Plasmonic Nanoparticle-Enhanced Optical Techniques for Cancer Biomarker Sensing
Abstract
:1. Introduction
2. Definition and Explanation of LSPR Phenomenon in Noble Metal Nanoparticles
3. Surface-Enhanced Raman Spectroscopy (SERS)
3.1. Concepts of SERS
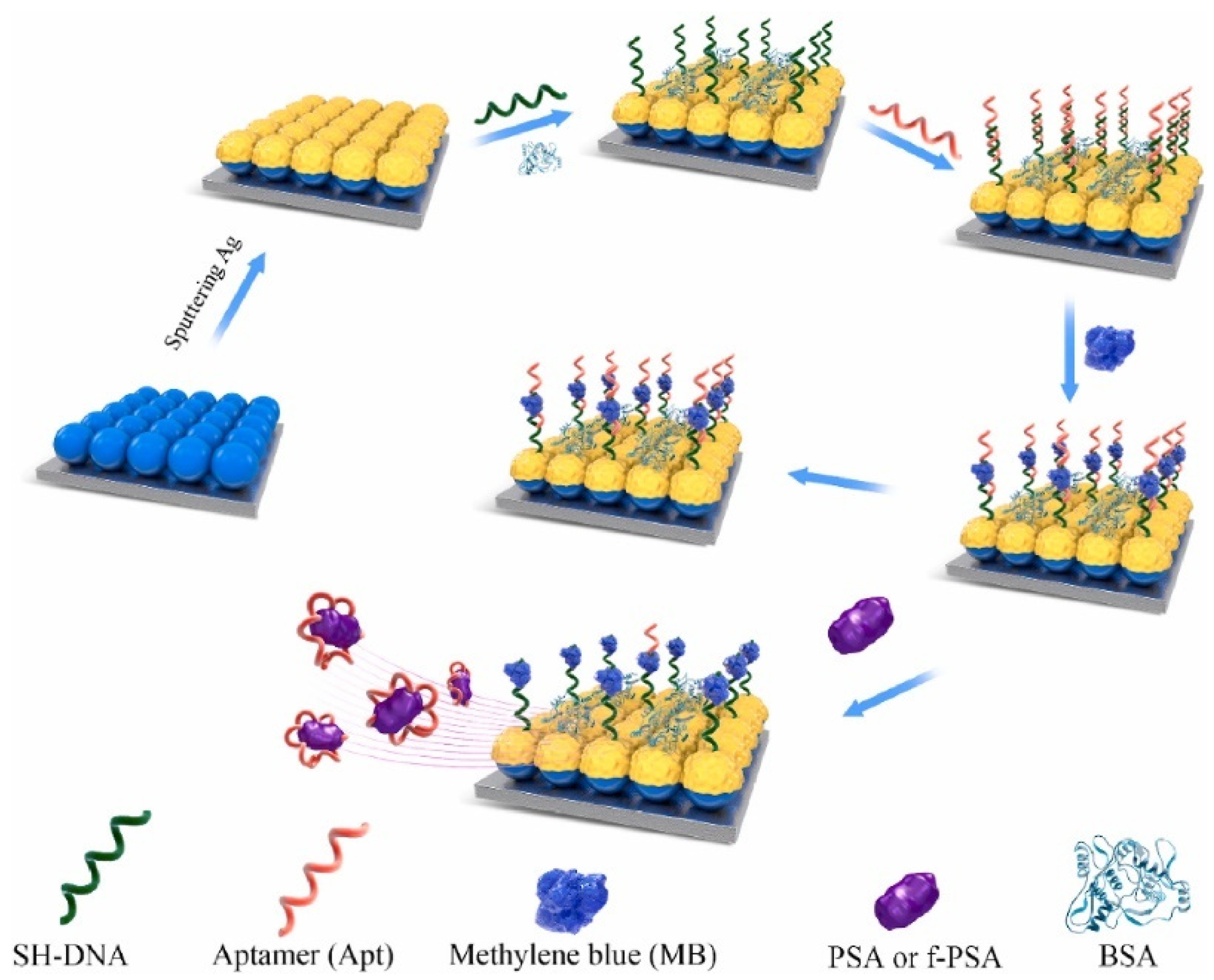
3.2. Direct and Indirect Detection of Cancer Biomarkers Using SERS
3.3. Materials Used for SERS
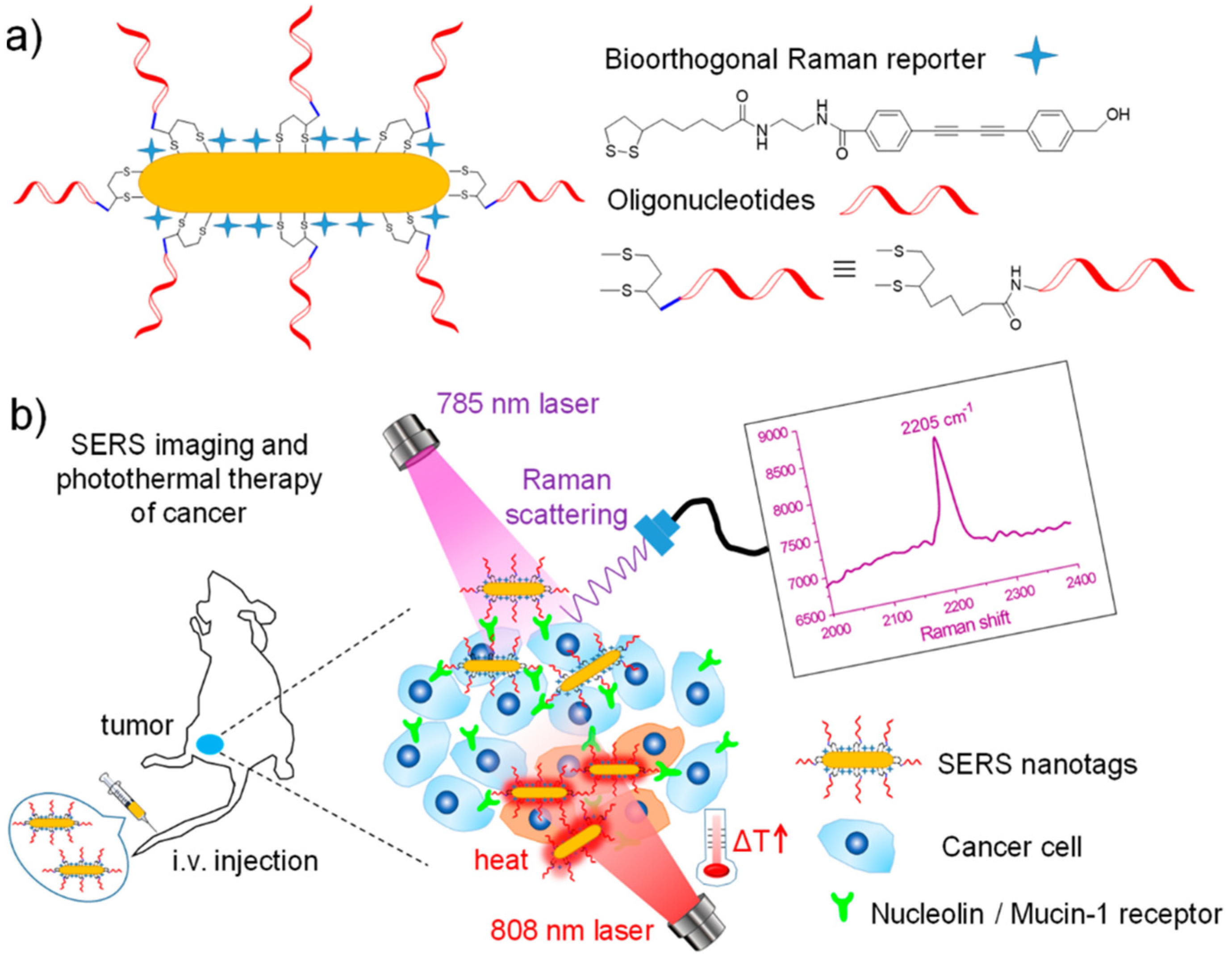
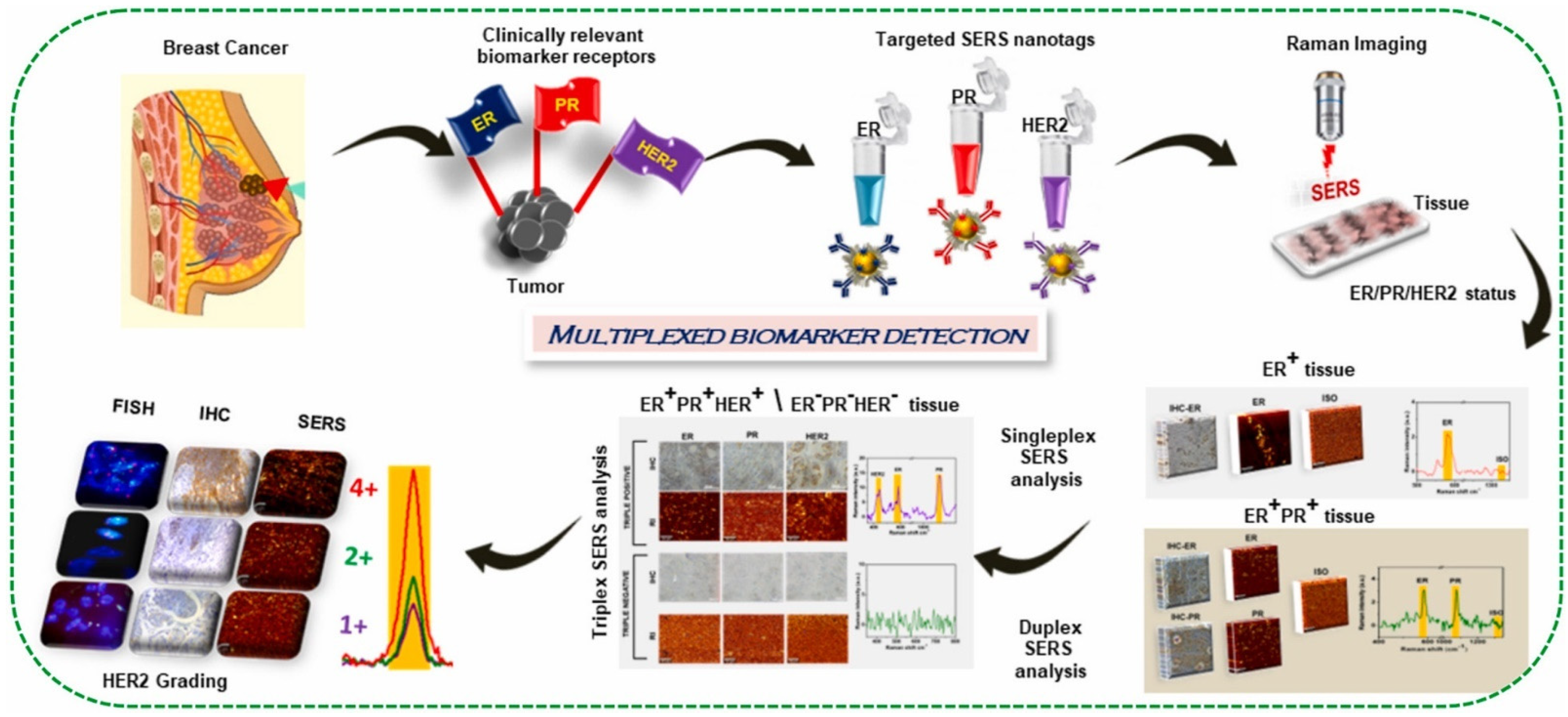
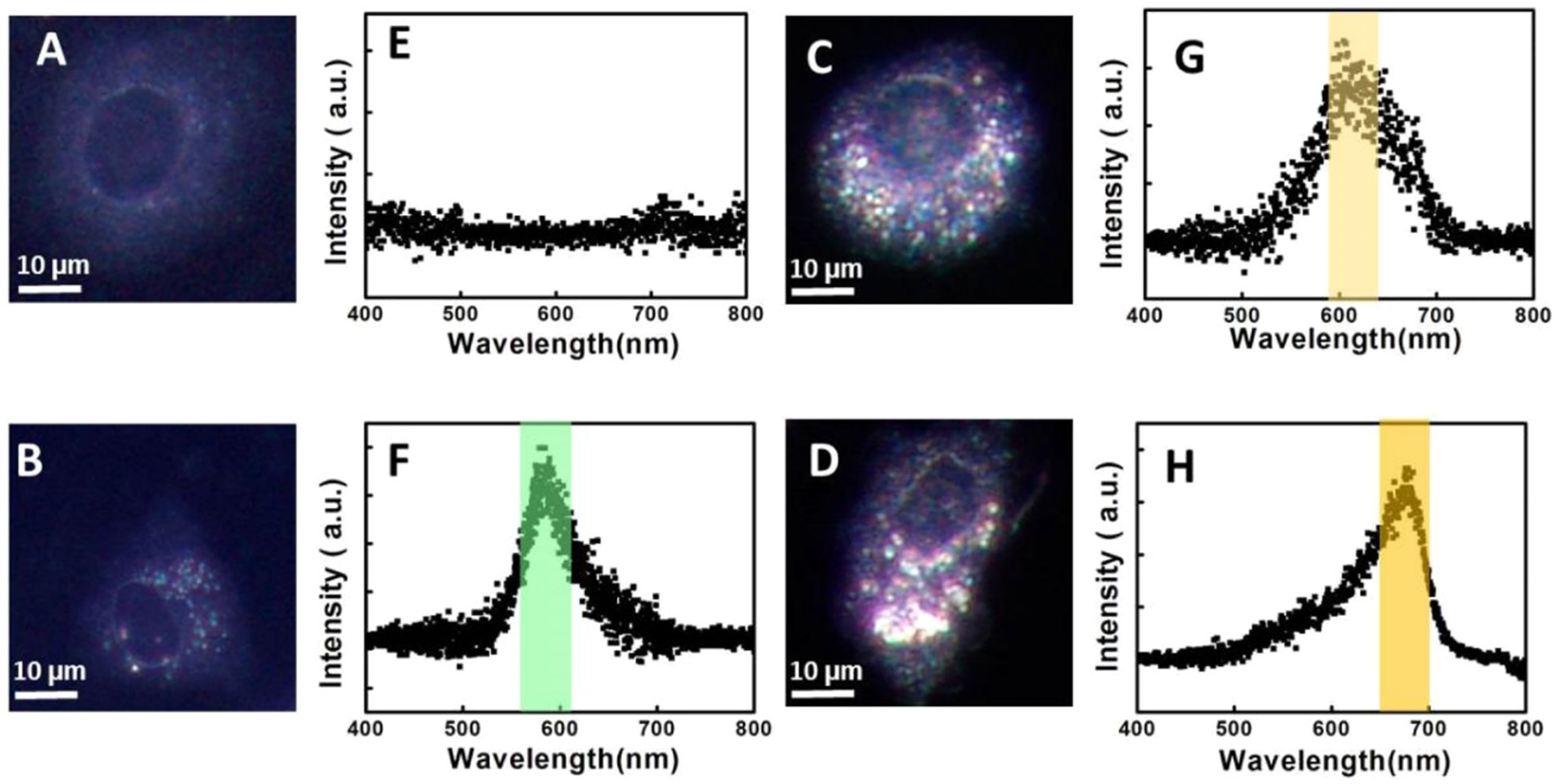
3.4. SERS Imaging
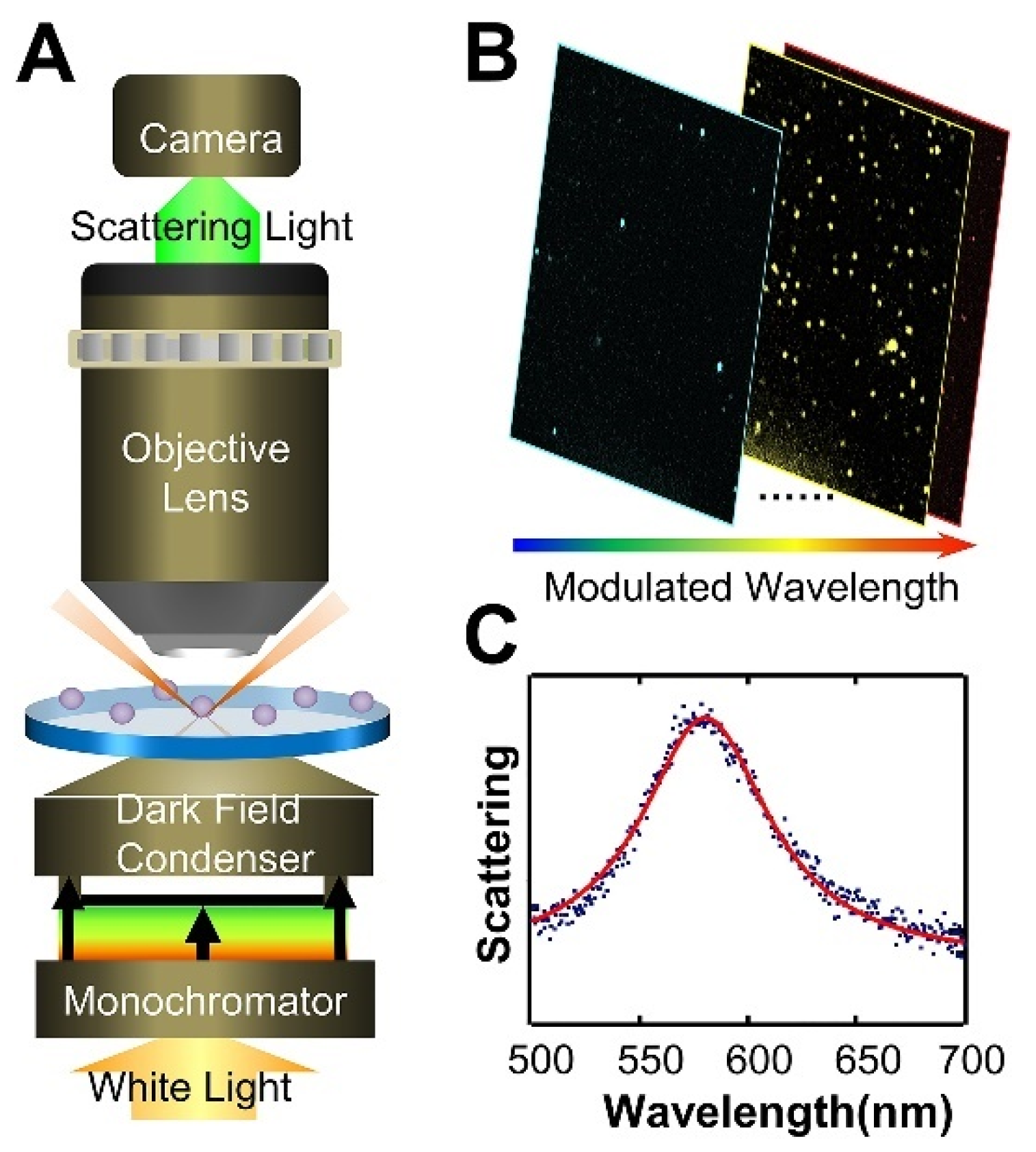
4. Dark-Field Microscopy
5. Photothermal Imaging
5.1. Concepts of Photothermal Imaging
5.2. Molecular Targeting Strategies
6. Photoacoustic Imaging
6.1. Concepts of Photoacoustic Imaging
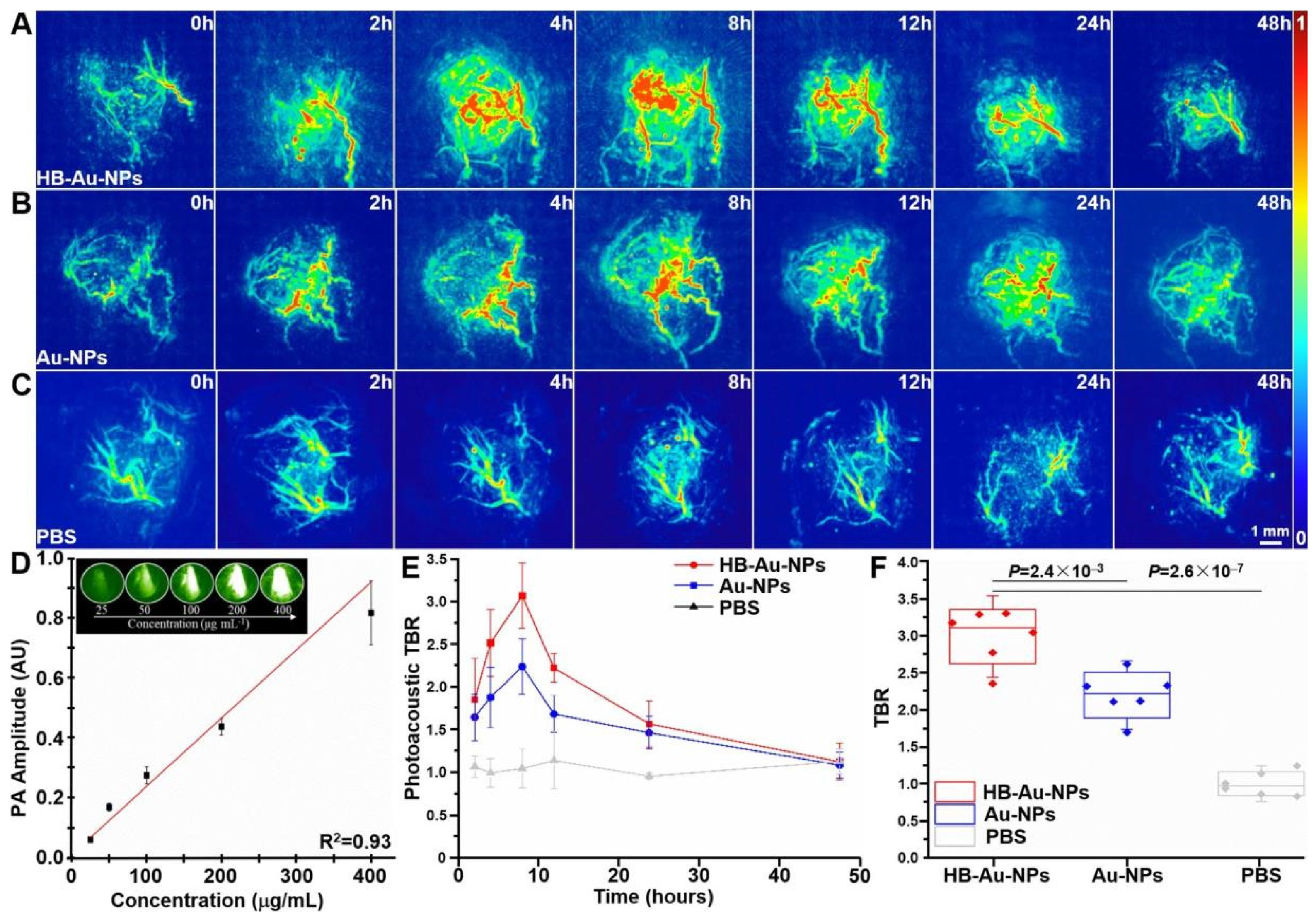
6.2. Photoacoustic Tomography (PAT)
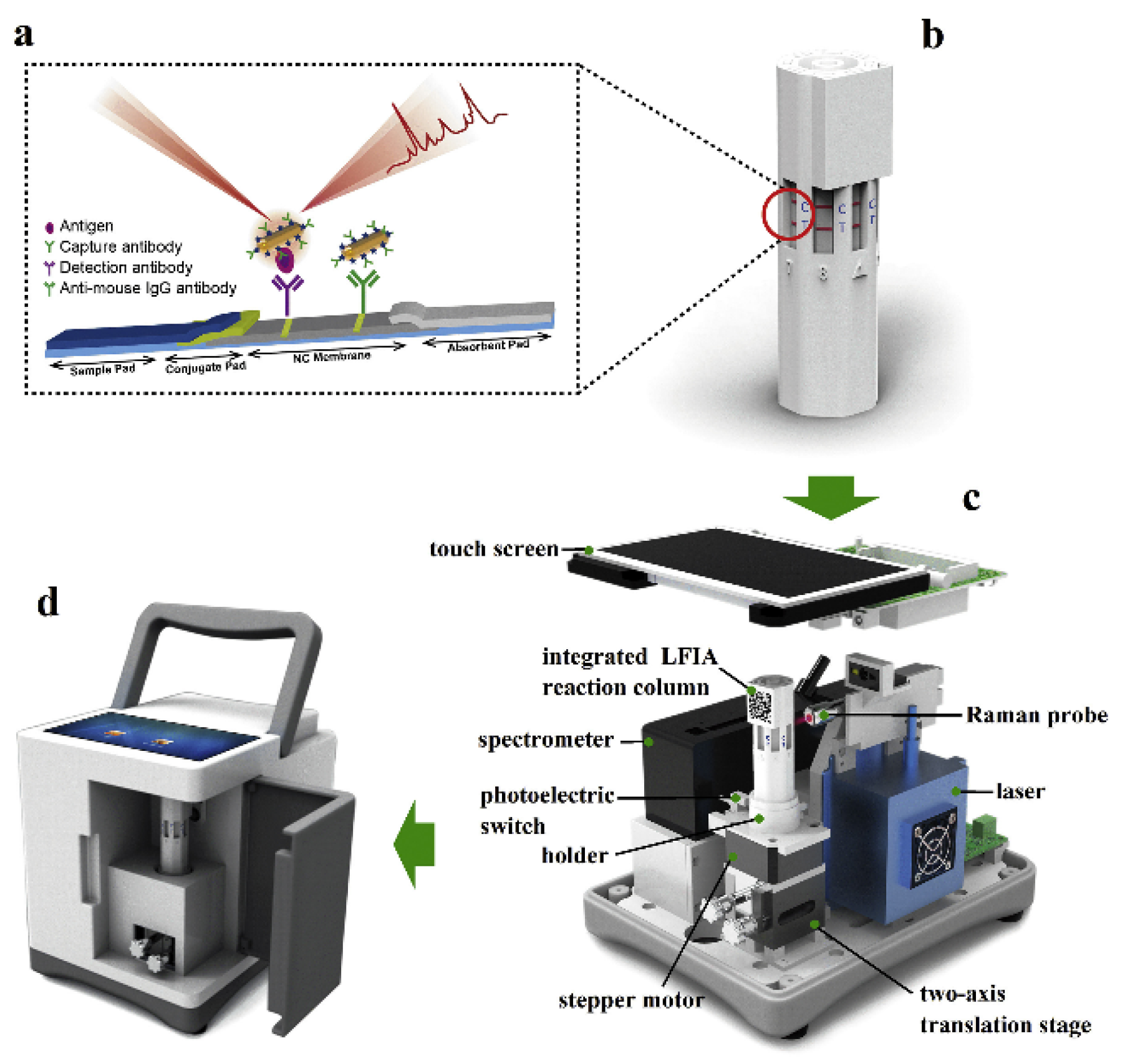
7. Multiplexing and Point-of-Care Detection
8. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, W.; Chen, H.-D.; Yu, Y.-W.; Li, N.; Chen, W.-Q. Changing Profiles of Cancer Burden Worldwide and in China: A Secondary Analysis of the Global Cancer Statistics 2020. Chin. Med. J. 2021, 134, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P. Early Detection of Cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef] [PubMed]
- Van Der Pol, Y.; Mouliere, F. Toward the Early Detection of Cancer by Decoding the Epigenetic and Environmental Fingerprints of Cell-Free DNA. Cancer Cell 2019, 36, 350–368. [Google Scholar] [CrossRef] [PubMed]
- Arshad, F.; Nabi, F.; Iqbal, S.; Khan, R.H. Applications of Graphene-Based Electrochemical and Optical Biosensors in Early Detection of Cancer Biomarkers. Colloids Surf. B Biointerfaces 2022, 212, 112356. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gole, J.; Gore, A.; He, Q.; Lu, M.; Min, J.; Yuan, Z.; Yang, X.; Jiang, Y.; Zhang, T. Non-Invasive Early Detection of Cancer Four Years before Conventional Diagnosis Using a Blood Test. Nat. Commun. 2020, 11, 3475. [Google Scholar] [CrossRef] [PubMed]
- Pashayan, N.; Pharoah, P.D. The Challenge of Early Detection in Cancer. Science 2020, 368, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Abbasi Kajani, A.; Haghjooy Javanmard, S.; Asadnia, M.; Razmjou, A. Recent Advances in Nanomaterials Development for Nanomedicine and Cancer. ACS Appl. Bio Mater. 2021, 4, 5908–5925. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, T.; Li, M.; Tao, Y. Recent Advances in Nanomaterials for Colorimetric Cancer Detection. J. Mater. Chem. B 2021, 9, 921–938. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Li, X.; Liu, X.; Lin, C.-T.; Karimi-Maleh, H. Strategies and Applications of Graphene and Its Derivatives-Based Electrochemical Sensors in Cancer Diagnosis. Molecules 2023, 28, 6719. [Google Scholar] [CrossRef]
- Karimi, F.; Karimi-Maleh, H.; Rouhi, J.; Zare, N.; Karaman, C.; Baghayeri, M.; Fu, L.; Rostamnia, S.; Dragoi, E.N.; Ayati, A. Revolutionizing Cancer Monitoring with Carbon-Based Electrochemical Biosensors. Environ. Res. 2023, 239, 117368. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Alizadeh, M.; Orooji, Y.; Karimi, F.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K. Guanine-Based DNA Biosensor Amplified with Pt/SWCNTs Nanocomposite as Analytical Tool for Nanomolar Determination of Daunorubicin as an Anticancer Drug: A Docking/Experimental Investigation. Ind. Eng. Chem. Res. 2021, 60, 816–823. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Khataee, A.; Karimi, F.; Baghayeri, M.; Fu, L.; Rouhi, J.; Karaman, C.; Karaman, O.; Boukherroub, R. A Green and Sensitive Guanine-Based DNA Biosensor for Idarubicin Anticancer Monitoring in Biological Samples: A Simple and Fast Strategy for Control of Health Quality in Chemotherapy Procedure Confirmed by Docking Investigation. Chemosphere 2022, 291, 132928. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Zhang, J.; He, W.; Ma, X. Au-Al Intermetallic Compounds: A Series of More Efficient LSPR Materials for Hot Carriers-Based Applications than Noble Metal Au. Nano Energy 2021, 82, 105763. [Google Scholar] [CrossRef]
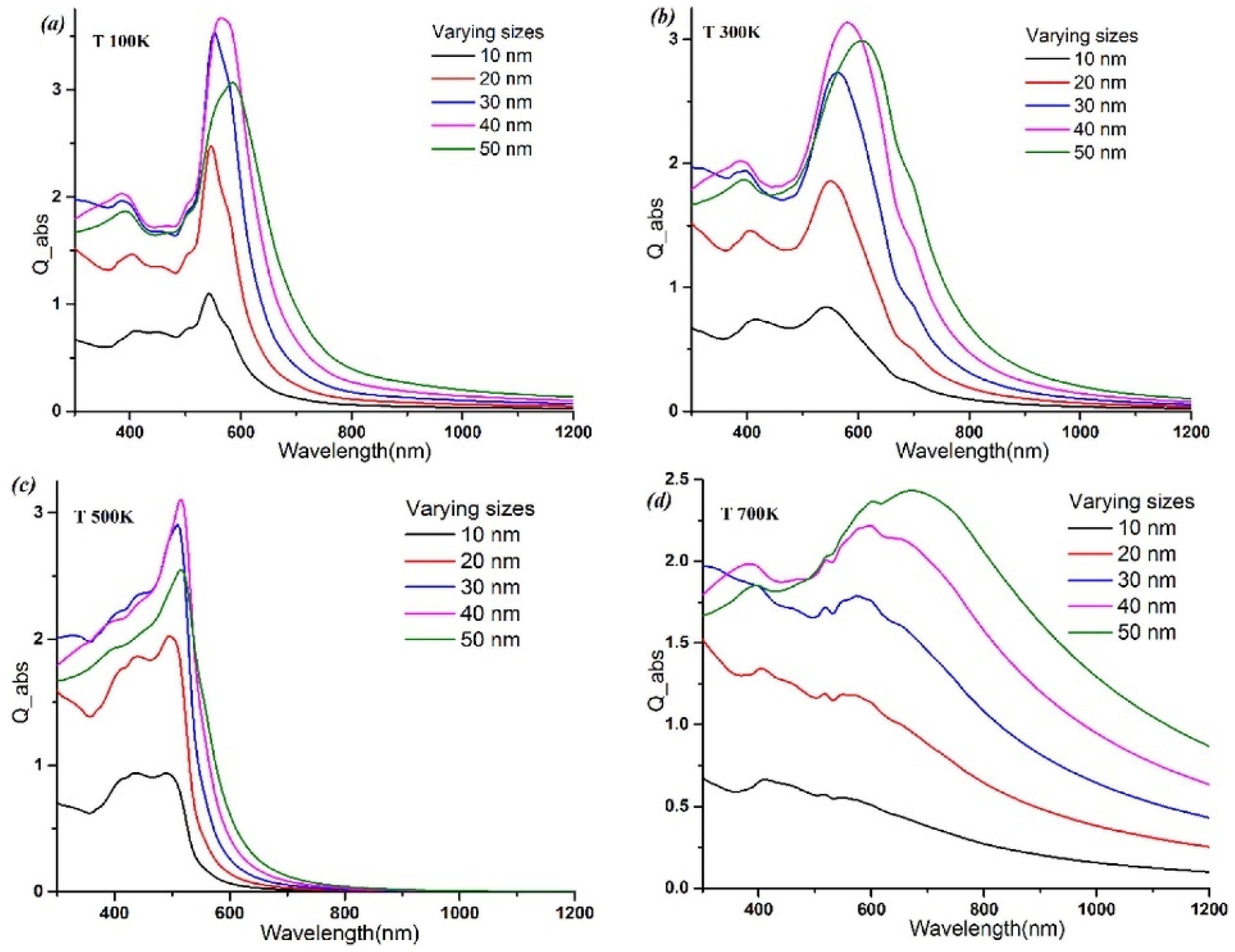
- He, W.; Huang, X.; Ma, X.; Zhang, J. Significant Temperature Effect on the LSPR Properties of Noble Metal Nanoparticles. J. Opt. 2022, 51, 142–153. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Huang, Y.; Luo, B.; Jing, L.; Jing, D. Noble-Metal Free Plasmonic Nanomaterials for Enhanced Photocatalytic Applications—A Review. Nano Res. 2022, 15, 10268–10291. [Google Scholar] [CrossRef]
- Fu, L.; Zhu, D.; Yu, A. Galvanic Replacement Synthesis of Silver Dendrites-Reduced Graphene Oxide Composites and Their Surface-Enhanced Raman Scattering Characteristics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Su, W.; Chen, F.; Fu, L.; Ding, S.; Song, K.; Huang, X.; Zhang, L. Atypical Defect-Mediated Photoluminescence and Resonance Raman Spectroscopy of Monolayer WS2. J. Phys. Chem. C 2019, 123, 3900–3907. [Google Scholar] [CrossRef]
- Zhou, Q.; Jin, M.; Wu, W.; Fu, L.; Yin, C.; Karimi-Maleh, H. Graphene-Based Surface-Enhanced Raman Scattering (SERS) Sensing: Bibliometrics Based Analysis and Review. Chemosensors 2022, 10, 317. [Google Scholar] [CrossRef]
- Fu, Y.; Zeng, G.; Lai, C.; Huang, D.; Qin, L.; Yi, H.; Liu, X.; Zhang, M.; Li, B.; Liu, S.; et al. Hybrid Architectures Based on Noble Metals and Carbon-Based Dots Nanomaterials: A Review of Recent Progress in Synthesis and Applications. Chem. Eng. J. 2020, 399, 125743. [Google Scholar] [CrossRef]
- Ma, X.; He, S.; Qiu, B.; Luo, F.; Guo, L.; Lin, Z. Noble Metal Nanoparticle-Based Multicolor Immunoassays: An Approach toward Visual Quantification of the Analytes with the Naked Eye. ACS Sens. 2019, 4, 782–791. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, B.Y.; Haque, F.; Ren, G.; Ou, J.Z. Plasmonic Metal Oxides and Their Biological Applications. Mater. Horiz. 2022, 9, 2288–2324. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Liu, Y.; Li, Z.; Darabi, R.; Orooji, Y.; Karaman, C.; Karimi, F.; Baghayeri, M.; Rouhi, J.; Fu, L.; et al. Calf Thymus Ds-DNA Intercalation with Pendimethalin Herbicide at the Surface of ZIF-8/Co/rGO/C3N4/Ds-DNA/SPCE; A Bio-Sensing Approach for Pendimethalin Quantification Confirmed by Molecular Docking Study. Chemosphere 2023, 332, 138815. [Google Scholar] [CrossRef] [PubMed]
- Khademalrasool, M.; Farbod, M.; Talebzadeh, M.D. Near-Field and Far-Field Optical Properties of Silver Nanospheres: Theoretical and Experimental Investigations of the Size, Shape, Dielectric Environment, and Composition Effects. Prot. Met. Phys. Chem. Surf. 2021, 57, 1180–1190. [Google Scholar] [CrossRef]
- Li, J.-J.; Qin, Q.-X.; Weng, G.-J.; Zhu, J.; Zhao, J.-W. Improve the Hole Size–Dependent Refractive Index Sensitivity of Au–Ag Nanocages by Tuning the Alloy Composition. Plasmonics 2022, 17, 597–612. [Google Scholar] [CrossRef]
- Ferhan, A.R.; Jackman, J.A.; Park, J.H.; Cho, N.-J.; Kim, D.-H. Nanoplasmonic Sensors for Detecting Circulating Cancer Biomarkers. Adv. Drug Deliv. Rev. 2018, 125, 48–77. [Google Scholar] [CrossRef] [PubMed]
- Bellassai, N.; D’Agata, R.; Jungbluth, V.; Spoto, G. Surface Plasmon Resonance for Biomarker Detection: Advances in Non-Invasive Cancer Diagnosis. Front. Chem. 2019, 7, 570. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Huh, Y.-M.; Yoon, D.S.; Yang, J. Nanobiosensors Based on Localized Surface Plasmon Resonance for Biomarker Detection. J. Nanomater. 2012, 2012, e759830. [Google Scholar] [CrossRef]
- Hwang, W.S.; Sim, S.J. A Strategy for the Ultrasensitive Detection of Cancer Biomarkers Based on the LSPR Response of a Single AuNP. J. Nanosci. Nanotechnol. 2011, 11, 5651–5656. [Google Scholar] [CrossRef]
- Lenzi, E.; Jimenez de Aberasturi, D.; Liz-Marzán, L.M. Surface-Enhanced Raman Scattering Tags for Three-Dimensional Bioimaging and Biomarker Detection. ACS Sens. 2019, 4, 1126–1137. [Google Scholar] [CrossRef]
- Azzouz, A.; Hejji, L.; Kim, K.-H.; Kukkar, D.; Souhail, B.; Bhardwaj, N.; Brown, R.J.C.; Zhang, W. Advances in Surface Plasmon Resonance–Based Biosensor Technologies for Cancer Biomarker Detection. Biosens. Bioelectron. 2022, 197, 113767. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, H.H.; Ru, C.; Zhu, F.; Zou, H.Y.; Gao, P.F.; Huang, C.Z.; Wang, J. Plasmonic Biosensor for the Highly Sensitive Detection of microRNA-21 via the Chemical Etching of Gold Nanorods under a Dark-Field Microscope. Biosens. Bioelectron. 2022, 201, 113942. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, M.; Wang, X.; Hao, Z.; Han, S.; Wang, T.; Zhang, H. Photothermal-Based Nanomaterials and Photothermal-Sensing: An Overview. Biosens. Bioelectron. 2023, 220, 114883. [Google Scholar] [CrossRef]
- Zhao, Z.; Swartchick, C.B.; Chan, J. Targeted Contrast Agents and Activatable Probes for Photoacoustic Imaging of Cancer. Chem. Soc. Rev. 2022, 51, 829–868. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.A.; Carneiro, M.; Ferreira, D.; Moreira, F.T.C.; Sales, M.G.F.; Rodrigues, L.R. Recent Advances in the Selection of Cancer-Specific Aptamers for the Development of Biosensors. Curr. Med. Chem. 2022, 29, 5850–5880. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Lee, J.-H.; Son, J.; Choi, J.-W. Noble Metal-Assisted Surface Plasmon Resonance Immunosensors. Sensors 2020, 20, 1003. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, Z.; Zuo, D.; Zhu, W.; Zhang, J.; Zeng, Q.; Yang, D.; Li, M.; Zhao, Y. Ultrasensitive Detection of Serum MicroRNA Using Branched DNA-Based SERS Platform Combining Simultaneous Detection of α-Fetoprotein for Early Diagnosis of Liver Cancer. ACS Appl. Mater. Interfaces 2018, 10, 34869–34877. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic Nanoparticles for Cancer Therapy: A Transition from Lab to Clinic. Curr. Med. Chem. 2018, 25, 4269–4303. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.V. Recent Progress in Photoacoustic Molecular Imaging. Curr. Opin. Chem. Biol. 2018, 45, 104–112. [Google Scholar]
- Gong, T.; Olivo, M.; Dinish, U.S.; Goh, D.; Kong, K.V.; Yong, K.-T. Engineering Bioconjugated Gold Nanospheres and Gold Nanorods as Label-Free Plasmon Scattering Probes for Ultrasensitive Multiplex Dark-Field Imaging of Cancer Cells. J. Biomed. Nanotechnol. 2013, 9, 985–991. [Google Scholar] [CrossRef]
- Pires, N.M.M.; Dong, T.; Hanke, U.; Hoivik, N. Recent Developments in Optical Detection Technologies in Lab-on-a-Chip Devices for Biosensing Applications. Sensors 2014, 14, 15458–15479. [Google Scholar] [CrossRef]
- Tai, J.; Fan, S.; Ding, S.; Ren, L. Gold Nanoparticles Based Optical Biosensors for Cancer Biomarker Proteins: A Review of the Current Practices. Front. Bioeng. Biotechnol. 2022, 10, 877193. [Google Scholar] [CrossRef] [PubMed]
- Mulyanti, B.; Nugroho, H.S.; Wulandari, C.; Rahmawati, Y.; Hasanah, L.; Hamidah, I.; Pawinanto, R.E.; Majlis, B.Y. SPR-Based Sensor for the Early Detection or Monitoring of Kidney Problems. Int. J. Biomater. 2022, 2022, e9135172. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Verma, S.S. Enhancement of LSPR Properties of Temperature-Dependent Gold Nanoparticles. Mater. Today Proc. 2023, 78, 871–876. [Google Scholar] [CrossRef]
- Amirjani, A.; Firouzi, F.; Haghshenas, D.F. Predicting the Size of Silver Nanoparticles from Their Optical Properties. Plasmonics 2020, 15, 1077–1082. [Google Scholar] [CrossRef]
- He, M.-Q.; Yu, Y.-L.; Wang, J.-H. Biomolecule-Tailored Assembly and Morphology of Gold Nanoparticles for LSPR Applications. Nano Today 2020, 35, 101005. [Google Scholar] [CrossRef]
- Shabaninezhad, M.; Ramakrishna, G. Theoretical Investigation of Size, Shape, and Aspect Ratio Effect on the LSPR Sensitivity of Hollow-Gold Nanoshells. J. Chem. Phys. 2019, 150, 144116. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Brown, P.K.; Wang, L.V.; Xia, Y. Gold Nanocages as Contrast Agents for Photoacoustic Imaging. Contrast Media Mol. Imaging 2011, 6, 370–377. [Google Scholar] [CrossRef]
- Auner, G.W.; Koya, S.K.; Huang, C.; Broadbent, B.; Trexler, M.; Auner, Z.; Elias, A.; Mehne, K.C.; Brusatori, M.A. Applications of Raman Spectroscopy in Cancer Diagnosis. Cancer Metastasis Rev. 2018, 37, 691–717. [Google Scholar] [CrossRef]
- Qian, X.-M.; Nie, S.M. Single-Molecule and Single-Nanoparticle SERS: From Fundamental Mechanisms to Biomedical Applications. Chem. Soc. Rev. 2008, 37, 912–920. [Google Scholar] [CrossRef]
- Poon, C.-Y.; Wei, L.; Xu, Y.; Chen, B.; Xiao, L.; Li, H.-W. Quantification of Cancer Biomarkers in Serum Using Scattering-Based Quantitative Single Particle Intensity Measurement with a Dark-Field Microscope. Anal. Chem. 2016, 88, 8849–8856. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal Therapy and Photoacoustic Imaging via Nanotheranostics in Fighting Cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Navas, M.P.; Soni, R.K. Laser-Generated Bimetallic Ag-Au and Ag-Cu Core-Shell Nanoparticles for Refractive Index Sensing. Plasmonics 2015, 10, 681–690. [Google Scholar] [CrossRef]
- Bansal, A.; Sekhon, J.S.; Verma, S.S. Scattering Efficiency and LSPR Tunability of Bimetallic Ag, Au, and Cu Nanoparticles. Plasmonics 2014, 9, 143–150. [Google Scholar] [CrossRef]
- Ma, J.; Liu, X.; Wang, R.; Zhang, J.; Jiang, P.; Wang, Y.; Tu, G. Bimetallic Core–Shell Nanostars with Tunable Surface Plasmon Resonance for Surface-Enhanced Raman Scattering. ACS Appl. Nano Mater. 2020, 3, 10885–10894. [Google Scholar] [CrossRef]
- Mahmud, S.; Satter, S.S.; Singh, A.K.; Rahman, M.M.; Mollah, M.Y.A.; Susan, M.A.B.H. Tailored Engineering of Bimetallic Plasmonic Au@Ag Core@Shell Nanoparticles. ACS Omega 2019, 4, 18061–18075. [Google Scholar] [CrossRef] [PubMed]
- Lismont, M.; Páez, C.A.; Dreesen, L. A One-Step Short-Time Synthesis of Ag@SiO2 Core–Shell Nanoparticles. J. Colloid Interface Sci. 2015, 447, 40–49. [Google Scholar] [CrossRef]
- Chen, J.-J.; Wu, J.C.S.; Wu, P.C.; Tsai, D.P. Improved Photocatalytic Activity of Shell-Isolated Plasmonic Photocatalyst Au@SiO2/TiO2 by Promoted LSPR. J. Phys. Chem. C 2012, 116, 26535–26542. [Google Scholar] [CrossRef]
- Ling, J.; Zhi Huang, C. Energy Transfer with Gold Nanoparticles for Analytical Applications in the Fields of Biochemical and Pharmaceutical Sciences. Anal. Methods 2010, 2, 1439–1447. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, J.I.L.; Ginger, D.S. Photoswitchable Oligonucleotide-Modified Gold Nanoparticles: Controlling Hybridization Stringency with Photon Dose. Nano Lett. 2012, 12, 2530–2536. [Google Scholar] [CrossRef]
- Lee, S.-W.; Lee, K.-S.; Ahn, J.; Lee, J.-J.; Kim, M.-G.; Shin, Y.-B. Highly Sensitive Biosensing Using Arrays of Plasmonic Au Nanodisks Realized by Nanoimprint Lithography. ACS Nano 2011, 5, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Quilis, N.G.; Lequeux, M.; Venugopalan, P.; Khan, I.; Knoll, W.; Boujday, S.; de la Chapelle, M.L.; Dostalek, J. Tunable Laser Interference Lithography Preparation of Plasmonic Nanoparticle Arrays Tailored for SERS. Nanoscale 2018, 10, 10268–10276. [Google Scholar] [CrossRef]
- Piantanida, L.; Naumenko, D.; Torelli, E.; Marini, M.; Bauer, D.M.; Fruk, L.; Firrao, G.; Lazzarino, M. Plasmon Resonance Tuning Using DNA Origami Actuation. Chem. Commun. 2015, 51, 4789–4792. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, Y.; Pan, J.; Zhou, C.; Wang, Q. Assembling Gold Nanobipyramids into Chiral Plasmonic Nanostructures with DNA Origami. Chem. Commun. 2021, 57, 6201–6204. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Peng, W.; Lin, M.; Wang, F.; Zhang, Y. Gold Nanoparticle-Enhanced Detection of DNA Hybridization by a Block Copolymer-Templating Fiber-Optic Localized Surface Plasmon Resonance Biosensor. Nanomaterials 2021, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Singh, R.; Zhang, B.; Cheng, S.; Kumar Kaushik, B.; Kumar, S. LSPR Based Uric Acid Sensor Using Graphene Oxide and Gold Nanoparticles Functionalized Tapered Fiber. Opt. Fiber Technol. 2019, 53, 102043. [Google Scholar] [CrossRef]
- Zangeneh Kamali, K.; Pandikumar, A.; Jayabal, S.; Ramaraj, R.; Lim, H.N.; Ong, B.H.; Bien, C.S.D.; Kee, Y.Y.; Huang, N.M. Amalgamation Based Optical and Colorimetric Sensing of Mercury(II) Ions with Silver@graphene Oxide Nanocomposite Materials. Microchim. Acta 2016, 183, 369–377. [Google Scholar] [CrossRef]
- Pons, T.; Medintz, I.L.; Sapsford, K.E.; Higashiya, S.; Grimes, A.F.; English, D.S.; Mattoussi, H. On the Quenching of Semiconductor Quantum Dot Photoluminescence by Proximal Gold Nanoparticles. Nano Lett. 2007, 7, 3157–3164. [Google Scholar] [CrossRef]
- Liu, J.; Detrembleur, C.; Mornet, S.; Jérôme, C.; Duguet, E. Design of Hybrid Nanovehicles for Remotely Triggered Drug Release: An Overview. J. Mater. Chem. B 2015, 3, 6117–6147. [Google Scholar] [CrossRef]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. “Smart” Materials-Based near-Infrared Light-Responsive Drug Delivery Systems for Cancer Treatment: A Review. J. Mater. Res. Technol. 2019, 8, 1497–1509. [Google Scholar] [CrossRef]
- Limonov, M.F.; Rybin, M.V.; Poddubny, A.N.; Kivshar, Y.S. Fano Resonances in Photonics. Nat. Photon. 2017, 11, 543–554. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, J. Highly Controllable Double Fano Resonances in Plasmonic Metasurfaces. Nanoscale 2016, 8, 17665–17674. [Google Scholar] [CrossRef]
- Du, M.; Shen, Z. Enhanced and Tunable Double Fano Resonances in Plasmonic Metasurfaces with Nanoring Dimers. J. Phys. D Appl. Phys. 2021, 54, 145106. [Google Scholar] [CrossRef]
- Campione, S.; Guclu, C.; Ragan, R.; Capolino, F. Enhanced Magnetic and Electric Fields via Fano Resonances in Metasurfaces of Circular Clusters of Plasmonic Nanoparticles. ACS Photonics 2014, 1, 254–260. [Google Scholar] [CrossRef]
- Liang, Y.; Koshelev, K.; Zhang, F.; Lin, H.; Lin, S.; Wu, J.; Jia, B.; Kivshar, Y. Bound States in the Continuum in Anisotropic Plasmonic Metasurfaces. Nano Lett. 2020, 20, 6351–6356. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liang, Y.; Yao, J.; Chen, M.K.; Lin, S.; Wang, Z.; Zhang, J.; Huang, X.G.; Yu, C.; Tsai, D.P. Chiral Bound States in the Continuum in Plasmonic Metasurfaces. Laser Photonics Rev. 2023, 17, 2200597. [Google Scholar] [CrossRef]
- Du, W.; Wen, X.; Gérard, D.; Qiu, C.-W.; Xiong, Q. Chiral Plasmonics and Enhanced Chiral Light-Matter Interactions. Sci. China Phys. Mech. Astron. 2019, 63, 244201. [Google Scholar] [CrossRef]
- Bochenkov, V.E.; Shabatina, T.I. Chiral Plasmonic Biosensors. Biosensors 2018, 8, 120. [Google Scholar] [CrossRef]
- Wu, X.; Xu, L.; Liu, L.; Ma, W.; Yin, H.; Kuang, H.; Wang, L.; Xu, C.; Kotov, N.A. Unexpected Chirality of Nanoparticle Dimers and Ultrasensitive Chiroplasmonic Bioanalysis. J. Am. Chem. Soc. 2013, 135, 18629–18636. [Google Scholar] [CrossRef]
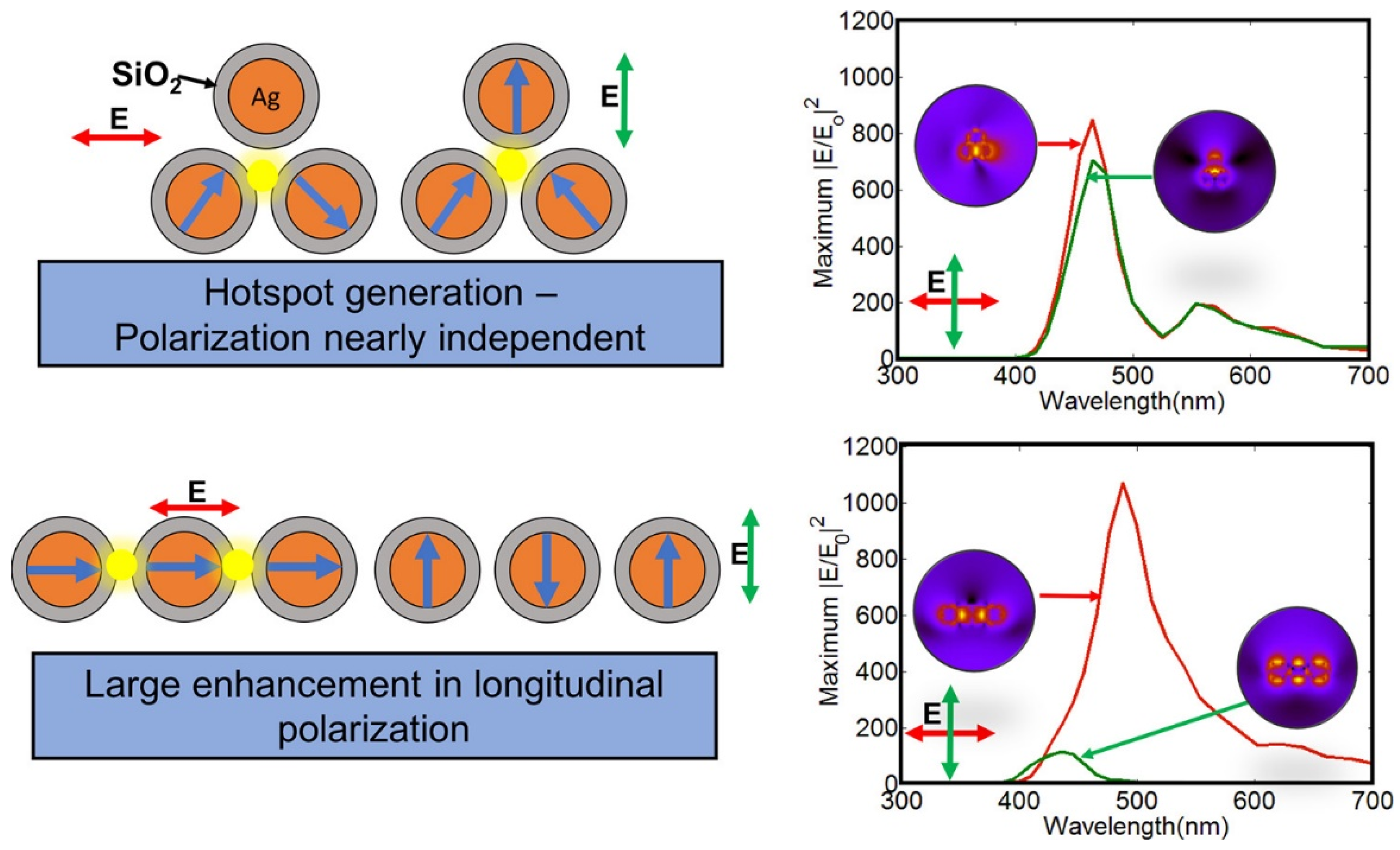
- Nagarajan, A.; Panchanathan, A.P.; Chelliah, P.; Satoh, H.; Inokawa, H. Optimization of Electric Field Enhancement of Ag@SiO2 Trimer Nanospheres by Finite Difference Time Domain Method. Appl. Surf. Sci. 2019, 495, 143547. [Google Scholar] [CrossRef]
- Bell, S.E.J.; Charron, G.; Cortés, E.; Kneipp, J.; de la Chapelle, M.L.; Langer, J.; Procházka, M.; Tran, V.; Schlücker, S. Towards Reliable and Quantitative Surface-Enhanced Raman Scattering (SERS): From Key Parameters to Good Analytical Practice. Angew. Chem. Int. Ed. 2020, 59, 5454–5462. [Google Scholar] [CrossRef]
- Litti, L.; Meneghetti, M. Predictions on the SERS Enhancement Factor of Gold Nanosphere Aggregate Samples. Phys. Chem. Chem. Phys. 2019, 21, 15515–15522. [Google Scholar] [CrossRef] [PubMed]
- Jung Seo, M.; Wan Kim, G.; Vuka Tsalu, P.; Woo Moon, S.; Won Ha, J. Role of Chemical Interface Damping for Tuning Chemical Enhancement in Resonance Surface-Enhanced Raman Scattering of Plasmonic Gold Nanorods. Nanoscale Horiz. 2020, 5, 345–349. [Google Scholar] [CrossRef]
- Ji, C.; Lu, J.; Shan, B.; Li, F.; Zhao, X.; Yu, J.; Xu, S.; Man, B.; Zhang, C.; Li, Z. The Origin of Mo2C Films for Surface-Enhanced Raman Scattering Analysis: Electromagnetic or Chemical Enhancement? J. Phys. Chem. Lett. 2022, 13, 8864–8871. [Google Scholar] [CrossRef] [PubMed]
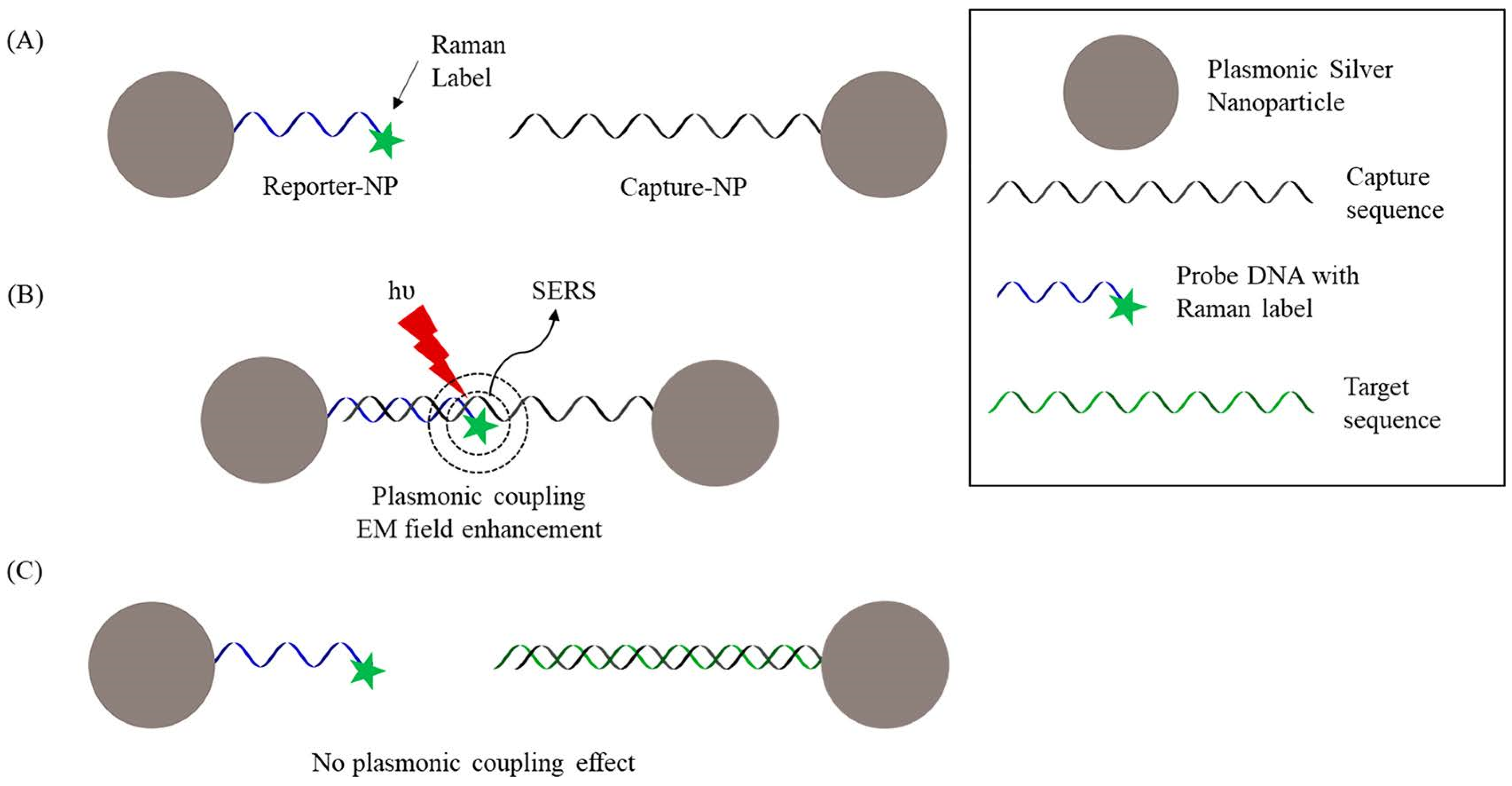
- Wang, H.-N.; Crawford, B.M.; Norton, S.J.; Vo-Dinh, T. Direct and Label-Free Detection of MicroRNA Cancer Biomarkers Using SERS-Based Plasmonic Coupling Interference (PCI) Nanoprobes. J. Phys. Chem. B 2019, 123, 10245–10251. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Guo, X.; Wu, Y.; Wang, H.; Fu, S.; Wen, Y.; Yang, H. Facile and Label-Free Detection of Lung Cancer Biomarker in Urine by Magnetically Assisted Surface-Enhanced Raman Scattering. ACS Appl. Mater. Interfaces 2014, 6, 20985–20993. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Kyaw, Y.M.E.; Tan, E.K.M.; Bekale, L.; Kang, M.W.C.; Kim, S.S.-Y.; Tan, I.; Lam, K.-P.; Kah, J.C.Y. Quantitative and Label-Free Detection of Protein Kinase a Activity Based on Surface-Enhanced Raman Spectroscopy with Gold Nanostars. Anal. Chem. 2018, 90, 6071–6080. [Google Scholar] [CrossRef] [PubMed]
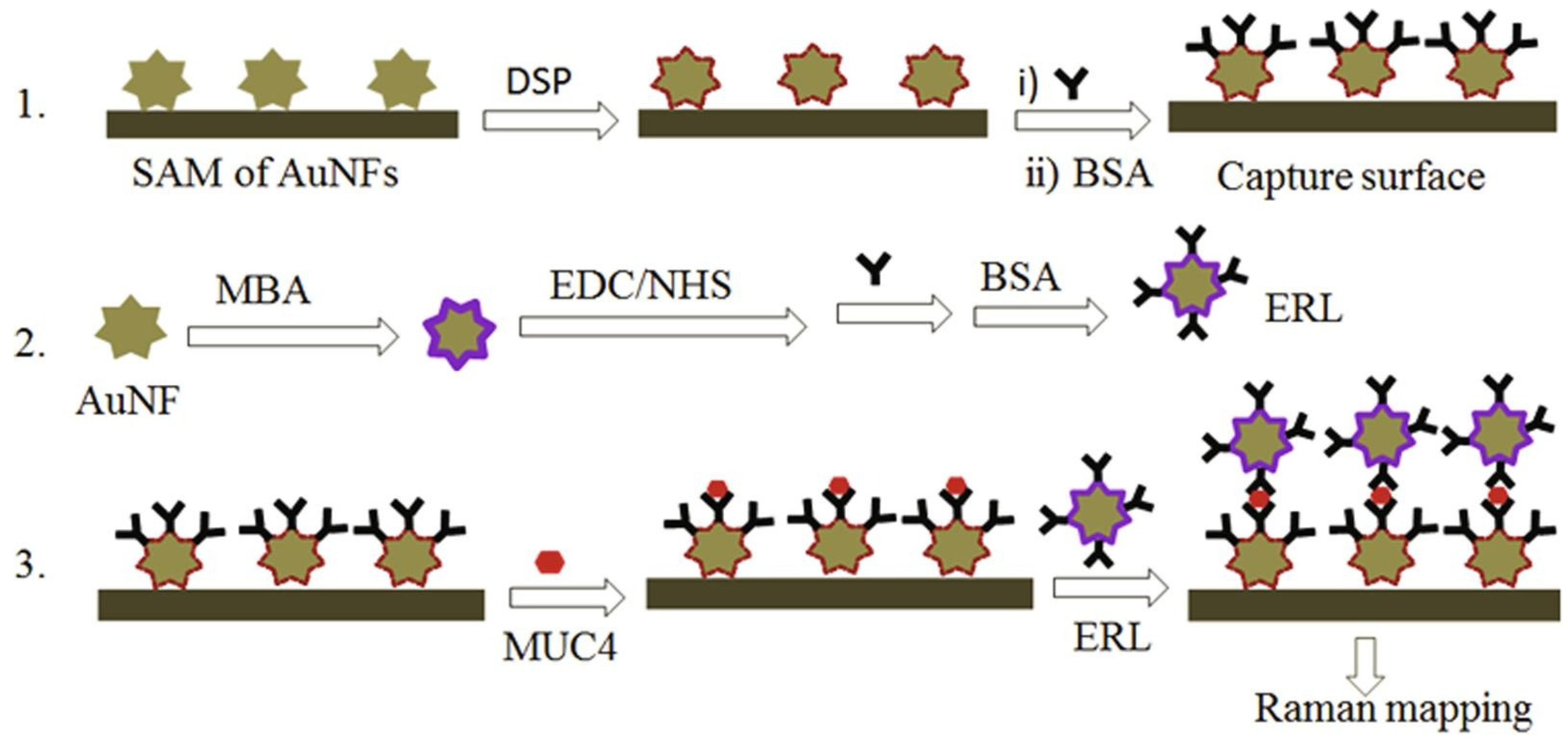
- Beyene, A.B.; Hwang, B.J.; Tegegne, W.A.; Wang, J.-S.; Tsai, H.-C.; Su, W.-N. Reliable and Sensitive Detection of Pancreatic Cancer Marker by Gold Nanoflower-Based SERS Mapping Immunoassay. Microchem. J. 2020, 158, 105099. [Google Scholar] [CrossRef]
- Verdin, A.; Malherbe, C.; Müller, W.H.; Bertrand, V.; Eppe, G. Multiplex Micro-SERS Imaging of Cancer-Related Markers in Cells and Tissues Using Poly(Allylamine)-Coated Au@Ag Nanoprobes. Anal. Bioanal. Chem. 2020, 412, 7739–7755. [Google Scholar] [CrossRef] [PubMed]
- Fălămaș, A.; Rotaru, H.; Hedeșiu, M. Surface-Enhanced Raman Spectroscopy (SERS) Investigations of Saliva for Oral Cancer Diagnosis. Lasers Med. Sci. 2020, 35, 1393–1401. [Google Scholar] [CrossRef]
- Bamrungsap, S.; Treetong, A.; Apiwat, C.; Wuttikhun, T.; Dharakul, T. SERS-Fluorescence Dual Mode Nanotags for Cervical Cancer Detection Using Aptamers Conjugated to Gold-Silver Nanorods. Microchim. Acta 2016, 183, 249–256. [Google Scholar] [CrossRef]
- Wang, X.-P.; Walkenfort, B.; König, M.; König, L.; Kasimir-Bauer, S.; Schlücker, S. Fast and Reproducible iSERS Microscopy of Single HER2-Positive Breast Cancer Cells Using Gold Nanostars as SERS Nanotags. Faraday Discuss. 2017, 205, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Eom, G.; Hwang, A.; Kim, H.; Moon, J.; Kang, H.; Jung, J.; Lim, E.-K.; Jeong, J.; Park, H.G.; Kang, T. Ultrasensitive Detection of Ovarian Cancer Biomarker Using Au Nanoplate SERS Immunoassay. BioChip J. 2021, 15, 348–355. [Google Scholar] [CrossRef]
- Hu, C.; Shen, J.; Yan, J.; Zhong, J.; Qin, W.; Liu, R.; Aldalbahi, A.; Zuo, X.; Song, S.; Fan, C.; et al. Highly Narrow Nanogap-Containing Au@Au Core–Shell SERS Nanoparticles: Size-Dependent Raman Enhancement and Applications in Cancer Cell Imaging. Nanoscale 2016, 8, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-H.; Pan, M.-J.; Jargalsaikhan, Z.; Ishdorj, T.-O.; Tseng, F.-G. Development of Surface-Enhanced Raman Scattering (SERS)-Based Surface-Corrugated Nanopillars for Biomolecular Detection of Colorectal Cancer. Biosensors 2020, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Omar, G.; Abd Ellah, R.G.; Elzayat, M.M.Y.; Afifi, G.; Imam, H. Superior Removal of Hazardous Dye Using Ag/Au Core–Shell Nanoparticles Prepared by Laser Ablation. Opt. Laser Technol. 2024, 168, 109868. [Google Scholar] [CrossRef]
- Awiaz, G.; Lin, J.; Wu, A. Recent Advances of Au@Ag Core–Shell SERS-Based Biosensors. Exploration 2023, 3, 20220072. [Google Scholar] [CrossRef]
- Dinish, U.S.; Balasundaram, G.; Chang, Y.-T.; Olivo, M. Actively Targeted In Vivo Multiplex Detection of Intrinsic Cancer Biomarkers Using Biocompatible SERS Nanotags. Sci. Rep. 2014, 4, 4075. [Google Scholar] [CrossRef]
- Banaei, N.; Foley, A.; Houghton, J.M.; Sun, Y.; Kim, B. Multiplex Detection of Pancreatic Cancer Biomarkers Using a SERS-Based Immunoassay. Nanotechnology 2017, 28, 455101. [Google Scholar] [CrossRef]
- Lyu, N.; Rajendran, V.K.; Diefenbach, R.J.; Charles, K.; Clarke, S.J.; Engel, A.; Rizos, H.; Molloy, M.P.; Wang, Y. Multiplex Detection of ctDNA Mutations in Plasma of Colorectal Cancer Patients by PCR/SERS Assay. Nanotheranostics 2020, 4, 224–232. [Google Scholar] [CrossRef]
- Saranya, G.; Joseph, M.M.; Karunakaran, V.; Nair, J.B.; Saritha, V.N.; Veena, V.S.; Sujathan, K.; Ajayaghosh, A.; Maiti, K.K. Enzyme-Driven Switchable Fluorescence-SERS Diagnostic Nanococktail for the Multiplex Detection of Lung Cancer Biomarkers. ACS Appl. Mater. Interfaces 2018, 10, 38807–38818. [Google Scholar] [CrossRef] [PubMed]
- Plou, J.; García, I.; Charconnet, M.; Astobiza, I.; García-Astrain, C.; Matricardi, C.; Mihi, A.; Carracedo, A.; Liz-Marzán, L.M. Multiplex SERS Detection of Metabolic Alterations in Tumor Extracellular Media. Adv. Funct. Mater. 2020, 30, 1910335. [Google Scholar] [CrossRef]
- Lee, S.; Chon, H.; Yoon, S.-Y.; Kyu Lee, E.; Chang, S.-I.; Woo Lim, D.; Choo, J. Fabrication of SERS-Fluorescence Dual Modal Nanoprobes and Application to Multiplex Cancer Cell Imaging. Nanoscale 2012, 4, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tabakman, S.M.; Goodwin, A.P.; Kattah, M.G.; Daranciang, D.; Wang, X.; Zhang, G.; Li, X.; Liu, Z.; Utz, P.J.; et al. Protein Microarrays with Carbon Nanotubes as Multicolor Raman Labels. Nat. Biotechnol. 2008, 26, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Reza, K.K.; Dey, S.; Wuethrich, A.; Sina, A.A.I.; Korbie, D.; Wang, Y.; Trau, M. Parallel Profiling of Cancer Cells and Proteins Using a Graphene Oxide Functionalized Ac-EHD SERS Immunoassay. Nanoscale 2018, 10, 18482–18491. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.K.M.R.H.; Tan, B.; Venkatakrishnan, K. SERS-Active 3D Interconnected Nanocarbon Web toward Nonplasmonic in Vitro Sensing of HeLa Cells and Fibroblasts. ACS Appl. Mater. Interfaces 2018, 10, 35715–35733. [Google Scholar] [CrossRef] [PubMed]
- Banaei, N.; Moshfegh, J.; Mohseni-Kabir, A.; Marie Houghton, J.; Sun, Y.; Kim, B. Machine Learning Algorithms Enhance the Specificity of Cancer Biomarker Detection Using SERS-Based Immunoassays in Microfluidic Chips. RSC Adv. 2019, 9, 1859–1868. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, J.; Liu, Y.; Han, X.X.; Xu, B.; Ozaki, Y.; Zhao, B. Detection of Prostate Cancer Biomarkers via a SERS-Based Aptasensor. Biosens. Bioelectron. 2022, 216, 114660. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, Y.; Zhu, W.; Xie, D.; Zhao, Y.; Yang, D.; Li, M. Ultrasensitive Detection of Circulating Tumor DNA of Lung Cancer via an Enzymatically Amplified SERS-Based Frequency Shift Assay. ACS Appl. Mater. Interfaces 2019, 11, 18145–18152. [Google Scholar] [CrossRef]
- Wang, J.; Liang, D.; Jin, Q.; Feng, J.; Tang, X. Bioorthogonal SERS Nanotags as a Precision Theranostic Platform for in Vivo SERS Imaging and Cancer Photothermal Therapy. Bioconjugate Chem. 2020, 31, 182–193. [Google Scholar] [CrossRef]
- Li, M.; Ray Banerjee, S.; Zheng, C.; Pomper, M.G.; Barman, I. Ultrahigh Affinity Raman Probe for Targeted Live Cell Imaging of Prostate Cancer. Chem. Sci. 2016, 7, 6779–6785. [Google Scholar] [CrossRef]
- Murali, V.P.; Karunakaran, V.; Murali, M.; Lekshmi, A.; Kottarathil, S.; Deepika, S.; Saritha, V.N.; Ramya, A.N.; Raghu, K.G.; Sujathan, K.; et al. A Clinically Feasible Diagnostic Spectro-Histology Built on SERS-Nanotags for Multiplex Detection and Grading of Breast Cancer Biomarkers. Biosens. Bioelectron. 2023, 227, 115177. [Google Scholar] [CrossRef] [PubMed]
- Mallia, R.J.; McVeigh, P.Z.; Fisher, C.J.; Veilleux, I.; Wilson, B.C. Wide-Field Multiplexed Imaging of EGFR-Targeted Cancers Using Topical Application of NIR SERS Nanoprobes. Nanomedicine 2015, 10, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Cheng, Z.; deMello, A.J.; Choo, J. Wash-Free Magnetic Immunoassay of the PSA Cancer Marker Using SERS and Droplet Microfluidics. Lab Chip 2016, 16, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Ngo, L.; Pham, L.Q.A.; Tukova, A.; Hassanzadeh-Barforoushi, A.; Zhang, W.; Wang, Y. Emerging Integrated SERS-Microfluidic Devices for Analysis of Cancer-Derived Small Extracellular Vesicles. Lab Chip 2023, 23, 2899–2921. [Google Scholar] [CrossRef] [PubMed]
- Știufiuc, G.F.; Toma, V.; Buse, M.; Mărginean, R.; Morar-Bolba, G.; Culic, B.; Tetean, R.; Leopold, N.; Pavel, I.; Lucaciu, C.M.; et al. Solid Plasmonic Substrates for Breast Cancer Detection by Means of SERS Analysis of Blood Plasma. Nanomaterials 2020, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Romo-Herrera, J.M.; Juarez-Moreno, K.; Guerrini, L.; Kang, Y.; Feliu, N.; Parak, W.J.; Alvarez-Puebla, R.A. Paper-Based Plasmonic Substrates as Surface-Enhanced Raman Scattering Spectroscopy Platforms for Cell Culture Applications. Mater. Today Bio 2021, 11, 100125. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Garcia-Rico, E.; O’Loghlen, A.; Giannini, V.; Alvarez-Puebla, R.A. Surface-Enhanced Raman Scattering (SERS) Spectroscopy for Sensing and Characterization of Exosomes in Cancer Diagnosis. Cancers 2021, 13, 2179. [Google Scholar] [CrossRef]
- Wang, Z.; Zong, S.; Wang, Y.; Li, N.; Li, L.; Lu, J.; Wang, Z.; Chen, B.; Cui, Y. Screening and Multiple Detection of Cancer Exosomes Using an SERS-Based Method. Nanoscale 2018, 10, 9053–9062. [Google Scholar] [CrossRef]
- Xie, Y.; Su, X.; Wen, Y.; Zheng, C.; Li, M. Artificial Intelligent Label-Free SERS Profiling of Serum Exosomes for Breast Cancer Diagnosis and Postoperative Assessment. Nano Lett. 2022, 22, 7910–7918. [Google Scholar] [CrossRef]
- Moisoiu, T.; Dragomir, M.P.; Iancu, S.D.; Schallenberg, S.; Birolo, G.; Ferrero, G.; Burghelea, D.; Stefancu, A.; Cozan, R.G.; Licarete, E.; et al. Combined miRNA and SERS Urine Liquid Biopsy for the Point-of-Care Diagnosis and Molecular Stratification of Bladder Cancer. Mol. Med. 2022, 28, 39. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, J.; Li, Y.; Wang, B.; Yang, S.; Chen, W.; Wu, X.; Guo, J.; Ma, X. SERS Substrate Fabrication for Biochemical Sensing: Towards Point-of-Care Diagnostics. J. Mater. Chem. B 2021, 9, 8378–8388. [Google Scholar] [CrossRef] [PubMed]
- Perumal, J.; Wang, Y.; Ebrahim Attia, A.B.; Dinish, U.S.; Olivo, M. Towards a Point-of-Care SERS Sensor for Biomedical and Agri-Food Analysis Applications: A Review of Recent Advancements. Nanoscale 2021, 13, 553–580. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Yong, K.-T.; Roy, I.; Ding, H.; He, S.; Prasad, P.N. Metallic Nanostructures as Localized Plasmon Resonance Enhanced Scattering Probes for Multiplex Dark-Field Targeted Imaging of Cancer Cells. J. Phys. Chem. C 2009, 113, 2676–2684. [Google Scholar] [CrossRef] [PubMed]
- El-Safadi, S.; Tinneberg, H.-R.; von Georgi, R.; Münstedt, K.; Brück, F. Does Dark Field Microscopy According to Enderlein Allow for Cancer Diagnosis? A Prospective Study. Forsch. Komplementarmedizin Klass. Naturheilkunde = Res. Complement. Nat. Class. Med. 2005, 12, 148–151. [Google Scholar]
- Gao, P.F.; Lei, G.; Huang, C.Z. Dark-Field Microscopy: Recent Advances in Accurate Analysis and Emerging Applications. Anal. Chem. 2021, 93, 4707–4726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, Q.; Dou, Z.; Ning, S.; Wang, Q.; Dong, Y.; Yue, Z.; Ye, W.; Liu, G. Ultrasensitive Detection of Lead (II) Ion by Dark-Field Spectroscopy and Glutathione Modified Gold Nanoparticles. Sens. Actuators B Chem. 2020, 321, 128548. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, F.; Hu, Y.; Zheng, X.; Cao, X.; Zhu, Y.; Zhang, X.; Li, D.; Zhang, Z.; Chen, S. Activated Plasmonic Nanoaggregates for Dark-Field in Situ Imaging for HER2 Protein Imaging on Cell Surfaces. Bioconjugate Chem. 2020, 31, 631–638. [Google Scholar] [CrossRef]
- Liu, M.L.; Zou, H.Y.; Li, C.M.; Li, R.S.; Huang, C.Z. Aptamer-Modified Selenium Nanoparticles for Dark-Field Microscopy Imaging of Nucleolin. Chem. Commun. 2017, 53, 13047–13050. [Google Scholar] [CrossRef]
- Bhat, A.; Huan, K.; Cooks, T.; Boukari, H.; Lu, Q. Probing Interactions between AuNPs/AgNPs and Giant Unilamellar Vesicles (GUVs) Using Hyperspectral Dark-Field Microscopy. Int. J. Mol. Sci. 2018, 19, 1014. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, X.; Ren, W.; Liu, J.; Irudayaraj, J. Optical Clearing Delivers Ultrasensitive Hyperspectral Dark-Field Imaging for Single-Cell Evaluation. ACS Nano 2016, 10, 3132–3143. [Google Scholar] [CrossRef] [PubMed]
- Crow, M.J.; Seekell, K.; Marinakos, S.; Ostrander, J.; Chilkoti, A.; Wax, A.P. Hyperspectral Molecular Imaging of Multiple Receptors Using Immunolabeled Plasmonic Nanoparticles. In Proceedings of the SPIE Conference, Plasmonics in Biology and Medicine VIII, San Francisco, CA, USA, 22–27 January 2011; Volume 7911, pp. 105–114. [Google Scholar]
- Akagi, T.; Hanamura, N.; Ichiki, T. Measurement of Individual Nanobioparticles on Microfluidic Chips by Laser Dark-Field Imaging. J. Photopolym. Sci. Technol. 2015, 28, 727–730. [Google Scholar] [CrossRef]
- Yang, Q.; Cheng, L.; Hu, L.; Lou, D.; Zhang, T.; Li, J.; Zhu, Q.; Liu, F. An Integrative Microfluidic Device for Isolation and Ultrasensitive Detection of Lung Cancer-Specific Exosomes from Patient Urine. Biosens. Bioelectron. 2020, 163, 112290. [Google Scholar] [CrossRef] [PubMed]
- Sikes, J.C.; Wonner, K.; Nicholson, A.; Cignoni, P.; Fritsch, I.; Tschulik, K. Characterization of Nanoparticles in Diverse Mixtures Using Localized Surface Plasmon Resonance and Nanoparticle Tracking by Dark-Field Microscopy with Redox Magnetohydrodynamics Microfluidics. ACS Phys. Chem. Au 2022, 2, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Hu, T.Y. A Low Cost Mobile Phone Dark-Field Microscope for Nanoparticle-Based Quantitative Studies. Biosens. Bioelectron. 2018, 99, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Nagy-Simon, T.; Tatar, A.-S.; Craciun, A.-M.; Vulpoi, A.; Jurj, M.-A.; Florea, A.; Tomuleasa, C.; Berindan-Neagoe, I.; Astilean, S.; Boca, S. Antibody Conjugated, Raman Tagged Hollow Gold–Silver Nanospheres for Specific Targeting and Multimodal Dark-Field/SERS/Two Photon-FLIM Imaging of CD19(+) B Lymphoblasts. ACS Appl. Mater. Interfaces 2017, 9, 21155–21168. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Zhu, C.; Han, L.; Liao, X.; Wang, C. SERS and Dark-Field Scattering Dual-Mode Detection of Intracellular Hydrogen Peroxide Using Biocompatible Au@COF Nanosensor. Sens. Actuators B Chem. 2022, 373, 132770. [Google Scholar] [CrossRef]
- Ip, S.; MacLaughlin, C.M.; Joseph, M.; Mullaithilaga, N.; Yang, G.; Wang, C.; Walker, G.C. Dual-Mode Dark Field and Surface-Enhanced Raman Scattering Liposomes for Lymphoma and Leukemia Cell Imaging. Langmuir 2019, 35, 1534–1543. [Google Scholar] [CrossRef]
- Boyer, D.; Tamarat, P.; Maali, A.; Lounis, B.; Orrit, M. Photothermal Imaging of Nanometer-Sized Metal Particles Among Scatterers. Science 2002, 297, 1160–1163. [Google Scholar] [CrossRef]
- He, S.; Song, J.; Qu, J.; Cheng, Z. Crucial Breakthrough of Second Near-Infrared Biological Window Fluorophores: Design and Synthesis toward Multimodal Imaging and Theranostics. Chem. Soc. Rev. 2018, 47, 4258–4278. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.-H.; Nam, J.-M. Plasmonic Photothermal Nanoparticles for Biomedical Applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Qu, J.; Swihart, M.T.; Prasad, P.N. Near-IR Responsive Nanostructures for Nanobiophotonics: Emerging Impacts on Nanomedicine. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Thummerer, G.; Mayr, G.; Haltmeier, M.; Burgholzer, P. Photoacoustic Reconstruction from Photothermal Measurements Including Prior Information. Photoacoustics 2020, 19, 100175. [Google Scholar] [CrossRef] [PubMed]
- Skala, M.C.; Crow, M.J.; Wax, A.; Izatt, J.A. Photothermal Optical Coherence Tomography of Epidermal Growth Factor Receptor in Live Cells Using Immunotargeted Gold Nanospheres. Nano Lett. 2008, 8, 3461–3467. [Google Scholar] [CrossRef] [PubMed]
- Lapierre-Landry, M.; Gordon, A.Y.; Penn, J.S.; Skala, M.C. In Vivo Photothermal Optical Coherence Tomography of Endogenous and Exogenous Contrast Agents in the Eye. Sci. Rep. 2017, 7, 9228. [Google Scholar] [CrossRef] [PubMed]
- Tucker-Schwartz, J.M.; Meyer, T.A.; Patil, C.A.; Duvall, C.L.; Skala, M.C. In Vivo Photothermal Optical Coherence Tomography of Gold Nanorod Contrast Agents. Biomed. Opt. Express 2012, 3, 2881–2895. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, D.; Wu, M.; Liu, Y.; Zhang, X.; Li, L.; Li, Z.; Han, X.; Wei, X.; Liu, X. Lipid-AuNPs@PDA Nanohybrid for MRI/CT Imaging and Photothermal Therapy of Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2014, 6, 14266–14277. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, Y.; Shen, J.; Wei, J.; Yu, C.; Kong, B.; Liu, W.; Yang, H.; Yang, S.; Wang, W. Iron/Iron Oxide Core/Shell Nanoparticles for Magnetic Targeting MRI and near-Infrared Photothermal Therapy. Biomaterials 2014, 35, 7470–7478. [Google Scholar] [CrossRef]
- MacDonald, T.D.; Liu, T.W.; Zheng, G. An MRI-Sensitive, Non-Photobleachable Porphysome Photothermal Agent. Angew. Chem. 2014, 126, 7076–7079. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Thompson, L.B.; Boulos, S.P.; Sisco, P.N.; Murphy, C.J. Gold Nanorods: Their Potential for Photothermal Therapeutics and Drug Delivery, Tempered by the Complexity of Their Biological Interactions. Adv. Drug Deliv. Rev. 2012, 64, 190–199. [Google Scholar] [CrossRef]
- Choi, W.I.; Sahu, A.; Kim, Y.H.; Tae, G. Photothermal Cancer Therapy and Imaging Based on Gold Nanorods. Ann. Biomed. Eng. 2012, 40, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Khoury, C.G.; Wilson, C.M.; Grant, G.A.; Bennett, A.J.; Vo-Dinh, T. In Vivo Particle Tracking and Photothermal Ablation Using Plasmon-Resonant Gold Nanostars. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, G.; Ding, Y.; Sun, S. Understanding the Photothermal Effect of Gold Nanostars and Nanorods for Biomedical Applications. RSC Adv. 2014, 4, 30375–30383. [Google Scholar] [CrossRef]
- Pérez-Hernández, M.; del Pino, P.; Mitchell, S.G.; Moros, M.; Stepien, G.; Pelaz, B.; Parak, W.J.; Gálvez, E.M.; Pardo, J.; de la Fuente, J.M. Dissecting the Molecular Mechanism of Apoptosis during Photothermal Therapy Using Gold Nanoprisms. ACS Nano 2015, 9, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Moros, M.; Lewinska, A.; Merola, F.; Ferraro, P.; Wnuk, M.; Tino, A.; Tortiglione, C. Gold Nanorods and Nanoprisms Mediate Different Photothermal Cell Death Mechanisms In Vitro and In Vivo. ACS Appl. Mater. Interfaces 2020, 12, 13718–13730. [Google Scholar] [CrossRef] [PubMed]
- Ambrosone, A.; del Pino, P.; Marchesano, V.; Parak, W.J.; de la Fuente, J.M.; Tortiglione, C. Gold Nanoprisms for Photothermal Cell Ablation In Vivo. Nanomedicine 2014, 9, 1913–1922. [Google Scholar] [CrossRef]
- Chen, M.; Tang, S.; Guo, Z.; Wang, X.; Mo, S.; Huang, X.; Liu, G.; Zheng, N. Core–Shell Pd@Au Nanoplates as Theranostic Agents for In-Vivo Photoacoustic Imaging, CT Imaging, and Photothermal Therapy. Adv. Mater. 2014, 26, 8210–8216. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Liu, L.; Cui, Z.; Liu, X.; Wang, L.; Li, Y.; Li, Q. Combined Chemo/Photothermal Therapy Based on Mesoporous Silica-Au Core-Shell Nanoparticles for Hepatocellular Carcinoma Treatment. Drug Dev. Ind. Pharm. 2019, 45, 1487–1495. [Google Scholar] [CrossRef]
- Dai, X.; Li, X.; Du, Y.; Han, M.; Wang, Z.; Wang, Y.; Yan, F.; Liu, Y. Gold Nanorod–Mesoporous Silica Core Shell Nanocomposites for NIR-II Photothermal Ablation and Dual PD-L1/VEGF Blockade Therapy in Hepatocellular Carcinoma. Chem. Eng. J. 2023, 459, 141426. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Yang, J.; Wei, P.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Hyaluronic Acid-Modified Fe3O4@Au Core/Shell Nanostars for Multimodal Imaging and Photothermal Therapy of Tumors. Biomaterials 2015, 38, 10–21. [Google Scholar] [CrossRef]
- Hu, R.; Zheng, M.; Wu, J.; Li, C.; Shen, D.; Yang, D.; Li, L.; Ge, M.; Chang, Z.; Dong, W. Core-Shell Magnetic Gold Nanoparticles for Magnetic Field-Enhanced Radio-Photothermal Therapy in Cervical Cancer. Nanomaterials 2017, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Xu, M.; Zhang, W.; Yang, Z.; Huang, L.; Liu, J.; Zhang, Y.; Gu, L.; Yu, Y.; Hao, W.; et al. Structurally Well-Defined Au@Cu2−xS Core–Shell Nanocrystals for Improved Cancer Treatment Based on Enhanced Photothermal Efficiency. Adv. Mater. 2016, 28, 3094–3101. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic Photothermal Therapy (PPTT) Using Gold Nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Austin, L.A.; Mackey, M.A.; Dreaden, E.C.; El-Sayed, M.A. The Optical, Photothermal, and Facile Surface Chemical Properties of Gold and Silver Nanoparticles in Biodiagnostics, Therapy, and Drug Delivery. Arch. Toxicol. 2014, 88, 1391–1417. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Lu, W.; Senapati, D.; Khan, S.A.; Fan, Z.; Senapati, T.; Demeritte, T.; Beqa, L.; Ray, P.C. Long-Range Nanoparticle Surface-Energy-Transfer Ruler for Monitoring Photothermal Therapy Response. Small 2011, 7, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Shelton, M.; Singh, A.K.; Senapati, D.; Khan, S.A.; Ray, P.C. Multifunctional Plasmonic Shell–Magnetic Core Nanoparticles for Targeted Diagnostics, Isolation, and Photothermal Destruction of Tumor Cells. ACS Nano 2012, 6, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Shipunova, V.O.; Belova, M.M.; Kotelnikova, P.A.; Shilova, O.N.; Mirkasymov, A.B.; Danilova, N.V.; Komedchikova, E.N.; Popovtzer, R.; Deyev, S.M.; Nikitin, M.P. Photothermal Therapy with HER2-Targeted Silver Nanoparticles Leading to Cancer Remission. Pharmaceutics 2022, 14, 1013. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, Y.; Wang, L.; Zhang, Q.; Cheng, Y. Melanin-like Nanoparticles Decorated with an Autophagy-Inducing Peptide for Efficient Targeted Photothermal Therapy. Biomaterials 2019, 203, 63–72. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, P.; Luo, Z.; Zheng, M.; Tian, H.; Gong, P.; Gao, G.; Pan, H.; Liu, L.; Ma, A.; et al. Cancer Cell Membrane–Biomimetic Nanoparticles for Homologous-Targeting Dual-Modal Imaging and Photothermal Therapy. ACS Nano 2016, 10, 10049–10057. [Google Scholar] [CrossRef]
- Pu, Y.; Wu, W.; Zhou, B.; Xiang, H.; Yu, J.; Yin, H.; Zhang, Y.; Du, D.; Chen, Y.; Xu, H. Starvation Therapy Enabled “Switch-on” NIR-II Photothermal Nanoagent for Synergistic in Situ Photothermal Immunotherapy. Nano Today 2022, 44, 101461. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, J.; Fu, Y.; Yang, Y.; Chen, M.; Zhang, Q. Near-Infrared Light-Triggered Nanobomb for in Situ on-Demand Maximization of Photothermal/Photodynamic Efficacy for Cancer Therapy. Biomater. Sci. 2021, 9, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Jakobsohn, K.; Motiei, M.; Sinvani, M.; Popovtzer, R. Towards Real-Time Detection of Tumor Margins Using Photothermal Imaging of Immune-Targeted Gold Nanoparticles. Int. J. Nanomed. 2012, 7, 4707–4713. [Google Scholar] [CrossRef]
- Nedosekin, D.A.; Juratli, M.A.; Sarimollaoglu, M.; Moore, C.L.; Rusch, N.J.; Smeltzer, M.S.; Zharov, V.P.; Galanzha, E.I. Photoacoustic and Photothermal Detection of Circulating Tumor Cells, Bacteria and Nanoparticles in Cerebrospinal Fluid In Vivo and Ex Vivo. J. Biophotonics 2013, 6, 523–533. [Google Scholar] [CrossRef] [PubMed]
- von Maltzahn, G.; Park, J.-H.; Agrawal, A.; Bandaru, N.K.; Das, S.K.; Sailor, M.J.; Bhatia, S.N. Computationally Guided Photothermal Tumor Therapy Using Long-Circulating Gold Nanorod Antennas. Cancer Res. 2009, 69, 3892–3900. [Google Scholar] [CrossRef]
- Lim, C.-K.; Shin, J.; Lee, Y.-D.; Kim, J.; Oh, K.S.; Yuk, S.H.; Jeong, S.Y.; Kwon, I.C.; Kim, S. Phthalocyanine-Aggregated Polymeric Nanoparticles as Tumor-Homing Near-Infrared Absorbers for Photothermal Therapy of Cancer. Theranostics 2012, 2, 871–879. [Google Scholar] [CrossRef]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.-S. Nanotheranostics ˗ Application and Further Development of Nanomedicine Strategies for Advanced Theranostics. Theranostics 2014, 4, 660–677. [Google Scholar] [CrossRef]
- Okuno, T.; Kato, S.; Hatakeyama, Y.; Okajima, J.; Maruyama, S.; Sakamoto, M.; Mori, S.; Kodama, T. Photothermal Therapy of Tumors in Lymph Nodes Using Gold Nanorods and Near-Infrared Laser Light. J. Control. Release 2013, 172, 879–884. [Google Scholar] [CrossRef]
- Ong, S.Y.; Zhang, C.; Dong, X.; Yao, S.Q. Recent Advances in Polymeric Nanoparticles for Enhanced Fluorescence and Photoacoustic Imaging. Angew. Chem. Int. Ed. 2021, 60, 17797–17809. [Google Scholar] [CrossRef]
- Zhen, X.; Pu, K.; Jiang, X. Photoacoustic Imaging and Photothermal Therapy of Semiconducting Polymer Nanoparticles: Signal Amplification and Second Near-Infrared Construction. Small 2021, 17, 2004723. [Google Scholar] [CrossRef]
- Mantri, Y.; Jokerst, J.V. Engineering Plasmonic Nanoparticles for Enhanced Photoacoustic Imaging. ACS Nano 2020, 14, 9408–9422. [Google Scholar] [CrossRef]
- Manohar, S.; Ungureanu, C.; Van Leeuwen, T.G. Gold Nanorods as Molecular Contrast Agents in Photoacoustic Imaging: The Promises and the Caveats. Contrast Media Mol. Imaging 2011, 6, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Yang, L.; Lin, Y.; Tian, Y.; Ni, Q.; Wang, S.; Ju, H.; Guo, J.; Lu, G. Gold Nanostar@Polyaniline Theranostic Agent with High Photothermal Conversion Efficiency for Photoacoustic Imaging-Guided Anticancer Phototherapy at a Low Dosage. ACS Appl. Mater. Interfaces 2022, 14, 28570–28580. [Google Scholar] [CrossRef] [PubMed]
- Manuel, L.D.B.; Vincely, V.D.; Bayer, C.L.; McPeak, K.M. Monodisperse Sub-100 Nm Au Nanoshells for Low-Fluence Deep-Tissue Photoacoustic Imaging. Nano Lett. 2023, 23, 7334–7340. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, C.; Cheng, Y.; Cheng, Q. Photostability Enhancement of Silica-Coated Gold Nanostars for Photoacoustic Imaging Guided Photothermal Therapy. Photoacoustics 2021, 23, 100284. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Frey, W.; Kim, S.; Kruizinga, P.; Homan, K.; Emelianov, S. Silica-Coated Gold Nanorods as Photoacoustic Signal Nanoamplifiers. Nano Lett. 2011, 11, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Frey, W.; Kim, S.; Homan, K.; Kruizinga, P.; Sokolov, K.; Emelianov, S. Enhanced Thermal Stability of Silica-Coated Gold Nanorods for Photoacoustic Imaging and Image-Guided Therapy. Opt. Express 2010, 18, 8867–8878. [Google Scholar] [CrossRef] [PubMed]
- Galanzha, E.I.; Shashkov, E.V.; Kelly, T.; Kim, J.-W.; Yang, L.; Zharov, V.P. In Vivo Magnetic Enrichment and Multiplex Photoacoustic Detection of Circulating Tumour Cells. Nat. Nanotechol. 2009, 4, 855–860. [Google Scholar] [CrossRef]
- Santiesteban, D.Y.; Hallam, K.A.; Yarmoska, S.K.; Emelianov, S.Y. Color-Coded Perfluorocarbon Nanodroplets for Multiplexed Ultrasound and Photoacoustic Imaging. Nano Res. 2019, 12, 741–747. [Google Scholar] [CrossRef]
- Luke, G.P.; Myers, J.N.; Emelianov, S.Y.; Sokolov, K.V. Sentinel Lymph Node Biopsy Revisited: Ultrasound-Guided Photoacoustic Detection of Micrometastases Using Molecularly Targeted Plasmonic Nanosensors. Cancer Res. 2014, 74, 5397–5408. [Google Scholar] [CrossRef]
- Liu, X.; Duan, Y.; Liu, B. Nanoparticles as Contrast Agents for Photoacoustic Brain Imaging. Aggregate 2021, 2, 4–19. [Google Scholar] [CrossRef]
- Li, H.; Shi, S.; Wu, M.; Shen, W.; Ren, J.; Mei, Z.; Ran, H.; Wang, Z.; Tian, Y.; Gao, J.; et al. iRGD Peptide-Mediated Liposomal Nanoparticles with Photoacoustic/Ultrasound Dual-Modality Imaging for Precision Theranostics Against Hepatocellular Carcinoma. Int. J. Nanomed. 2021, 16, 6455–6475. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zeng, S.; Xue, Q.; Hong, Y.; Liu, L.; Song, L.; Fang, C.; Zhang, H.; Wang, B.; Sedgwick, A.C.; et al. Photoacoustic Image-Guided Biomimetic Nanoparticles Targeting Rheumatoid Arthritis. Proc. Natl. Acad. Sci. USA 2022, 119, e2213373119. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Jiang, D.; Lan, H.; Duan, T.; Gao, F.; Gao, F. Low-Cost Multi-Wavelength Photoacoustic Imaging Based on Portable Continuous-Wave Laser Diode Module. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sanders, J.L.; Dundar, M.M.; Oralkan, Ö. Deep-Learning-Based High-Intensity Focused Ultrasound Lesion Segmentation in Multi-Wavelength Photoacoustic Imaging. Bioengineering 2023, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Guo, L.; Chen, S.; Yu, Y.-L.; Wang, J.-H. A Portable Photoacoustic Device for Facile and Sensitive Detection of Serum Alkaline Phosphatase Activity. Anal. Chim. Acta 2020, 1108, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, S.; Wang, T.; Zhang, C.; Feng, T.; Tian, C. Three-Dimensional Interventional Photoacoustic Imaging for Biopsy Needle Guidance with a Linear Array Transducer. J. Biophotonics 2019, 12, e201900212. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; West, S.J.; Nikitichev, D.I.; Ourselin, S.; Beard, P.C.; Desjardins, A.E. Interventional Multispectral Photoacoustic Imaging with a Clinical Linear Array Ultrasound Probe for Guiding Nerve Blocks. In Proceedings of the SPIE Conference, Photons Plus Ultrasound: Imaging and Sensing 2016, San Francisco, CA, USA, 13–18 February 2016; Volume 9708, pp. 87–92. [Google Scholar]
- Liu, R.; Jing, L.; Peng, D.; Li, Y.; Tian, J.; Dai, Z. Manganese (II) Chelate Functionalized Copper Sulfide Nanoparticles for Efficient Magnetic Resonance/Photoacoustic Dual-Modal Imaging Guided Photothermal Therapy. Theranostics 2015, 5, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Iwakuma, N.; Sharma, P.; Moudgil, B.M.; Wu, C.; McNeill, J.; Jiang, H.; Grobmyer, S.R. Gold Nanoparticles as a Contrast Agent for In Vivo Tumor Imaging with Photoacoustic Tomography. Nanotechnology 2009, 20, 395102. [Google Scholar] [CrossRef]
- Chen, J.; Nguyen, V.P.; Jaiswal, S.; Kang, X.; Lee, M.; Paulus, Y.M.; Wang, T.D. Thin Layer-Protected Gold Nanoparticles for Targeted Multimodal Imaging with Photoacoustic and CT. Pharmaceuticals 2021, 14, 1075. [Google Scholar] [CrossRef]
- Jo, J.; Lee, C.H.; Kopelman, R.; Wang, X. In Vivo Quantitative Imaging of Tumor pH by Nanosonophore Assisted Multispectral Photoacoustic Imaging. Nat. Commun. 2017, 8, 471. [Google Scholar] [CrossRef]
- Haisch, C. Raman-Based Microarray Readout: A Review. Anal. Bioanal. Chem. 2016, 408, 4535–4545. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Yaraki, M.T.; Wang, Y. Enabling Spectral Barcoding of SERS Nanotags Using Gold Nanostars. Mol. Syst. Des. Eng. 2023, 8, 251–260. [Google Scholar] [CrossRef]
- Xiao, R.; Lu, L.; Rong, Z.; Wang, C.; Peng, Y.; Wang, F.; Wang, J.; Sun, M.; Dong, J.; Wang, D.; et al. Portable and Multiplexed Lateral Flow Immunoassay Reader Based on SERS for Highly Sensitive Point-of-Care Testing. Biosens. Bioelectron. 2020, 168, 112524. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, T.; Li, C.S.; Song, Y.; Lou, H.; Guan, D.; Jin, L. Surface Enhanced Raman Spectroscopy (SERS) for the Multiplex Detection of Braf, Kras, and Pik3ca Mutations in Plasma of Colorectal Cancer Patients. Theranostics 2018, 8, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Nyayapathi, N.; Xia, J. Photoacoustic Imaging of Breast Cancer: A Mini Review of System Design and Image Features. J. Biomed. Opt. 2019, 24, 121911. [Google Scholar] [CrossRef] [PubMed]
- García-Hernández, L.A.; Martínez-Martínez, E.; Pazos-Solís, D.; Aguado-Preciado, J.; Dutt, A.; Chávez-Ramírez, A.U.; Korgel, B.; Sharma, A.; Oza, G. Optical Detection of Cancer Cells Using Lab-on-a-Chip. Biosensors 2023, 13, 439. [Google Scholar] [CrossRef] [PubMed]
- Marquez, S.; Morales-Narváez, E. Nanoplasmonics in Paper-Based Analytical Devices. Front. Bioeng. Biotechnol. 2019, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Chen, G.; Deng, J.; Gu, Y.; Mao, Y.; Zhou, X.; Li, G. Multiplex Signal Amplification Strategy-Based Early-Stage Diagnosis of Parkinson’s Disease on a SERS-Enabled LoC System. Anal. Chim. Acta 2023, 1247, 340890. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Geng, Z.; Fang, W.; Lv, X.; Su, Y.; Wang, S.; Chen, H. Smartphone Biosensor System with Multi-Testing Unit Based on Localized Surface Plasmon Resonance Integrated with Microfluidics Chip. Sensors 2020, 20, 446. [Google Scholar] [CrossRef]
- Pinheiro, T.; Marques, A.C.; Carvalho, P.; Martins, R.; Fortunato, E. Paper Microfluidics and Tailored Gold Nanoparticles for Nonenzymatic, Colorimetric Multiplex Biomarker Detection. ACS Appl. Mater. Interfaces 2021, 13, 3576–3590. [Google Scholar] [CrossRef]
- He, J.-L.; Wang, D.-S.; Fan, S.-K. Opto-Microfluidic Immunosensors: From Colorimetric to Plasmonic. Micromachines 2016, 7, 29. [Google Scholar] [CrossRef]
- Cong, S.; Yuan, Y.; Chen, Z.; Hou, J.; Yang, M.; Su, Y.; Zhang, Y.; Li, L.; Li, Q.; Geng, F.; et al. Noble Metal-Comparable SERS Enhancement from Semiconducting Metal Oxides by Making Oxygen Vacancies. Nat. Commun. 2015, 6, 7800. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Yin, P.; Hu, Y.; Szydzik, C.; Khan, M.W.; Xu, K.; Thurgood, P.; Mahmood, N.; Dekiwadia, C.; Afrin, S.; et al. Highly Accurate and Label-Free Discrimination of Single Cancer Cell Using a Plasmonic Oxide-Based Nanoprobe. Biosens. Bioelectron. 2022, 198, 113814. [Google Scholar] [CrossRef]





| Term | Definition | Typical Calculation/Measurement |
|---|---|---|
| Sensitivity | The ability of a biosensor to detect small changes in the concentration of an analyte. It is expressed as the slope of the calibration curve or the ratio of the change in sensor response to the change in analyte concentration. A higher sensitivity indicates a more responsive biosensor. | Slope of the linear region of calibration curve (signal vs. analyte concentration) |
| Limit of detection (LOD) | The lowest concentration of an analyte that can be reliably detected by a biosensor. It is an important figure of merit, with a lower LOD indicating a more sensitive biosensor. | Concentration corresponding to signal equivalent to blank mean + 3 × standard deviation of blank |
| Linear detection range | Concentration range over which the biosensor response is directly proportional to the analyte concentration. Wider linear range allows for quantifying analytes over a broader concentration span. | Concentration range over which calibration curve is linear (R2~1) |
| Selectivity | The ability of a biosensor to respond specifically to the target analyte in the presence of other interfering species. A highly selective biosensor has minimal cross-reactivity with non-target analytes. | Measured by comparing the sensor response to the target analyte versus non-target analytes at the same concentration. |
| Quality factor (Q factor) | A measure of the sharpness of the resonance peak of a plasmonic nanostructure. A higher Q factor indicates a narrower resonance peak and a more sensitive response to changes in the local environment. | Q = λres/Δλ, where λres = LSPR peak wavelength, Δλ = full width at half max |
| EF | The ratio of the sensor response (e.g., Raman scattering intensity) with a plasmonic nanostructure to the response without it. A higher EF indicates greater signal amplification by the plasmonic nanostructure. | EF = Ienhanced/Ioriginal, where I = intensity of enhanced and original signals |
| Reproducibility | Variability in response when the same experiment is repeated multiple times under same conditions, expressed using relative standard deviation. Lower variability indicates more consistent performance. | Standard deviation/mean of repeated measurements under same conditions |
| Response time | Time required to achieve stable sensor response after analyte introduction. Shorter response time enables faster detection. | Time from analyte introduction to achievement of stable sensor signal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, L.; Lin, C.-T.; Karimi-Maleh, H.; Chen, F.; Zhao, S. Plasmonic Nanoparticle-Enhanced Optical Techniques for Cancer Biomarker Sensing. Biosensors 2023, 13, 977. https://doi.org/10.3390/bios13110977
Fu L, Lin C-T, Karimi-Maleh H, Chen F, Zhao S. Plasmonic Nanoparticle-Enhanced Optical Techniques for Cancer Biomarker Sensing. Biosensors. 2023; 13(11):977. https://doi.org/10.3390/bios13110977
Chicago/Turabian StyleFu, Li, Cheng-Te Lin, Hassan Karimi-Maleh, Fei Chen, and Shichao Zhao. 2023. "Plasmonic Nanoparticle-Enhanced Optical Techniques for Cancer Biomarker Sensing" Biosensors 13, no. 11: 977. https://doi.org/10.3390/bios13110977
APA StyleFu, L., Lin, C.-T., Karimi-Maleh, H., Chen, F., & Zhao, S. (2023). Plasmonic Nanoparticle-Enhanced Optical Techniques for Cancer Biomarker Sensing. Biosensors, 13(11), 977. https://doi.org/10.3390/bios13110977








