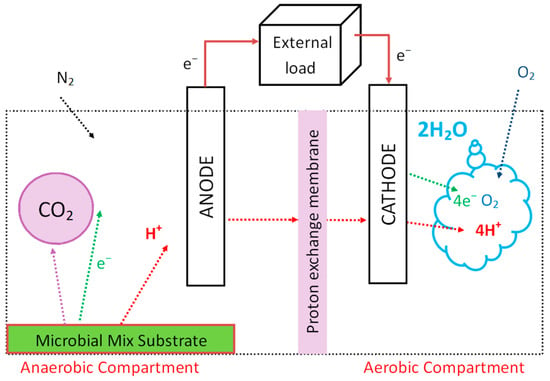
Figure 2.
Scheme of the microbial fuel cell. The electrodes are connected by a wire and electrons from the redox reactions in the anode compartment are passed through the wire to the cathode. As the microbial mix substrate is providing protons in the anaerobic chamber to transfer to the aerobic one, the electrical circuit is complete, and the charge is generated.
Currently, there are limited resources on MFCs applications since they are in their early development stages. The most important issue is low power density output coming from inefficient CT. The most popular anodes from carbon felt provide a large surface area, which can be modified with electrically conductive materials to improve CT. Moreover, they have good surface physical characteristics suitable for microbial attachment which can result in more efficient direct electron transfer from biocatalyst to anode [20,21]. The highest power density was achieved by using carbon felt anode decoration with gold nanoparticles [21], manganese oxide, or iron oxide nano-flowers [20], following modification by S. cerevisiae (Table 1). In both cases, S. cerevisiae combined with an electrode surface covered with nanostructures provided a durable direct electrochemical ‘wiring’. In many cases, S. cerevisiae was used as a model system. Nevertheless, the microorganism can be chosen depending on the available fuel, or the purpose of the whole system.
For example, an organic toxin para-aminophenol is an excellent fuel for the S. dehoogii-based MFCs. Such MFC could be used to reduce this toxicity in wastewater, and it can operate for up to eight days [22,23]. The MFCs based on S. loihica could be used as a nontoxic and environmentally friendly method for the remediation of chromium, and its pollution by the production of chromium nanoparticles since S. loihica is known to reduce metals [24]. Furthermore, MFCs based on Negativicutes and Gammaproteobacteria were used for purification and energy generation from sewage silt [25]. Many papers emphasize the modification of electrode surfaces with different materials for improved performance [14,18,20,21,26,27,28].
3. Mediators Used in MFCs
MFCs nowadays produce relatively low current and power, even though significant amounts of energy could be created during the metabolic redox process that takes place in live microorganisms while purifying waste [24,29,30,31,32]. The main limiting factor is hindered CT capacity by the cell walls and membrane [32]. It can be significantly improved using appropriate redox mediators [32]. Redox mediators can be hydrophilic or lipophilic, [33,34], and redox polymer-based matrices [32,35,36]. Some of the best-performing artificial mediators (including thionin, methylene blue, and neutral red) have been reviewed in [37].
Hydrophilic mediators usually enhance MFC performance by interacting with the cell trans-plasma redox system [38]. This interaction takes place between cytoplasmic mediators and redox enzymes. The most typical examples are membrane-bound cytochromes [39]. Cytochromes typically carry a co-enzyme, a functional group, a redox-active center, or a combination of them [40]. Generally, the hydrophilic mediator cannot pass through the membrane. Hence, there is a need for lipophilic mediators that play the vital role of CT through the cell membrane. These redox mediators can dissolve into the plasma membrane and can easily transport charge from cell internals to the outer leaflet of the cell membrane. Lipophilic mediators execute CT via functional groups. When lipophilic mediators in combination with hydrophilic are used, a significant improvement in CT is achieved [38]. As lipophilic mediators, several quinones can be used. Their drawbacks, however, include toxicity and damage to microbial cells which can vary significantly depending on their chemical properties and the conditions of cellular exposure [41,42,43,44,45]. For example, 9,10-phenanthrenequinone (PQ), which is a lipophilic redox compound, can be used in MFC, while PQ is immobilized on the anode, and hydrophilic ferricyanide can be dissolved in a working solution [11,12,46].
Metal or carbon-based nanomaterials can be synthesized in the required size and shape to facilitate CT from cells’ metabolic processes [14,47,48]. Nanoparticles form an electric channel from the cell to the electrodes. Modification with nanoparticles can take place either on the electrodes or by cell conjugation with nanoparticles [49,50]. In such systems, it is crucial that the nanocomposites do not kill the microorganisms [51]. Hence, only biocompatible nanomaterials should be used. These substances, such as gold nanoparticles [52] or carbon nanotubes [53], enhance the CT of the final MFC product. Mediators used in MFCs should have the following properties:
- Electrochemical activity.
- Biocompatibility with microorganisms used in the MFCs.
- Cell membrane permeability.
- Redox potential should be suitable for mediated electron transfer.
- Stable and soluble in both oxidized and reduced forms.
- Fast oxidation kinetics at the electrode surface [32,37].
Our investigations of MFCs mostly include yeast. Hence, Table 1 represents the most often utilized mediators for this kind of cell culture. Based on the data, Methylene Blue and Tetramethyl-phenylenediamine seem to transfer the most power output, 500 and 1000 mW m−2 maximum accordingly, although others are also considerable for utilization and the transferred power output varies from 0.408 to mentioned 1000 mW m−2.

Table 1.
Description of the performance of various yeast-based MFCs using respective mediators for the systems. Abbreviation YEPD stands for “yeast extract peptone dextrose”.
4. Modification of Microorganisms by Conductive Polymers
Electrochemical sedimentation of conductive polymers (CPs) is a relatively simple method for modifying electrode surfaces and has become a popular choice when designing bioelectronic devices [67] (Figure 3). Its popularity comes from the ease of controlling physical characteristics, where layer thickness, density, and ion permeability can be adjusted by changing the electrochemical conditions required for the polymerization reaction [15,68,69]. Biologically active molecules, such as proteins [70,71,72,73], DNA [74,75], and even live cells and bacteria, can be immobilized within CP layers. However, it is important to mention that other chemical factors: solvents, monomers, polymerization bulk composition, and pH, also have a significant impact on the features of produced CP layers.
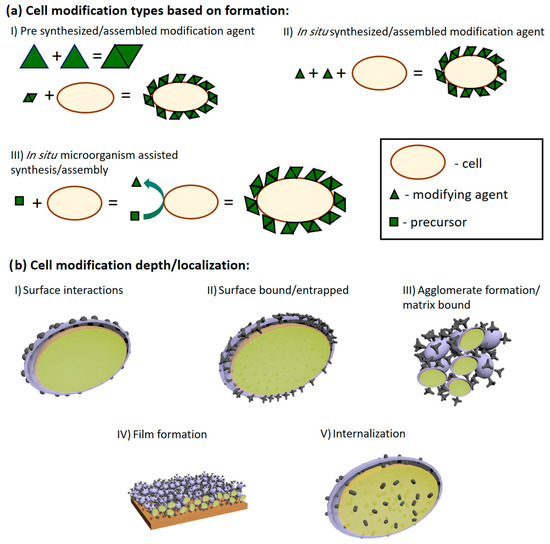
Figure 3.
(a) Schematic depiction of cell modification by agent formation principle (a). Cells can be modified using pre-synthesized compounds (I), assembled/synthesized in situ in the presence of living cells (II), and in situ when the cells assist/catalyze the synthesis assembly of the modifying agent (III). (b) Schematic representation of modifying agent localization in MBFC applications: (I) surface interactions as adsorption and electrostatic interactions; (II) modifying agent is either covalently bonded or forms interlacing and inseparable structures with cell walls or other similar structures; (III) when modifying agent forms aggregates from its matrix and cells; (IV) higher agglomerate organization onto surfaces; (V) internalization of modification agent. In picture (b), the purple surface represents the cell wall; the light green part of the picture represents the inside of the cells; the dark green parts of the picture show the modifying agents. Adapted from [7].
Conductivity is one of the most important characteristics when designing highly efficient bioelectronic devices. Some research groups established methods to evaluate the conductivity of electrochemically deposited polypyrrole (PPy) layers [76] and polyaniline (PANI)-based layers [75,77]. Our group proposed a mathematical model [78] to predict the conductivity of formed layers. Using this information, properties of multi-PPy layer electrodes can be predicted. Therefore, only the most efficient structures can be constructed. Even though CP layers may increase efficiency [79,80,81], formed layers on the electrode itself could hinder the diffusion of nutrients and destabilize metabolic processes. To relieve this problem, organic ‘spacers’ are introduced to CP layers to alter the porosity while interlinking various polymeric chains [82]. For this reason, several types of microorganisms were entangled in the structure of various polymers, including conductive polymers [11,12,17,46,75,83,84] (Figure 4).
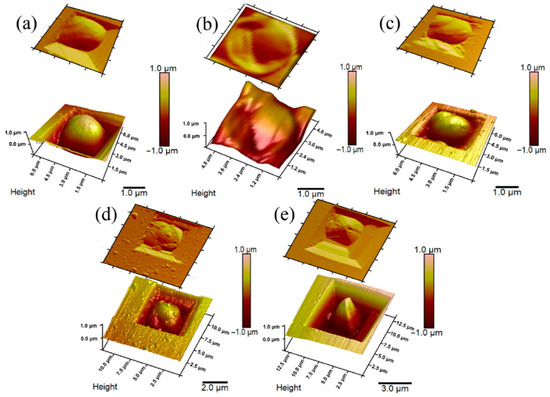
Figure 4.
AFM images: (a) non-modified yeast cell; (b) inactivated yeast cell; (c) PPy0.05-modified yeast cell; (d) PPy0.1-modified yeast cell; (e) PPy0.3-modified yeast cell. Adapted from [46].
However, the electron transfer from microorganisms to the electrode is infrequently observed even when various microorganisms [85,86] and mammalian cells (specifically lymphocytes [87] and erythrocytes [88]), are used for BFCs construction. To enhance the electron transfer, microorganisms could be modified with CP (Figure 5). Cells can be modified by exploiting their metabolic processes to initialize the polymerization of CPs [13,14,84,89]. Microorganisms (yeast, stem cells) modified with CPs typically preserve their viability, remain metabolically active, and form a stable system [90,91]. It was found that such systems’ functional lifetime is prolonged compared to polymer-modified enzymes [92,93,94].
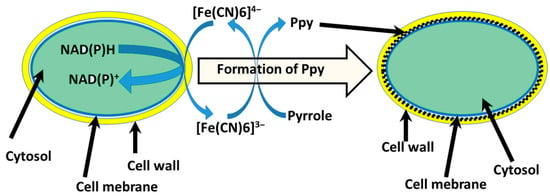
Figure 5.
Schematic representation of PPy synthesis in the cell wall of yeast [13]. Redox enzymes located in the plasma membrane oxidize [Fe(CN)6]4− into [Fe(CN)6]3− and induce a polymerization reaction of pyrrole [95].
Currently, the PPy application is gaining extra attention in the field of cell self-encapsulation [89]. Polymer matrices can be prepared in situ with cell culture or produced through metabolic/chemical processes within the cell structure. To our best knowledge, the first work on PPy bio-assisted polymer synthesis was performed in 2016 by our group [83]. The capacity of Streptomyces spp. to release redox enzymes (e.g., phenol-oxidase) to extracellular media enables the bacteria to initialize the creation of spherical PPy particles without the need for additional chemicals. For instance, it was identified that phenol-oxidases could be used to synthesize polypyrrole. After six days of multiplying, Streptomyces spp. bacteria favorable conditions are established for the arrangement of hollow PPy microspheres with a diameter of 10–20 µm [83]. Particle shapes appeared to have been influenced by organic compounds present in the growth medium [83].
Afterward, it was described that encapsulation of yeast S. cerevisiae cells by PPy could be achieved [13,95]. In this instance, yeast cell metabolic processes were employed to cycle redox mediator ([Fe(CN)6]4−/[Fe(CN)6]3−) that initializes the polymerization process in situ under controlled conditions (Figure 5). Cell shape, diameter, and roughness of the surface after the modification with PPy are related to the viability of cells [46]. Designing MFC based on CP-modified microorganisms’ cells that sustain viability after the modifications is the most desirable. Increasing the concentration of pyrrole during the modification stage causes cells to become smaller in diameter, surface roughness also increased, and small clusters of formed polymers can be observed. A minimal change in cells’ physiological state was observed at the lowest 0.05 M pyrrole concentration, suggesting yeast cells sustained their viability. Therefore, the system was used for the MFC design. Constructed MFCs generated power (47.12 mW/m2), compared to the non-modified system, was higher by 8.32 mW/m2.
In addition to yeast modification, methodologies to achieve similar results were developed. By introducing iron nitride, iron (III) nitrate nonahydrate, various bacteria: Streptococcus thermophiles, Ochrobacterium anthropic, Escherichia coli, or Shewanella oneidensis, MR-1 can form similar PPy layers [96]. In preparation, bacterial cells were saturated with iron (III) nitrate nonahydrate, which was placed in cell outer layers, and then polymerization was initiated upon the addition of pyrrole [96]. It was reported that bacterial cells retained viability, and the coating procedure did not affect cell proliferation. Moreover, in terms of electrical characteristics, the treatment of cells with conductive polymer (PPy) has resulted in a 14,1-fold improvement in power density comparison to unmodified S. oneidensis (147.9 µW cm−2) [96]. Analogous self-encapsulation was applied for microorganisms Aspergillus Niger and Rhizoctonia sp., and successfully used for MFC applications [32,84,97].
Scanning electrochemical microscopy (SECM) could be applied for MFC assessment [89]. During electrochemical probing over immobilized modified white-rot fungal cell culture, the current production (Imax = 0.86 nA) was nearly three times higher than control groups (Imax = 0.30 nA) [89]. Results were obtained from the surface approach curves. In addition, these studies revealed that the charge transfer efficiency, which is critical for the current production of MFC, is dependent on several variables: (1) the distance between the ultra-micro electrode of SECM and the cells and (2) the modification of microorganisms. The current recorded when the ultra-micro electrode distance from the sample surface was 20 µm (0.47 nA) was 1.5 times greater compared to the control sample [97]. Researchers noted that PPy production in fungal hyphae was facilitated by the laccase enzyme, which Trametes spp. fungus synthesizes and releases into the growing media. Utilizing crude enzyme extract with cell culture in a nutrient broth, polymerization of pyrrole was detected. At that time, bio-assisted polymer synthesis was very novel [98], and to the best of our knowledge, this was one of the first research that enabled the practical use of enzyme-assisted creation of conductive polymers [98,99,100,101,102]. Later, it led to polymer-based coating formation in cell culture [13,14,84,89]. Thus, it was demonstrated that cells modified with conductive polymer have advanced electron transferability, which enables to use of these microorganisms in microbial biofuel cells (MFCs) [32].
Furthermore, researchers reported that bacteria capable of metal reduction: Clostridium sporogenes, Cupriavidus metallidurans, and Escherichia coli, could use FeCl3 to initialize atom transfer radical polymerization of (poly(ethylene glycol methyl ether methacrylate); N-Hydroxyethyl acrylamide; hydroxyethyl methacrylate; 2-(methacryloyloxy) ethyl dimethyl-(3-sulfopropyl) ammonium hydroxide and 2-Acrylamido-2-methyl-1-propane sulfonic sodium) [103]. These cultures reduce Fe+3 to Fe+2 in a controlled way and initiate the polymerization of monomers. It is important to mention that monomers must be nontoxic to cells and engage in redox processes of Fe+2/Fe+3. After polymerization cells preserve high viability [103]. Along with PPy, various polymers are also employed to improve the performance of MFCs. Similarly, S. xiamenensis were coated with polydopamine (PDA) [103]. Selected bacteria can adhere to PDA during biofilm on MFC formation via oxidative polymerization in aerobic and slight alkali (pH 8) conditions. Researchers reported that PDA-modified bacteria S. xiamenensis cells were able to generate a much higher 452.8 mW/m2 power density, which was 6.1 times greater than the MFC system using nonmodified cells (74.7 mW/m2) [103]. Moreover, within three hours, conductive PDA additives were generated, which is quite quick. In addition, it appears that the modification of bacteria had insignificant effect on cell viability, which decreased only by 2–3% [103]. A prevalent bacterium for MFC design is Shewanella oneidensis MR-1, coated with PDA. In their study [104], Yu et al. reported that it is possible to use cell-assisted synthesis to form conductive PDA and use the same bacteria to exploit the biomineralization of FeS nanoparticles. Results showed that different interfaces wire up a cell at different levels. Thus, their electric/electrochemical properties are different. Polysulfide reductase mineralized FeS nanoparticle interface boosted the efficiency of MFC anodes up to 3.2 W/m2, and this was 14.5 times more than anodes modified by native S. oneidensis cells (0.2 W/m2), although the power output of PDA coated anodes was roughly 0.6 W/m2 [104].
Researchers developed an alternate strategy by internalizing the feeding process of pre-synthesized carbon dots (CD) and carbon nanoparticles into S. oneidensis and Shewanella xiamensis, respectively [105,106]. Both studies showed remarkable effects of CD that turned out to be highly biocompatible. Furthermore, CD could enhance metabolic activity by significantly increasing internal ATP (Adenosine 5′-triphosphate) levels. Overall, it was believed that a boosted metabolic rate might generate harmful reactive oxygen species. However, it was not the case. Moreover, CD generated photoactive particles that stimulate lactate consumption and result in a current generation when illuminated. With Shewanella oneidensis, MR-1’s maximum current density of 1.23 A/m2 was achieved, compared to the control of 0.19 A/m2 [105]. Meanwhile, the maximum power density of the MFC with CD was 0.491 W/m2 and was 6.46 folds higher compared to the control using the same nonmodified bacteria (0.076 W/m2). Shewanella xiamenensis attained a current density of 329.4 µA/cm2 under illuminance with lactate (as the only carbon source), which was 4.8-fold more than the control (68.1 µA/cm2) [106].
Osmium redox polymers can also be applied in developing MFCs [27,107,108,109,110]. The researchers attained the highest charge density of 15.079 mA/cm2 and an open circuit potential of 176 mV.
To compare the employed anodes and their materials for MFCs, as well as the power density of each version, the data in Table 2 are presented, with the order from lowest to highest power density.

Table 2.
Description of the MFC anode modification method and performance. Abbreviations are provided below the table [7].
Here, we explored and overviewed emerging technologies and methodologies for enhanced performance of MFCs by introducing some agents into cells themselves or covering them. In summary, these technologies fall under the headings of cell surface engineering, internalization, and artificial biofilm synthesis. Polymeric coating formation and polymer inclusion inside live cells represent the most promising approaches for cell manipulation. Even though there is clear proof of such a modification-based influence on charge transfer [32], there are still a few disadvantages. Some improvements are rather intricate, and their implementation in real-world MFCs might be challenging [27,107,108,109,110]. The primary disadvantages are microbe survival and proliferation since newly created cells in MFC must either inherit the change or experience it. These drawbacks compromise the longevity and stable electricity output of the MFCs. Consequently, the net electricity output of MFCs should be increased by a synergistic impact resulting from the combination of cell surface changes and other techniques described in this paper.
5. Conclusions and Future Aspects
Microbial fuel cells (MFCs) are a developing technology suitable to produce ‘green’ power and support bioremediation for the rising use of fossil fuels generating a worldwide energy crisis and a heightened awareness of environmental issues. However, the power generated by MFCs is still low for practical applications. Thus, MFC performance must be enhanced. The anode and current-generating bacteria are two crucial MFC structural components. The anode arranges the medium for microorganism attachment, while the living cells undertake bacteria-electrode charge transfer mechanisms. The low performance of the anode in MFC is the most significant challenge for its proper utilization these days. Effective anode modifications are presumed to increase the surface area and provide for the efficient attachment of biofilm, which subsequently intensifies the electrical power production by MFC. The microorganism-based biofuel cells show a relatively poor power density, mainly because charge transfer from the cells to the electrodes is restricted. These restrictions are caused by natural cell barriers (membrane and cell wall) that insulate the cell from an outer environment. This inconvenience can be very effectively exploited in the structure of microbial biofuel cells, as the immobilized cells can use various materials for fuel to generate electrical energy and be compatible with high cell viability and metabolic activity. To significantly increase the electron transfer rate and power density of MFCs, several chemical modifications of the cell wall or membrane are used. Carbon nanotubes, conductive polymers, metal nanoparticles, and other metal-based nanostructures have been used to increase the performance of MFCs by modifying the anode and different cell walls and plasma membranes.
Electrochemically covering the electrodes of BFCs with conductive polymers, such as PPy or PANI, or the mixtures of conductive polymers with chitosan or hydrogels might alleviate the biocompatibility difficulties of implantable MFCs. Furthermore, diverse properties of produced layers may be readily manipulated by selecting appropriate chemical and electrochemical conditions for effective electrode modification to minimize inflammatory responses while in touch with human tissues.
In summary, the future of BFCs and MFCs seems promising since the capabilities of such devices’ applications are immeasurable. Furthermore, although designs of BFCs and MFCs deliver poor electrical output, various studies have shown that there are no limits to their diversity. Each modification suggests a novel approach to the higher efficacy of these devices, and each modification leads one step closer to the application of such devices in daily life.
Author Contributions
Conceptualization, I.M.-V. and A.R.; software, K.K.; resources, I.M.-V.; writing—original draft preparation, K.K., A.Z., B.J., I.B., E.B., M.P., A.R., and I.M.-V.; writing—review and editing, K.K., A.Z., A.R., and I.M.-V.; visualization, K.K.; supervision, I.M.-V.; funding acquisition, I.M.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Council of Lithuania (LMTLT), agreement No S-MIP-22-87.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial Fuel Cells: An Overview of Cur-rent Technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Dwivedi, K.A.; Huang, S.J.; Wang, C.T.; Kumar, S. Fundamental Understanding of Microbial Fuel Cell Technology: Recent Development and Challenges. Chemosphere 2022, 288, 132446. [Google Scholar] [CrossRef]
- Rewatkar, P.; Goel, S. Shewanella Putrefaciens Powered Microfluidic Microbial Fuel Cell with Printed Circuit Board Electrodes and Soft-Lithographic Microchannel. Chemosphere 2022, 286, 131855. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, H.S.; Hyun, M.S.; Chang, I.S.; Kim, M.; Kim, B.H. A Mediator-Less Microbial Fuel Cell Using a Metal Reducing Bacterium, Shewanella putrefaciens. Enzym. Microb. Technol. 2002, 30, 145–152. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, G.; Kuang, B.; Jia, J.; Liu, C.; Cai, G.; Li, C. Novel Insights into the Anaerobic Digestion of Propionate via Syntrophobacter fumaroxidans and Geobacter sulfurreducens: Process and Mechanism. Water Res. 2021, 200, 117270. [Google Scholar] [CrossRef]
- Yi, H.; Nevin, K.P.; Kim, B.C.; Franks, A.E.; Klimes, A.; Tender, L.M.; Lovley, D.R. Selection of a Variant of Geobacter sulfurreducens with Enhanced Capacity for Current Production in Microbial Fuel Cells. Biosens. Bioelectron. 2009, 24, 3498–3503. [Google Scholar] [CrossRef]
- Andriukonis, E.; Celiesiute-Germaniene, R.; Ramanavicius, S.; Viter, R.; Ramanavicius, A. From Microorganism-based Aperometric Biosensors towards Microbial Fuel Cells. Sensors 2021, 21, 2442. [Google Scholar] [CrossRef]
- Li, S.W.; He, H.; Zeng, R.J.; Sheng, G.P. Chitin Degradation and Electricity Generation by Aeromonas Hydrophila in Microbial Fuel Cells. Chemosphere 2017, 168, 293–299. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, M.; Guo, J.; Sun, G. Bacterial Extracellular Electron Transfer in Bioelectrochemical Systems. Process. Biochem. 2012, 47, 1707–1714. [Google Scholar] [CrossRef]
- Raghavulu, S.V.; Goud, R.K.; Sarma, P.N.; Mohan, S.V. Saccharomyces Cerevisiae as Anodic Biocatalyst for Power Generation in Biofuel Cell: Influence of Redox Condition and Substrate Load. Bioresour. Technol. 2011, 102, 2751–2757. [Google Scholar] [CrossRef]
- Rozene, J.; Morkvenaite-Vilkonciene, I.; Bruzaite, I.; Zinovicius, A.; Ramanavicius, A. Baker’s Yeast-Based Microbial Fuel Cell Mediated by 2-Methyl-1,4-Naphthoquinone. Membranes 2021, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Rozene, J.; Morkvenaite-Vilkonciene, I.; Bruzaite, I.; Dzedzickis, A.; Ramanavicius, A. Yeast-Based Microbial Biofuel Cell Mediated by 9,10-Phenantrenequinone. Electrochim. Acta 2021, 373, 137918. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Andriukonis, E.; Stirke, A.; Mikoliunaite, L.; Balevicius, Z.; Ramanaviciene, A. Synthesis of Polypyrrole within the Cell Wall of Yeast by Redox-Cycling of [Fe(CN)6]3−/[Fe(CN)6]4−. Enzym. Microb. Technol. 2016, 83, 40–47. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef]
- Bruzaite, I.; Rozene, J.; Morkvenaite-Vilkonciene, I.; Ramanavicius, A. Towards Microorganism-Based Biofuel Cells: The Viability of Saccharomyces Cerevisiae Modified by Multiwalled Carbon Nanotubes. Nanomaterials 2020, 10, 954. [Google Scholar] [CrossRef]
- Gal, I.; Schlesinger, O.; Amir, L.; Alfonta, L. Yeast Surface Display of Dehydrogenases in Microbial Fuel-Cells. Bioelectrochemistry 2016, 112, 53–60. [Google Scholar] [CrossRef]
- Niu, J.; Lunn, D.J.; Pusuluri, A.; Yoo, J.I.; O’Malley, M.A.; Mitragotri, S.; Soh, H.T.; Hawker, C.J. Engi-neering Live Cell Surfaces with Functional Polymers via Cytocompatible Controlled Radical Polymerization. Nat. Chem. 2017, 9, 537–545. [Google Scholar] [CrossRef]
- de Oliveira, A.H.P.; Alcaraz-Espinoza, J.J.; da Costa, M.M.; Nascimento, M.L.F.; Swager, T.M.; de Oliveira, H.P. Improvement of Baker’s Yeast-Based Fuel Cell Power Output by Electrodes and Proton Exchange Membrane Modification. Mater. Sci. Eng. C 2019, 105, 110082. [Google Scholar] [CrossRef]
- Flimban, S.G.A.; Ismail, I.M.I.; Kim, T.; Oh, S.-E. Review Overview of Recent Advancements in the Microbial Fuel Cell from Fundamentals to Applications. Energies 2019, 12, 3390. [Google Scholar] [CrossRef]
- Duarte, K.D.Z.; Kwon, Y. Enhanced Extracellular Electron Transfer of Yeast-Based Microbial Fuel Cells via One Pot Substrate-Bound Growth Iron-Manganese Oxide Nanoflowers. J. Power Sources 2020, 474, 228496. [Google Scholar] [CrossRef]
- Duarte, K.D.Z.; Frattini, D.; Kwon, Y. High Performance Yeast-Based Microbial Fuel Cells by Surfactant-Mediated Gold Nanoparticles Grown atop a Carbon Felt Anode. Appl. Energy 2019, 256, 113912. [Google Scholar] [CrossRef]
- Mbokou, S.F.; Tonle, I.K.; Pontié, M. Development of a Novel Hybrid Biofuel Cell Type APAP/O2 Based on a Fungal Bioanode with a Scedosporium dehoogii Biofilm. J. Appl. Electrochem. 2017, 47, 273–280. [Google Scholar] [CrossRef]
- Pontié, M.; Jaspard, E.; Friant, C.; Kilani, J.; Fix-Tailler, A.; Innocent, C.; Chery, D.; Mbokou, S.F.; Som-rani, A.; Cagnon, B.; et al. A Sustainable Fungal Microbial Fuel Cell (FMFC) for the Bioremediation of Acetaminophen (APAP) and Its Main by-Product (PAP) and Energy Production from Biomass. BioCatal. Agric. Biotechnol. 2019, 22, 101376. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Liu, Q.; Du, P.; Liu, W.; He, Z. Biosynthesis of Palladium Nanoparticles Using: Shewanella loihica PV-4 for Excellent Catalytic Reduction of Chromium(VI). Environ. Sci. Nano 2018, 5, 730–739. [Google Scholar] [CrossRef]
- Wu, X.; Xiong, X.; Owens, G.; Brunetti, G.; Zhou, J.; Yong, X.; Xie, X.; Zhang, L.; Wei, P.; Jia, H. Anode Modification by Biogenic Gold Nanoparticles for the Improved Performance of Microbial Fuel Cells and Microbial Community Shift. Bioresour. Technol. 2018, 270, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Christwardana, M.; Kwon, Y. Yeast and Carbon Nanotube Based Biocatalyst Developed by Synergetic Effects of Covalent Bonding and Hydrophobic Interaction for Performance Enhancement of Membraneless Microbial Fuel Cell. Bioresour. Technol. 2017, 225, 175–182. [Google Scholar] [CrossRef]
- Coman, V.; Gustavsson, T.; Finkelsteinas, A.; Von Wachenfeldt, C.; Hägerhäll, C.; Gorton, L. Electrical Wiring of Live, Metabolically Enhanced Bacillus Subtilis Cells with Flexible Osmium-Redox Polymers. J. Am. Chem. Soc. 2009, 131, 16171–16176. [Google Scholar] [CrossRef]
- Christwardana, M.; Frattini, D.; Duarte, K.D.Z.; Accardo, G.; Kwon, Y. Carbon Felt Molecular Modification and Biofilm Augmentation via Quorum Sensing Approach in Yeast-Based Microbial Fuel Cells. Appl. Energy 2019, 238, 239–248. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Ramanaviciene, A. Hemoproteins in Design of Biofuel Cells. Fuel Cells 2009, 9, 25–36. [Google Scholar] [CrossRef]
- Sekrecka-Belniak, A.; Toczyłowska-Maminska, R. Fungi-Based Microbial Fuel Cells. Energies 2018, 11, 2827. [Google Scholar] [CrossRef]
- Davis, F.; Higson, S.P.J. Biofuel Cells-Recent Advances and Applications. Biosens. Bioelectron. 2007, 22, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Kisieliute, A.; Popov, A.; Apetrei, R.M.; Cârâc, G.; Morkvenaite-Vilkonciene, I.; Ramanaviciene, A.; Ramanavicius, A. Towards Microbial Biofuel Cells: Improvement of Charge Transfer by Self-Modification of Microoganisms with Conducting Polymer—Polypyrrole. Chem. Eng. J. 2019, 356, 1014–1021. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Nastajute, G.; Snitka, V.; Kausaite, A.; German, N.; Barauskas-Memenas, D.; Ra-manavicius, A. Spectrophotometric Evaluation of Gold Nanoparticles as Red-Ox Mediator for Glucose Oxidase. Sens. Actuators B-Chem. 2009, 137, 483–489. [Google Scholar] [CrossRef]
- Li, M.; Zhou, M.; Tian, X.; Tan, C.; McDaniel, C.T.; Hassett, D.J.; Gu, T. Microbial Fuel Cell (MFC) Power Performance Improvement through Enhanced Microbial Electrogenicity. Biotechnol. Adv. 2018, 36, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Higham, T.E. Author’s Personal Copy Automatica Author’s Personal Copy. Encycl. Toxicol. 2014, 50, 952–961. [Google Scholar]
- Rudra, R. Conducting Polymer-Based Microbial Fuel Cells. In Enzymatic Fuel Cells: Materials and Applications; Chapter 8; Materials Research Forum LLC: Millersville, PA, USA, 2019; Volume 44, pp. 173–186. ISBN 9781644900079. [Google Scholar]
- Babanova, S.; Hubenova, Y.; Mitov, M. Influence of Artificial Mediators on Yeast-Based Fuel Cell Performance. J. Biosci. Bioeng. 2011, 112, 379–387. [Google Scholar] [CrossRef]
- Rawson, F.J.; Downard, A.J.; Baronian, K.H. Electrochemical Detection of Intracellular and Cell Membrane Redox Systems in Saccharomyces Cerevisiae. Sci. Rep. 2014, 4, 5216. [Google Scholar] [CrossRef]
- Holmes, D.E.; Ueki, T.; Tang, H.; Zhou, J.; Smith, J.A.; Chaput, G.; Lovley, D.R. A Membrane-Bound Cytochrome Enables from Extracellular Electron Transfer. Am. Soc. Microbiol. 2019, 10, 1–12. [Google Scholar]
- Okamoto, A.; Kalathil, S.; Deng, X.; Hashimoto, K.; Nakamura, R.; Nealson, K.H. Cell-Secreted Flavins Bound to Membrane Cytochromes Dictate Electron Transfer Reactions to Surfaces with Diverse Charge and PH. Sci. Rep. 2014, 4, 5628. [Google Scholar] [CrossRef]
- Ishioka, T.; Uchida, T.; Teramae, N. Analysis of the Redox Reaction of 9,10-Phenanthrenequinone on a Gold Electrode Surface by Cyclic Voltammetry and Time-Resolved Fourier Transform Surface-Enhanced Raman Scattering Spectroscopy. Anal. Chim. Acta 2001, 449, 253–260. [Google Scholar] [CrossRef]
- Le Comte, A.; Chhin, D.; Gagnon, A.; Retoux, R.; Brousse, T.; Bélanger, D. Spontaneous Grafting of 9,10-Phenanthrenequinone on Porous Carbon as an Active Electrode Material in an Electrochemical Capacitor in an Alkaline Electrolyte. J. Mater. Chem. A Mater. 2015, 3, 6146–6156. [Google Scholar] [CrossRef]
- Genys, P.; Aksun, E.; Tereshchenko, A.; Valiūnienė, A.; Ramanaviciene, A.; Ramanavicius, A. Electro-chemical Deposition and Investigation of Poly-9,10-Phenanthrenequinone Layer. Nanomaterials 2019, 9, 702. [Google Scholar] [CrossRef]
- Hossain, M.S.; Tryk, D.; Yeager, E. The Electrochemistry of Graphite and Modified Graphite Surfaces: The Reduction of O2. Electrochim. Acta 1989, 34, 1733–1737. [Google Scholar] [CrossRef]
- Brousse, T.; Cougnon, C.; Bélanger, D. Grafting of Quinones on Carbons as Active Electrode Materials in Electrochemical Capacitors. J. Braz. Chem. Soc. 2018, 29, 989–997. [Google Scholar] [CrossRef]
- Zinovicius, A.; Rozene, J.; Merkelis, T.; Bruzaitė, I.; Ramanavicius, A.; Morkvenaite-Vilkonciene, I. Evaluation of a Yeast–Polypyrrole Biocomposite Used in Microbial Fuel Cells. Sensors 2022, 22, 327. [Google Scholar] [CrossRef]
- Jiang, X.; Hu, J.; Lieber, A.M.; Jackan, C.S.; Biffinger, J.C.; Fitzgerald, L.A.; Ringeisen, B.R.; Lieber, C.M. Nanoparticle Facilitated Extracellular Electron Transfer in Microbial Fuel Cells. Nano Lett. 2014, 14, 6737–6742. [Google Scholar] [CrossRef]
- Sharma, T.; Mohana Reddy, A.L.; Chandra, T.S.; Ramaprabhu, S. Development of Carbon Nanotubes and Nanofluids Based Microbial Fuel Cell. Int. J. Hydrogen Energy 2008, 33, 6749–6754. [Google Scholar] [CrossRef]
- Zhao, C.E.; Chen, J.; Ding, Y.; Wang, V.B.; Bao, B.; Kjelleberg, S.; Cao, B.; Loo, S.C.J.; Wang, L.; Huang, W.; et al. Chemically Functionalized Conjugated Oligoelectrolyte Nanoparticles for Enhancement of Current Generation in Microbial Fuel Cells. ACS Appl. Mater. Interfaces 2015, 7, 14501–14505. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, X.; Yang, Y.; Li, S.; Li, L.; Wang, S. Binding-Directed Energy Transfer of Conjugated Polymer Materials for Dual-Color Imaging of Cell Membrane. Chem. Mater. 2016, 28, 4661–4669. [Google Scholar] [CrossRef]
- Mammari, N.; Lamouroux, E.; Boudier, A.; Duval, R.E. Current Knowledge on the Oxidative-Stress-Mediated Antimicrobial Properties of Metal-Based Nanoparticles. Microorganisms 2022, 10, 437. [Google Scholar] [CrossRef]
- Guo, W.; Pi, Y.; Song, H.; Tang, W.; Sun, J. Layer-by-Layer Assembled Gold Nanoparticles Modified Anode and Its Application in Microbial Fuel Cells. Colloids Surf. A Phys. Eng. Asp. 2012, 415, 105–111. [Google Scholar] [CrossRef]
- Hindatu, Y.; Annuar, M.S.M.; Gumel, A.M. Mini-Review: Anode Modification for Improved Performance of Microbial Fuel Cell. Renew. Sustain. Energy Rev. 2017, 73, 236–248. [Google Scholar] [CrossRef]
- Bennetto, H.P.; Stirling, J.L.; Vega, C.A. Anodic Reactions in Microbial Fuel Cells. Biotechnol. Bioeng. 1983, 25, 559–568. [Google Scholar] [CrossRef]
- Gunawardena, A.; Fernando, S.; To, F. Performance of a Yeast-Mediated Biological Fuel Cell. Int. J. Mol. Sci. 2008, 9, 1893–1907. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Najafpour, G.D.; Ghoreyshi, A.A.; Talebnia, F.; Premier, G.C.; Bakeri, G.; Kim, J.R.; Oh, S.E. Thionine Increases Electricity Generation from Microbial Fuel Cell Using Saccharomyces Cerevisiae and Exoelectrogenic Mixed Culture. J. Microbiol. 2012, 50, 575–580. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Najafpour, G.D.; Ghoreyshi, A.A.; Shakeri, M.; Zare, H. Methylene Blue as Electron Promoters in Microbial Fuel Cell. Int. J. Hydrogen Energy 2011, 36, 13335–13341. [Google Scholar] [CrossRef]
- Walker, A.L.; Walker, C.W. Biological Fuel Cell and an Application as a Reserve Power Source. J. Power Sources 2006, 160, 123–129. [Google Scholar] [CrossRef]
- Permana, D.; Rosdianti, D.; Ishmayana, S.; Rachman, S.D.; Putra, H.E.; Rahayuningwulan, D.; Hari-yadi, H.R. Preliminary Investigation of Electricity Production Using Dual Chamber Microbial Fuel Cell (DCMFC) with Saccharomyces Cerevisiae as Biocatalyst and Methylene Blue as an Electron Mediator. Procedia Chem. 2015, 17, 36–43. [Google Scholar] [CrossRef]
- Wilkinson, S.; Klar, J.; Applegarth, S. Optimizing Biofuel Cell Performance Using a Targeted Mixed Mediator Combination. Electroanalysis 2006, 18, 2001–2007. [Google Scholar] [CrossRef]
- Ganguli, R.; Dunn, B.S. Kinetics of Anode Reactions for a Yeast-Catalysed Microbial Fuel Cell. Fuel Cells 2009, 9, 44–52. [Google Scholar] [CrossRef]
- Christwardana, M.; Frattini, D.; Accardo, G.; Yoon, S.P.; Kwon, Y. Effects of Methylene Blue and Methyl Red Mediators on Performance of Yeast Based Microbial Fuel Cells Adopting Polyethylenimine Coated Carbon Felt as Anode. J. Power Sources 2018, 396, 1–11. [Google Scholar] [CrossRef]
- Hubenova, Y.; Mitov, M. Potential Application of Candida Melibiosica in Biofuel Cells. Bioelectrochemistry 2010, 78, 57–61. [Google Scholar] [CrossRef]
- Moradian, J.M.; Xu, Z.A.; Shi, Y.T.; Fang, Z.; Yong, Y.C. Efficient Biohydrogen and Bioelectricity Production from Xylose by Microbial Fuel Cell with Newly Isolated Yeast of Cystobasidium slooffiae. Int. J. Energy Res. 2020, 44, 325–333. [Google Scholar] [CrossRef]
- Pal, M.; Sharma, R.K. Exoelectrogenic Response of Pichia fermentans Influenced by Mediator and Reactor Design. J. Biosci. Bioeng. 2019, 127, 714–720. [Google Scholar] [CrossRef]
- Haslett, N.D.; Rawson, F.J.; Barriëre, F.; Kunze, G.; Pasco, N.; Gooneratne, R.; Baronian, K.H.R. Characterisation of Yeast Microbial Fuel Cell with the Yeast Arxula adeninivorans as the Biocatalyst. Biosens. Bioelectron. 2011, 26, 3742–3747. [Google Scholar] [CrossRef]
- Emir, G.; Dilgin, Y.; Ramanaviciene, A.; Ramanavicius, A. Amperometric Nonenzymatic Glucose Biosensor Based on Graphite Rod Electrode Modified by Ni-Nanoparticle/Polypyrrole Composite. Microchem. J. 2021, 161, 105751. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Oztekin, Y.; Ramanaviciene, A. Electrochemical Formation of Polypyrrole-Based Layer for Immunosensor Design. Sens. Actuators B Chem. 2014, 197, 237–243. [Google Scholar] [CrossRef]
- Long, Y.Z.; Li, M.M.; Gu, C.; Wan, M.; Duvail, J.L.; Liu, Z.; Fan, Z. Recent Advances in Synthesis, Physical Properties and Applications of Conducting Polymer Nanotubes and Nanofibers. Prog. Polym. Sci. 2011, 36, 1415–1442. [Google Scholar] [CrossRef]
- Rahman, M.A.; Kumar, P.; Park, D.S.; Shim, Y.B. Electrochemical Sensors Based on Organic Conjugated Polymers. Sensors 2008, 8, 118–141. [Google Scholar] [CrossRef]
- Bredas, J.L.; Street, G.B. Polarons, Bipolarons, and Solitons in Conducting Polymers. Acc. Chem. Res. 1985, 18, 309–315. [Google Scholar] [CrossRef]
- Le, T.-H.H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Ratautaite, V.; Topkaya, S.N.; Mikoliunaite, L.; Ozsoz, M.; Oztekin, Y.; Ramanaviciene, A.; Ramanavicius, A. Molecularly Imprinted Polypyrrole for DNA Determination. Electroanalysis 2013, 25, 1169–1177. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Ramanavicius, A. Pulsed Amperometric Detection of DNA with an ssDNA/Polypyrrole-Modified Electrode. Anal. Bioanal. Chem. 2004, 379, 287–293. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Charge Transfer and Biocompatibility Aspects in Conducting Polymer-Based Enzymatic Biosensors and Biofuel Cells. Nanomaterials 2021, 11, 371. [Google Scholar] [CrossRef]
- Patois, T.; Lakard, B.; Martin, N.; Fievet, P. Effect of Various Parameters on the Conductivity of Free Standing Electrosynthesized Polypyrrole Films. Synth. Met. 2010, 160, 2180–2185. [Google Scholar] [CrossRef]
- Lete, C.; Lakard, B.; Hihn, J.-Y.; del Campo, F.J.; Lupu, S. Use of Sinusoidal Voltages with Fixed Frequency in the Preparation of Tyrosinase Based Electrochemical Biosensors for Dopamine Electroanalysis. Sens. Actuators B Chem. 2017, 240, 801–809. [Google Scholar] [CrossRef]
- Leonavicius, K.; Ramanaviciene, A.; Ramanavicius, A. Polymerization Model for Hydrogen Peroxide Initiated Synthesis of Polypyrrole Nanoparticles. Langmuir 2011, 27, 10970–10976. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Kausaite, A.; Ramanaviciene, A. Self-Encapsulation of Oxidases as a Basic Approach to Tune the Upper Detection Limit of Amperometric Biosensors. Analyst 2008, 133, 1083. [Google Scholar] [CrossRef]
- Bai, S.; Hu, Q.; Zeng, Q.; Wang, M.; Wang, L. Variations in Surface Morphologies, Properties, and Electrochemical Responses to Nitro-Analyte by Controlled Electropolymerization of Thiophene Derivatives. ACS Appl. Mater. Interfaces 2018, 10, 11319–11327. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Ivy, M.A.; Anslyn, E.V. The Use of Principal Component Analysis and Discriminant Analysis in Differential Sensing Routines. Chem. Soc. Rev. 2014, 43, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-X.; Su, F.; Trewin, A.; Wood, C.D.; Campbell, N.L.; Niu, H.; Dickinson, C.; Ganin, A.Y.; Rosseinsky, M.J.; Khimyak, Y.Z.; et al. Conjugated Microporous Poly(Aryleneethynylene) Networks. Angew. Chem. 2007, 119, 8728–8732. [Google Scholar] [CrossRef]
- Apetrei, R.M.; Carac, G.; Bahrim, G.; Ramanaviciene, A.; Ramanavicius, A. Modification of Aspergillus Niger by Conducting Polymer, Polypyrrole, and the Evaluation of Electrochemical Properties of Modified Cells. Bioelectrochemistry 2018, 121, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Stirke, A.; Apetrei, R.M.; Kirsnyte, M.; Dedelaite, L.; Bondarenka, V.; Jasulaitiene, V.; Pucetaite, M.; Selskis, A.; Carac, G.; Bahrim, G.; et al. Synthesis of Polypyrrole Microspheres by Streptomyces spp. Polymer 2016, 84, 99–106. [Google Scholar] [CrossRef]
- Pankratova, G.; Hederstedt, L.; Gorton, L. Extracellular Electron Transfer Features of Gram-Positive Bacteria. Anal. Chim Acta 2019, 1076, 32–47. [Google Scholar] [CrossRef]
- Pankratova, G.; Pankratov, D.; Milton, R.D.; Minteer, S.D.; Gorton, L. Following Nature: Bioinspired Mediation Strategy for Gram-Positive Bacterial Cells. Adv. Energy Mater. 2019, 9, 1900215. [Google Scholar] [CrossRef]
- Güven, G.; Lozano-Sanchez, P.; Güven, A. Power Generation from Human Leukocytes/Lymphocytes in Mammalian Biofuel Cell. Int. J. Electrochem. 2013, 2013, 706792. [Google Scholar] [CrossRef]
- Ayato, Y.; Sakurai, K.; Fukunaga, S.; Suganuma, T.; Yamagiwa, K.; Shiroishi, H.; Kuwano, J. A Simple Biofuel Cell Cathode with Human Red Blood Cells as Electrocatalysts for Oxygen Reduction Reaction. Biosens. Bioelectron. 2014, 55, 14–18. [Google Scholar] [CrossRef]
- Apetrei, R.M.; Carac, G.; Ramanaviciene, A.; Bahrim, G.; Tanase, C.; Ramanavicius, A. Cell-Assisted Synthesis of Conducting Polymer—Polypyrrole—For the Improvement of Electric Charge Transfer through Fungal Cell Wall. Colloids Surf. B Biointerfaces 2019, 175, 671–679. [Google Scholar] [CrossRef]
- Vaitkuviene, A.; Kaseta, V.; Voronovic, J.; Ramanauskaite, G.; Biziuleviciene, G.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of Cytotoxicity of Polypyrrole Nanoparticles Synthesized by Oxidative Polymerization. J. Hazard. Mater. 2013, 250–251, 167–174. [Google Scholar] [CrossRef]
- Vaitkuviene, A.; Ratautaite, V.; Mikoliunaite, L.; Kaseta, V.; Ramanauskaite, G.; Biziuleviciene, G.; Ramanaviciene, A.; Ramanavicius, A. Some Biocompatibility Aspects of Conducting Polymer Polypyrrole Evaluated with Bone Marrow-Derived Stem Cells. Colloids Surf. A Phys. Eng. Asp. 2014, 442, 152–156. [Google Scholar] [CrossRef]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation and Electrochemical Evaluation of Polyaniline and Polypyrrole Nanocomposites Based on Glucose Oxidase and Gold Nanostructures. Polymers 2020, 12, 3026. [Google Scholar] [CrossRef]
- Van der Zee, F.P.; Cervantes, F.J. Impact and Application of Electron Shuttles on the Redox (Bio)Transformation of Contaminants: A Review. Biotechnol. Adv. 2009, 27, 256–277. [Google Scholar] [CrossRef]
- Magennis, E.P.; Fernandez-Trillo, F.; Sui, C.; Spain, S.G.; Bradshaw, D.J.; Churchley, D.; Mantovani, G.; Winzer, K.; Alexander, C. Bacteria-Instructed Synthesis of Polymers for Self-Selective Microbial Binding and Labelling. Nat. Mater. 2014, 13, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Andriukonis, E.; Stirke, A.; Garbaras, A.; Mikoliunaite, L.; Ramanaviciene, A.; Remeikis, V.; Thornton, B.; Ramanavicius, A. Yeast-Assisted Synthesis of Polypyrrole: Quantification and Influence on the Mechanical Properties of the Cell Wall. Colloids Surf. B Biointerfaces 2018, 164, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-B.; Wu, Y.; Lin, Z.-Q.; Xie, J.; Tan, C.H.; Loo, J.S.C.; Cao, B.; Zhang, J.-R.; Zhu, J.-J.; Zhang, Q. Living and Conducting: Coating Individual Bacterial Cells with In Situ Formed Polypyrrole. Angew. Chem. 2017, 129, 10652–10656. [Google Scholar] [CrossRef]
- Apetrei, R.M.; Cârâc, G.; Bahrim, G.; Camurlu, P. Sensitivity Enhancement for Microbial Biosensors through Cell Self-Coating with Polypyrrole. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 1058–1067. [Google Scholar] [CrossRef]
- Ramanavičius, A.; Kaušaite, A.; Ramanavičiene, A. Polypyrrole-Coated Glucose Oxidase Nanoparticles for Biosensor Design. Sens. Actuators B Chem. 2005, 111–112, 532–539. [Google Scholar] [CrossRef]
- Olea, D.; Viratelle, O.; Faure, C. Polypyrrole-Glucose Oxidase Biosensor. Effect of Enzyme Encapsulation in Multilamellar Vesicles on Analytical Properties. Biosens. Bioelectron. 2008, 23, 788–794. [Google Scholar] [CrossRef]
- Mazur, M.; Krywko-Cendrowska, A.; Krysiński, P.; Rogalski, J. Encapsulation of Laccase in a Conducting Polymer Matrix: A Simple Route towards Polypyrrole Microcontainers. Synth. Met. 2009, 159, 1731–1738. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Kausaite, A.; Ramanaviciene, A.; Acaite, J.; Malinauskas, A. Redox Enzyme—Glu-cose Oxidase—Initiated Synthesis of Polypyrrole. Synth. Met. 2006, 156, 409–413. [Google Scholar] [CrossRef]
- Apetrei, R.M.; Cârâc, G.; Bahrim, G.; Camurlu, P. Utilization of Enzyme Extract Self-Encapsulated within Polypyrrole in Sensitive Detection of Catechol. Enzym. Microb. Technol. 2019, 128, 34–39. [Google Scholar] [CrossRef]
- Liu, S.; Cai, L.; Wang, L.; Yi, X.; Peng, Y. Polydopamine Nanocoating on Individual Cells for Enhanced Extracellular Electron Transfer. Chem. Commun. 2019, 55, 10535–10538. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Wang, Y.Z.; Fang, Z.; Shi, Y.T.; Cheng, Q.W.; Chen, Y.X.; Shi, W.; Yong, Y.C. Single Cell Elec-tron Collectors for Highly Efficient Wiring-up Electronic Abiotic/Biotic Interfaces. Nat. Commun. 2020, 11, 4087. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Aslan, H.; Zhang, P.; Zhu, S.; Xiao, Y.; Chen, L.; Khan, N.; Boesen, T.; Wang, Y.; Liu, Y.; et al. Carbon Dots-Fed Shewanella Oneidensis MR-1 for Bioelectricity Enhancement. Nat. Commun. 2020, 11, 1379. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yi, X.; Wu, X.; Li, Q.; Wang, Y. Internalized Carbon Dots for Enhanced Extracellular Electron Transfer in the Dark and Light. Small 2020, 16, e2004194. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Conghaile, P.; Leech, D.; Gorton, L.; Timur, S.; Anik, U. Development of an Osmium Redox Polymer Mediated Bioanode and Examination of Its Performance in Gluconobacter oxydans Based Microbial Fuel Cell. Electroanalysis 2017, 29, 1651–1657. [Google Scholar] [CrossRef]
- Yuan, Y.; Shin, H.; Kang, C.; Kim, S. Wiring Microbial Biofilms to the Electrode by Osmium Redox Polymer for the Performance Enhancement of Microbial Fuel Cells. Bioelectrochemistry 2016, 108, 8–12. [Google Scholar] [CrossRef]
- Timur, S.; Haghighi, B.; Tkac, J.; Pazarlioǧlu, N.; Telefoncu, A.; Gorton, L. Electrical Wiring of Pseudomonas putida and Pseudomonas fluorescens with Osmium Redox Polymers. Bioelectrochemistry 2007, 71, 38–45. [Google Scholar] [CrossRef]
- Hasan, K.; Çevik, E.; Sperling, E.; Packer, M.A.; Leech, D.; Gorton, L. Photoelectrochemical Wiring of Paulschulzia Pseudovolvox (Algae) to Osmium Polymer Modified Electrodes for Harnessing Solar Energy. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Sayed, E.T.; Tsujiguchi, T.; Nakagawa, N. Catalytic Activity of Baker’s Yeast in a Mediatorless Microbial Fuel Cell. Bioelectrochemistry 2012, 86, 97–101. [Google Scholar] [CrossRef]
- Wu, W.; Niu, H.; Yang, D.; Wang, S.; Jiang, N.; Wang, J.; Lin, J.; Hu, C. Polyaniline/Carbon Nanotubes Composite Modified Anode via Graft Polymerization and Self-Assembling for Microbial Fuel Cells. Polymers 2018, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.W.; May, H.D. Electrochemical Evidence of Direct Electrode Reduction by a Thermophilic Gram-Positive Bacterium, Thermincola ferriacetica. Energy Environ. Sci. 2009, 2, 699–705. [Google Scholar] [CrossRef]
- Herrero-Hernandez, E.; Smith, T.J.; Akid, R. Electricity Generation from Wastewaters with Starch as Carbon Source Using a Mediatorless Microbial Fuel Cell. Biosens. Bioelectron. 2013, 39, 194–198. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, J.; Qiao, Y.; Li, C.M.; Tan, T.T.Y. Architecture Engineering of Hierarchically Porous Chitosan/Vacuum-Stripped Graphene Scaffold as Bioanode for High Performance Microbial Fuel Cell. Nano Lett. 2012, 12, 4738–4741. [Google Scholar] [CrossRef] [PubMed]
- Mardiana, U.; Innocent, C.; Cretin, M.; Buchari; Setiyanto, H.; Nurpalah, R.; Kusmiati, M. Applicability of Alginate Film Entrapped Yeast for Microbial Fuel Cell. Russ. J. Electrochem. 2019, 55, 78–87. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).