Insights into Chemical Bonds for Eliminating the Depletion Region and Accelerating the Photo-Induced Charge Efficient Separation toward Ultrasensitive Photoelectrochemical Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Characterizations
2.2. Synthesis of 3D HWT and HWT-C Arrays
2.3. Fabrication of the PEC Sensor
2.4. Assay Procedure of the PEC Sensor
3. Results and Discussions
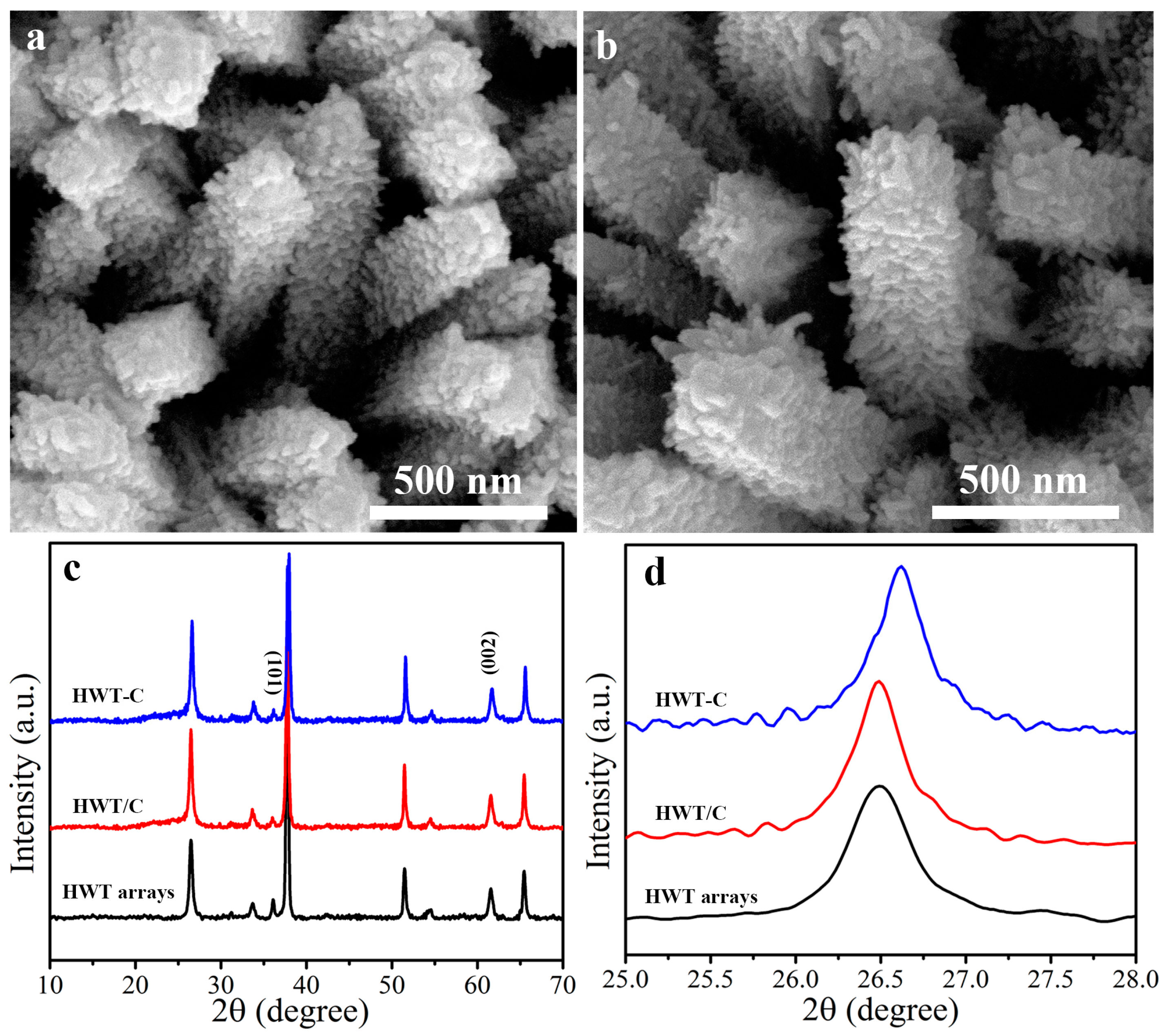
3.1. Characterization of HWT and HWT-C Samples
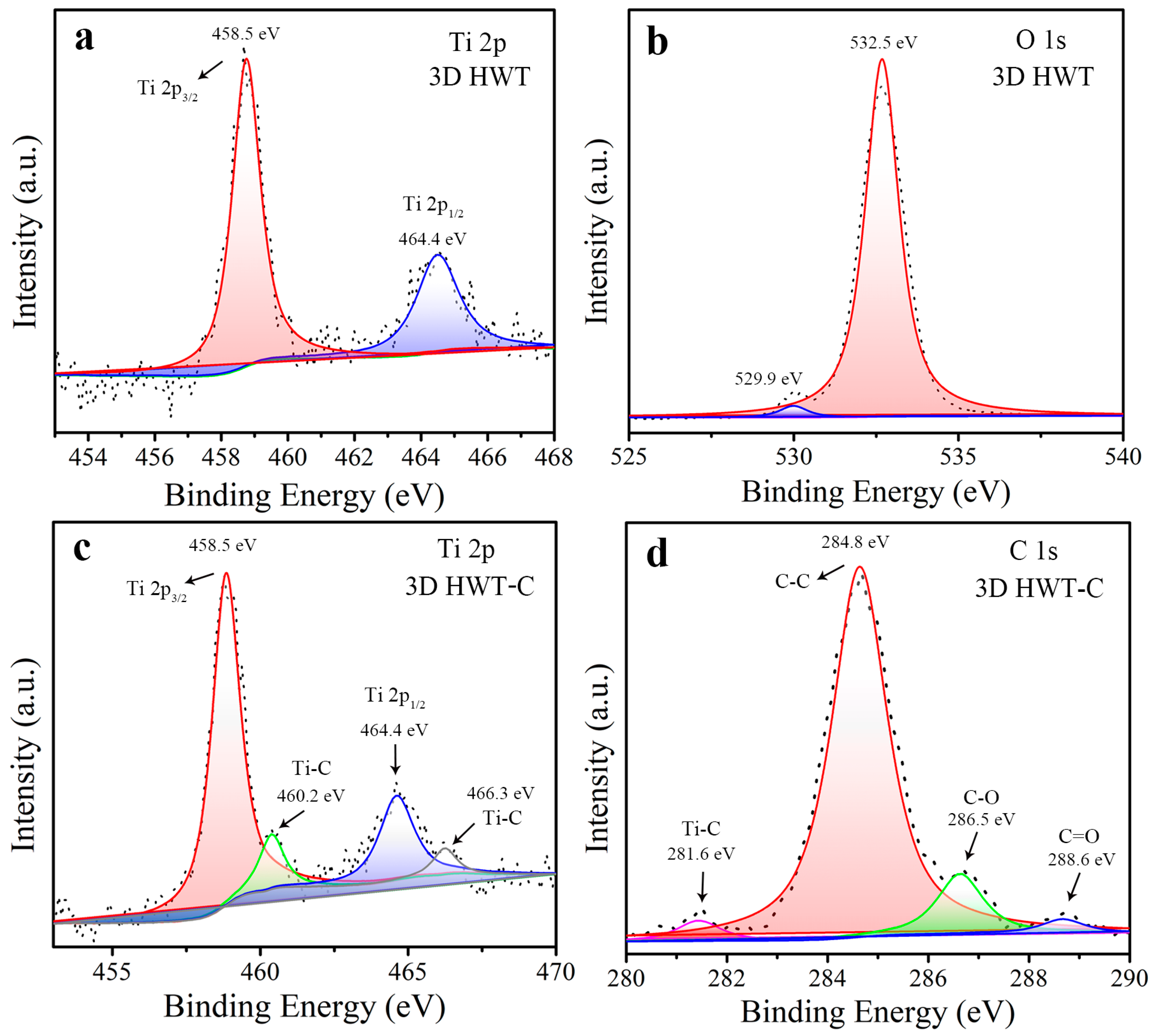
3.2. Investigation on the Promotion of Chemical Bonds on Carrier’s Separation
3.3. Feasibility of the PEC Sensor
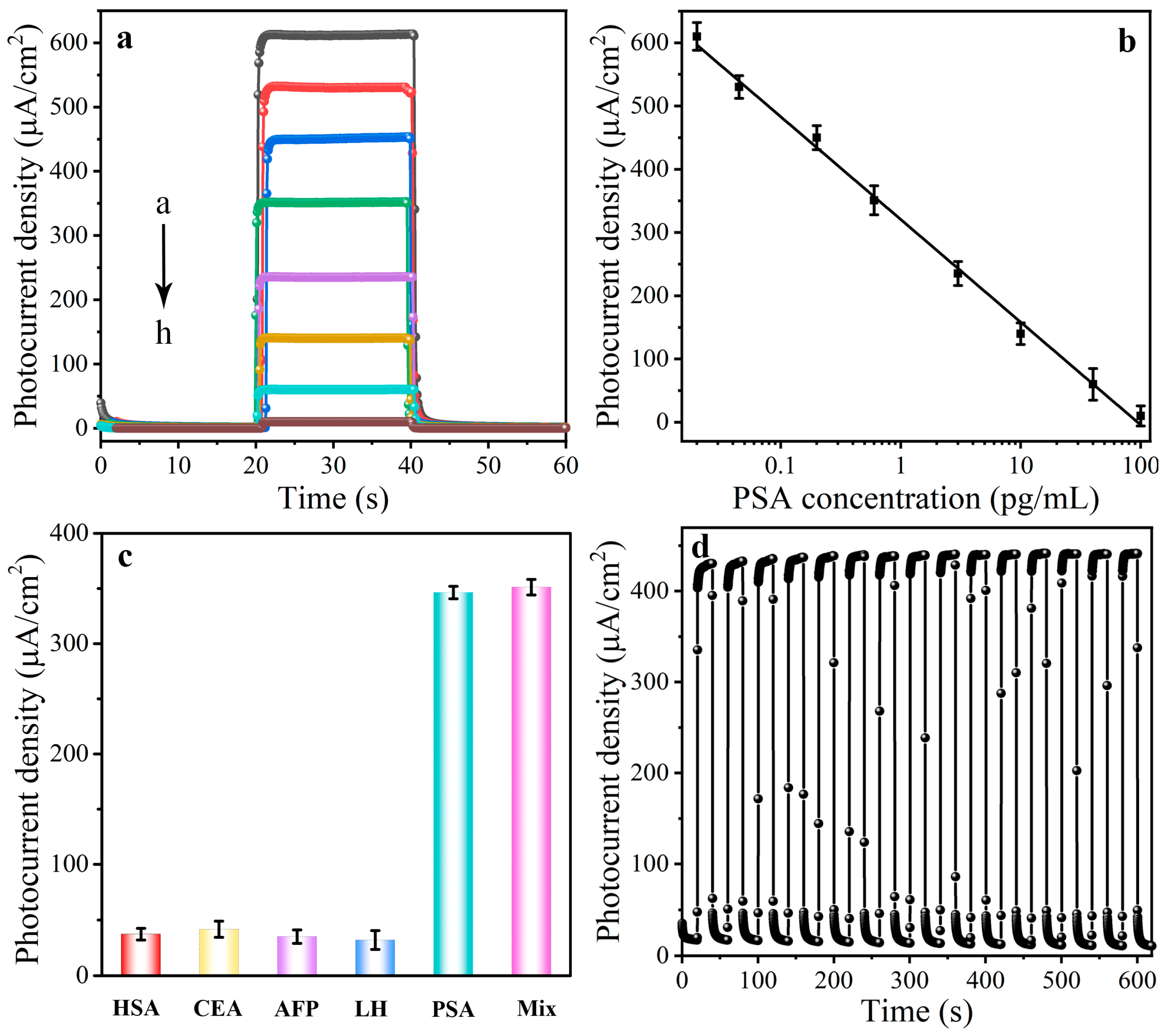
3.4. Optimization of the Detection Conditions
3.5. Selectivity, Reproducibility, and Stability
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Victorious, A.; Saha, S.; Pandey, R.; Soleymani, L. Enhancing the Sensitivity of Photoelectrochemical DNA Biosensing Using Plasmonic DNA Barcodes and Differential Signal Readout. Angew. Chem. Int. Ed. 2021, 60, 7316–7322. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Gong, H.; Xue, F.; Zeng, Y.; Liu, X.; Tang, D. Flexible and High-Throughput Photothermal Biosensors for Rapid Screening of Acute Myocardial Infarction Using Thermochromic Paper-Based Image Analysis. Anal. Chem. 2022, 94, 13233–13242. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wang, X.; Kong, Y.; Dai, M.; Han, D.; Liu, Z. FRET Modulated Signaling: A Versatile Strategy to Construct Photoelectrochemical Microsensors for In Vivo Analysis. Angew. Chem. Int. Ed. 2021, 60, 11774–11778. [Google Scholar] [CrossRef]
- Hu, C.; Zheng, J.; Su, X.; Wang, J.; Wu, W.; Hu, S. Ultrasensitive All-Carbon Photoelectrochemical Bioprobes for Zeptomole Immunosensing of Tumor Markers by an Inexpensive Visible Laser Light. Anal. Chem. 2013, 85, 10612–10619. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zou, K.; Liu, M.; Zhang, X.; Du, C.; Chen, J. Highly Selective and Sensitive Photoelectrochemical Sensing Platform for VEGF165 Assay Based on the Switching of Photocurrent Polarity of CdS QDs by Porous Cu2O-CuO Flower. Anal. Chem. 2019, 92, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Li, P.P.; Cao, Y.; Mao, C.J.; Jin, B.K.; Zhu, J.J. TiO2/g-C3N4/CdS Nanocomposite-Based Photoelectrochemical Biosensor for Ultrasensitive Evaluation of T4 Polynucleotide Kinase Activity. Anal. Chem. 2019, 91, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lin, Q.; Gong, H.; Li, M.; Tang, D. Integrated solar-powered MEMS-based photoelectrochemical immunoassay for point-of-care testing of cTnI protein. Biosens. Bioelectron. 2023, 223, 115028. [Google Scholar] [CrossRef]
- Jiao, S.; Liu, L.; Wang, J.; Ma, K.; Lv, J. A Novel Biosensor Based on Molybdenum Disulfide MoS2 Modified Porous Anodic Aluminum Oxide Nanochannels for Ultrasensitive microRNA-155 Detection. Small 2020, 16, e2001223. [Google Scholar] [CrossRef]
- Ding, L.; Liu, Y.; Lai, J.; Zhu, W.; Fan, C.; Hao, N.; Wei, J.; Qian, J.; Wang, K. Turning on High-Sensitive Organic Electrochemical Transistor-Based Photoelectrochemical-Type Sensor over Modulation of Fe-MOF by PEDOT. Adv. Funct. Mater. 2022, 32, 2202735. [Google Scholar] [CrossRef]
- Ge, L.; Hong, Q.; Li, H.; Liu, C.; Li, F. Direct-Laser-Writing of Metal Sulfide-Graphene Nanocomposite Photoelectrode toward Sensitive Photoelectrochemical Sensing. Adv. Funct. Mater. 2019, 29, 1904000. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Shen, Y.; Guo, L.; Yin, H.; Fang, X.; Yang, Z.; Xu, Q.; Li, H. Superparamagnetic Nanostructures for Split-Type and Competitive-Mode Photoelectrochemical Aptasensing. Anal. Chem. 2020, 92, 8607–8613. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Z.; Guan, R.; Wu, D.; Shi, W.; Yu, L.; Li, P.; Wei, W.; Zhao, Z.; Sun, Z. Synergistic CO2 reduction and tetracycline degradation by CuInZnS-Ti3C2Tx in one photoredox cycle. Nano Res. 2022, 15, 8010–8018. [Google Scholar] [CrossRef]
- Wei, J.J.; Li, H.B.; Wang, G.Q.; Zheng, J.Y.; Wang, A.J.; Mei, L.P.; Zhao, T.; Feng, J.J. Novel Ultrasensitive Photoelectrochemical Cytosensor Based on Hollow CdIn2S4/In2S3 Heterostructured Microspheres for HepG2 Cells Detection and Inhibitor Screening. Anal. Chem. 2022, 94, 12240–12247. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, M.; Zeng, Y.; Liu, X.; Tang, D. Tunable Competitive Absorption-Induced Signal-On Photoelectrochemical Immunoassay for Cardiac Troponin I Based on Z-Scheme Metal-Organic Framework Heterojunctions. Anal. Chem. 2022, 94, 13582–13589. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, Y.; Jiang, L.; Liu, M.; Liu, F.; Jia, M.; Li, J.; Lai, Y. Photoelectrochemical Determination of Cu2+ Using a WO3/CdS Heterojunction Photoanode. ACS Appl. Mater. Interfaces 2019, 11, 37541–37549. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Xue, J.; Zhang, L.; Cui, K.; Li, H.; Yu, J. Paper-Based Origami Photoelectrochemical Sensing Platform with TiO2/Bi4NbO8Cl/Co-Pi Cascade Structure Enabling of Bidirectional Modulation of Charge Carrier Separation. Anal. Chem. 2018, 90, 14116–14120. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Yu, H.; Zhang, L.; Zhao, Y.; Xie, J.; Li, C.; Cui, K.; Yu, J. Ultrasensitive Paper-Based Photoelectrochemical Sensing Platform Enabled by the Polar Charge Carriers-Created Electric Field. Anal. Chem. 2020, 92, 2902–2906. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Z.; Zeng, R.; Zhou, Q.; Tang, D. All-solid-state metal-mediated Z-scheme photoelectrochemical immunoassay with enhanced photoexcited charge-separation for monitoring of prostate-specific antigen. Biosens. Bioelectron. 2019, 134, 1–7. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, C.; Li, P.; Wu, T.; Yang, N.; Wang, X.; Wang, Y.; Li, C. ZnS/C/MoS2 Nanocomposite Derived from Metal-Organic Framework for High-Performance Photo-Electrochemical Immunosensing of Carcinoembryonic Antigen. Small 2019, 15, e1902086. [Google Scholar] [CrossRef]
- Tang, H.; Xiong, M.; Qu, D.; Liu, D.; Zhang, Z.; Xie, Z.; Wei, X.; Tu, W.; Qu, D. Enhanced supercapacitive performance on TiO2@C coaxial nano-rod array through a bio-inspired approach. Nano Energy 2015, 15, 75–82. [Google Scholar] [CrossRef]
- Zhou, G.; Li, T.; Huang, R.; Wang, P.; Hu, B.; Li, H.; Liu, L.; Sun, Y. Recharged Catalyst with Memristive Nitrogen Reduction Activity through Learning Networks of Spiking Neurons. J. Am. Chem. Soc. 2021, 143, 5378–5385. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Tian, S.; Zeng, D.; Wang, X.; Song, W.; Li, Y.; Xiao, W.; Xie, C. Enhanced Photocatalytic Activity of Chemically Bonded TiO2/Graphene Composites Based on the Effective Interfacial Charge Transfer through the C-Ti Bond. ACS Catal. 2013, 3, 1477–1485. [Google Scholar] [CrossRef]
- Xu, F.; Meng, K.; Zhu, B.; Liu, H.; Xu, J.; Yu, J. Graphdiyne: A New Photocatalytic CO2 Reduction Cocatalyst. Adv. Funct. Mater. 2019, 29, 1904256. [Google Scholar] [CrossRef]
- Li, N.; Tao, S.; Chen, Y.; Niu, X.; Onwudinanti, C.K.; Hu, C.; Qiu, Z.; Xu, Z.; Zheng, G.; Wang, L.; et al. Cation and anion immobilization through chemical bonding enhancement with fluorides for stable halide perovskite solar cells. Nat. Energy 2019, 4, 408–415. [Google Scholar] [CrossRef]
- Peng, C.; Luo, G.; Xu, Z.; Yan, S.; Zhang, J.; Chen, M.; Qian, L.; Wei, W.; Han, Q.; Zheng, G. Lithiation-Enabled High-Density Nitrogen Vacancies Electrocatalyze CO2 to C2 Products. Adv. Mater. 2021, 33, 2103150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, P.; Hu, B.; Shen, X.; Liu, C.; Tao, W.; Huang, P.; Liu, L. Spin-related symmetry breaking induced by half-disordered hybridization in BixEr2-xRu2O7 pyrochlores for acidic oxygen evolution. Nat. Commun. 2022, 13, 4106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Huang, H.; Liu, L.; Chen, F.; Tian, N.; Zhang, Y.; Yu, H. Chemically Bonded alpha-Fe2O3/Bi4MO8Cl Dot-on-Plate Z-Scheme Junction with Strong Internal Electric Field for Selective Photo-oxidation of Aromatic Alcohols. Angew. Chem. Int. Ed. 2022, 61, 202203519. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Shen, Z.-K.; Song, S.; Guan, J.; Bao, L.; Pei, L.; Su, Y.; Wu, S.; Bai, W.; Yu, Z.-T.; et al. Co-P Bonds as Atomic-Level Charge Transfer Channel To Boost Photocatalytic H2 Production of Co2P/Black Phosphorus Nanosheets Photocatalyst. ACS Catal. 2019, 9, 7801–7807. [Google Scholar] [CrossRef]
- Feng, N.; Lin, H.; Deng, F.; Ye, J. Interfacial-Bonding Ti-N-C Boosts Efficient Photocatalytic H2 Evolution in Close Coupling g-C3N4/TiO2. J. Phys. Chem. C 2021, 125, 12012–12018. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-Treated TiO2 Nanowire Arrays for Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
- Shi, Y.; Li, T.; Zhao, L.; Liu, Y.; Ding, K.; Li, D.; He, P.; Jiang, D.; Liu, J.; Zhou, H. Ultrathin MXene nanosheet-based TiO2/CdS heterostructure as a photoelectrochemical sensor for detection of CEA in human serum samples. Biosens. Bioelectron. 2023, 230, 115287. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Huang, P.; Miao, Y.; Liang, A.; Wang, X.; Tang, B.; Hou, H.; Ren, M.; Gao, S.; Geng, L.; et al. Novel photoelectrochemical sensor for cholesterol based on CH3NH3PbBr3 perovskite /TiO2 inverse opal heterojunction coated with molecularly imprinted polymers. Sens. Actuators B Chem. 2022, 368, 132121. [Google Scholar] [CrossRef]
- Luo, S.; Liu, F.; Gu, S.; Chen, K.; Yang, G.; Gu, Y.; Cao, J.; Qu, L.L. Nanozyme-mediated signal amplification for ultrasensitive photoelectrochemical sensing of Staphylococcus aureus based on Cu-C3N4-TiO2 heterostructure. Biosens. Bioelectron. 2022, 216, 114593. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, L.; Li, S.; Liu, R. Built-in electric field-assisted charge separation over carbon dots-modified Bi2WO6 nanoplates for photodegradation. Appl. Surf. Sci. 2019, 465, 164–171. [Google Scholar] [CrossRef]
- Khan, S.U.; Trashin, S.; Beltran, V.; Korostei, Y.S.; Pelmus, M.; Gorun, S.M.; Dubinina, T.V.; Verbruggen, S.W.; De Wael, K. Photoelectrochemical Behavior of Phthalocyanine-Sensitized TiO2 in the Presence of Electron-Shuttling Mediators. Anal. Chem. 2022, 94, 12723–12731. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Meng, K.; Cheng, B.; Wang, S.; Xu, J.; Yu, J. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat. Commun. 2020, 11, 4613. [Google Scholar] [CrossRef] [PubMed]
- Bie, C.; Zhu, B.; Xu, F.; Zhang, L.; Yu, J. In Situ Grown Monolayer N-Doped Graphene on CdS Hollow Spheres with Seamless Contact for Photocatalytic CO2 Reduction. Adv. Mater. 2019, 31, 1902868. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wei, T.; Zhang, Y.; Song, X.; Huan, Y.; Liu, H.; Zhao, M.; Yu, J.; Chen, X. A Photoresponsive Rutile TiO2 Heterojunction with Enhanced Electron-Hole Separation for High-Performance Hydrogen Evolution. Adv. Mater. 2019, 31, 1806596. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, C.; Jian, H.; Cheng, X.; Hu, T.; Wang, D.; Shang, L.; Chen, G.; Schaaf, P.; Wang, X.; et al. Substitutionally Dispersed High-Oxidation CoOx Clusters in the Lattice of Rutile TiO2 Triggering Efficient CoTi Cooperative Catalytic Centers for Oxygen Evolution Reactions. Adv. Funct. Mater. 2020, 31, 2009610. [Google Scholar] [CrossRef]
- Chen, X.; Peng, X.; Jiang, L.; Yuan, X.; Fei, J.; Zhang, W. Photocatalytic removal of antibiotics by MOF-derived Ti3+-and oxygen vacancy-doped anatase/rutile TiO2 distributed in a carbon matrix. Chem. Eng. J. 2022, 427, 130945. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, P.; Wang, Y.; Sha, L.; Ren, X.; Zhang, J.; Chen, Y.; Wu, T.; Yang, P.; Li, X. A direct charger transfer from interface to surface for the highly efficient spatial separation of electrons and holes: The construction of Ti-C bonded interfaces in TiO2-C composite as a touchstone for photocatalytic water splitting. Nano Energy 2017, 33, 29–36. [Google Scholar] [CrossRef]
- Wu, S.; Li, X.; Tian, Y.; Lin, Y.; Hu, Y.H. Excellent photocatalytic degradation of tetracycline over black anatase-TiO2 under visible light. Chem. Eng. J. 2021, 406, 126747. [Google Scholar] [CrossRef]
- Zhu, J.H.; Feng, Y.G.; Wang, A.J.; Mei, L.P.; Luo, X.; Feng, J.J. A signal-on photoelectrochemical aptasensor for chloramphenicol assay based on 3D self-supporting AgI/Ag/BiOI Z-scheme heterojunction arrays. Biosens. Bioelectron. 2021, 181, 113158. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, O.A.a.E. Photocatalytic Reduction of Graphene Oxide Nanosheets on TiO2 Thin Film for Photoinactivation of Bacteria in Solar Light Irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar]
- Rockafellow, E.M.; Fang, X.; Trewyn, B.G.; Schmidt-Rohr, K.; Jenks, W.S. Solid-State 13C NMR Characterization of Carbon-Modified TiO2. Chem. Mater. 2009, 21, 1187–1197. [Google Scholar] [CrossRef][Green Version]
- Yi, Q.; Tan, J.; Liu, W.; Lu, H.; Xing, M.; Zhang, J. Peroxymonosulfate activation by three-dimensional cobalt hydroxide/graphene oxide hydrogel for wastewater treatment through an automated process. Chem. Eng. J. 2020, 400, 125965. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, Y.; Liu, S.; Korzeniewski, C.L.; Wang, S.; Fan, Z. Comparing graphene-TiO2 nanowire and graphene-TiO2 nanoparticle composite photocatalysts. ACS Appl. Mater. Interfaces 2012, 4, 3944–3950. [Google Scholar] [CrossRef]
- Xu, Y.F.; Yang, M.Z.; Chen, B.X.; Wang, X.D.; Chen, H.Y.; Kuang, D.B.; Su, C.Y. A CsPbBr3 Perovskite Quantum Dot/Graphene Oxide Composite for Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 5660–5663. [Google Scholar] [CrossRef]
- Ou, M.; Tu, W.; Yin, S.; Xing, W.; Wu, S.; Wang, H.; Wan, S.; Zhong, Q.; Xu, R. Amino-Assisted Anchoring of CsPbBr3 Perovskite Quantum Dots on Porous g-C3N4 for Enhanced Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2018, 57, 13570–13574. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, B.; Wang, L.; Cheng, B.; Zhang, L.; Yu, J. In-situ grown N, S co-doped graphene on TiO2 fiber for artificial photosynthesis of H2O2 and mechanism study. Appl. Catal. B Environ. 2022, 317, 121788. [Google Scholar] [CrossRef]
- Tan, L.; Xu, S.M.; Wang, Z.; Xu, Y.; Wang, X.; Hao, X.; Bai, S.; Ning, C.; Wang, Y.; Zhang, W.; et al. Highly Selective Photoreduction of CO2 with Suppressing H2 Evolution over Monolayer Layered Double Hydroxide under Irradiation above 600 nm. Angew. Chem. Int. Ed. 2019, 58, 11860–11867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Wang, Z.K.; Yuan, S.; Wang, R.; Li, M.; Jimoh, M.F.; Liao, L.S.; Yang, Y. Polarized Ferroelectric Polymers for High-Performance Perovskite Solar Cells. Adv. Mater. 2019, 31, 1902222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Fan, G.C.; Chen, J.J.; Shi, J.J.; Zhu, J.J. Highly sensitive and selective photoelectrochemical biosensor for Hg2+ detection based on dual signal amplification by exciton energy transfer coupled with sensitization effect. Anal. Chem. 2015, 87, 12340–12347. [Google Scholar] [CrossRef] [PubMed]
- Kavosi, B.; Salimi, A.; Hallaj, R.; Amani, K. A highly sensitive prostate-specific antigen immunosensor based on gold nanoparticles/PAMAM dendrimer loaded on MWCNTS/chitosan/ionic liquid nanocomposite. Biosens. Bioelectron. 2014, 52, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Gao, H.; Xu, C.H.; Cheng, Y.; Chen, H.Y.; Xu, J.J. An Efficient Electrochemiluminescence Enhancement Strategy on Bipolar Electrode for Bioanalysis. Anal. Chem. 2019, 91, 12553–12559. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.C.; Chou, C.C.; Yang, Y.Q.; Wei-Kai, T.; Wang, Y.T.; Chan, Y.H. Multiplexed Detection of Tumor Markers with Multicolor Polymer Dot-Based Immunochromatography Test Strip. Anal. Chem. 2018, 90, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhang, L.; Wang, Y.; Yu, J.; Song, X. Visible-light driven biofuel cell based on hierarchically branched titanium dioxide nanorods photoanode for tumor marker detection. Biosens. Bioelectron. 2016, 83, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Guo, L.; Chen, M.; Guo, Y.; Ge, L.; Kwok, H.F. Single-atom Pt-anchored Zn0.5Cd0.5S boosted photoelectrochemical immunoassay of prostate-specific antigen. Biosens. Bioelectron. 2022, 202, 114006. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, B.; Tian, Y.; Guo, Q.; Nie, G. Ultrasensitive ratiometric photoelectrochemical immunoassay for prostate specific antigen based on nanoscale heterojunction. Sens. Actuators B Chem. 2021, 326, 128994. [Google Scholar] [CrossRef]
- Zhang, K.; Lv, S.; Lin, Z.; Li, M.; Tang, D. Bio-bar-code-based photoelectrochemical immunoassay for sensitive detection of prostate-specific antigen using rolling circle amplification and enzymatic biocatalytic precipitation. Biosens. Bioelectron. 2018, 101, 159–166. [Google Scholar] [CrossRef]
- Yao, L.; Xu, J.; Shi, M.; Huang, Y.; Fang, L.; Zhao, S.; Chen, Z.F.; Liang, H. Polydopamine nanoparticle-based multicolor proximity immunoassays for ultrasensitive, multiplexed analysis of proteins using isothermal quadratic amplification. Sens. Actuators B Chem. 2019, 282, 626–635. [Google Scholar] [CrossRef]
- Zhou, Q.; Lin, Y.; Zhang, K.; Li, M.; Tang, D. Reduced graphene oxide/BiFeO3 nanohybrids-based signal-on photoelectrochemical sensing system for prostate-specific antigen detection coupling with magnetic microfluidic device. Biosens. Bioelectron. 2018, 101, 146–152. [Google Scholar] [CrossRef]
- Chen, M.; Wang, C.; Meng, H.; Mo, F.; Fu, Y. A novel signal self-enhancement photoelectrochemical immunosensor without addition of a sacrificial agent in solution based on Ag2S/CuS/α-Fe2O3 n-p-n heterostructure films. Chem. Commun. 2020, 56, 2300–2303. [Google Scholar] [CrossRef]
- Deng, K.; Wang, H.; Xiao, J.; Li, C.; Zhang, S.; Huang, H. Polydopamine nanospheres loaded with l-cysteine-coated cadmium sulfide quantum dots as photoelectrochemical signal amplifier for PSA detection. Anal. Chim. Acta 2019, 1090, 143–150. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Yu, H.; Ge, S.; Wang, Y.; Gao, C.; Yu, J. Insights into Chemical Bonds for Eliminating the Depletion Region and Accelerating the Photo-Induced Charge Efficient Separation toward Ultrasensitive Photoelectrochemical Sensing. Biosensors 2023, 13, 984. https://doi.org/10.3390/bios13110984
Wang S, Yu H, Ge S, Wang Y, Gao C, Yu J. Insights into Chemical Bonds for Eliminating the Depletion Region and Accelerating the Photo-Induced Charge Efficient Separation toward Ultrasensitive Photoelectrochemical Sensing. Biosensors. 2023; 13(11):984. https://doi.org/10.3390/bios13110984
Chicago/Turabian StyleWang, Shuai, Haihan Yu, Shenguang Ge, Yanhu Wang, Chaomin Gao, and Jinghua Yu. 2023. "Insights into Chemical Bonds for Eliminating the Depletion Region and Accelerating the Photo-Induced Charge Efficient Separation toward Ultrasensitive Photoelectrochemical Sensing" Biosensors 13, no. 11: 984. https://doi.org/10.3390/bios13110984
APA StyleWang, S., Yu, H., Ge, S., Wang, Y., Gao, C., & Yu, J. (2023). Insights into Chemical Bonds for Eliminating the Depletion Region and Accelerating the Photo-Induced Charge Efficient Separation toward Ultrasensitive Photoelectrochemical Sensing. Biosensors, 13(11), 984. https://doi.org/10.3390/bios13110984





