Recent Progress in Electrochemical Nano-Biosensors for Detection of Pesticides and Mycotoxins in Foods
Abstract
1. Introduction
| Mycotoxin | Fungal Sources | Health Hazards | IARC a Classification | Reference |
|---|---|---|---|---|
| Aflatoxins B1, B2, G1, G2 | Aspergillus flflavus A. parasiticus | Acutely toxic, carcinogenic, immunosuppressive, reproductive toxicity | Group 1 | [22] |
| Ochratoxin A | A. ochraceus Penicillium verrucosum A. carbonarius | Carcinogen, nephrotoxic | Group 2B | [23] |
| Fumonisins B1, B2 | Fusarium verticillioides F. proliferatum | Acutely toxic, carcinogenic, immunosuppressive, hepatotoxic, and nephrotoxic | Group 2B | [24,25,26,27] |
| Zearalenone | F. graminearum F. culmorum | Reproductive toxicity and immunosuppressive | Group 3 | [28,29] |
| Deoxynivalenol | F. graminearum F. culmorum | DON contamination of grains has been linked to human cases of fever, stomach pain, headache, vomiting, and diarrhea. | Group 3 | [30,31,32] |
| Patulin | P. expansum | Immunotoxic, neurotoxic, hepatotoxic, and nephrotoxic | Group 3 | [33,34,35] |
2. Nano-Electrochemical Biosensors for Pesticides
2.1. Metal Nanomaterials
2.1.1. Gold Nanoparticle (AuNPs)
2.1.2. Sliver Nanomaterials (AgNMs)
2.1.3. Magnetic Nanomaterials

2.1.4. Metal–Organic Framework
2.1.5. Other Metal/Metal Oxide Nanoparticles
2.2. Carbon-Based Nanomaterials
2.2.1. Carbon Nanotubes
2.2.2. Graphene and Its Derivatives
2.3. Aptamer-Based Nanoparticles
3. Nano-Electrochemical Biosensor for Mycotoxins
3.1. Metal Nanomaterials
3.1.1. Gold Nanoparticle (AuNPs)
3.1.2. Gold Nanorods (AuNRs)
3.1.3. Sliver Nanomaterials (AgNMs)
3.1.4. Bimetallic Nanomaterials
3.1.5. Magnetic Nanomaterials
3.1.6. Metal–Organic Framework
3.1.7. Other Metal/Metal Oxide Nanoparticles
3.2. Carbon-Based Nanomaterials
3.2.1. Carbon Nanotubes
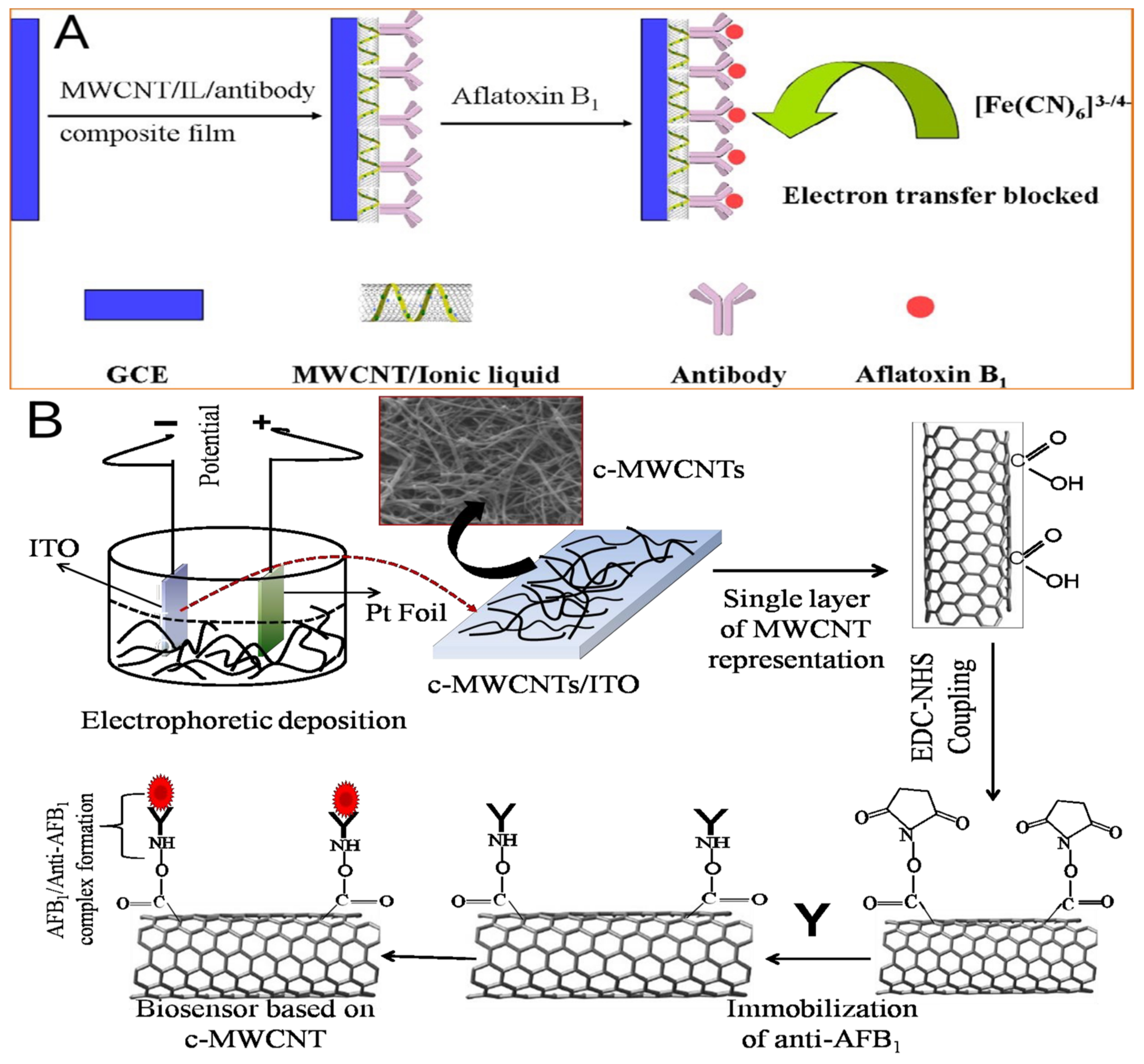
3.2.2. Graphene and Its Derivatives
3.2.3. Other Carbon Nanomaterials
3.3. Other Nanomaterials
3.3.1. Quantum Dots (QDs)
3.3.2. Black Phosphorus and Black Phosphene (BP)
4. Roles of Nanomaterials in Electrochemical Biosensor for Pesticide and Mycotoxin Detection
4.1. Immobilization of Biomolecules
4.2. Signal Generation
4.3. Signal Amplification
5. Discussion
6. Future Prospects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Full Form | Abbreviations | Full Form |
| Ab | antibody | hcPtAuNFs | hollow cubic gold-platinum nanoframes |
| AChE | acetylcholinesterase | HMDA | hexamethylenediamine |
| AF | aflatoxin | HRP | horseradish peroxidase enzyme |
| AFO | aflatoxin oxidase | IgG | immunoglobulin G |
| AgNMs | sliver nanomaterials | ILs | ionic liquids |
| AgNPs | Ag nanoparticles | IMB | immunoaffinity magnetic beads |
| AgNRs | Ag nanorods | ITO | indium tin oxide |
| AgNWs | Ag nanowires | JECFA | Joint Expert Committee on Food Additives |
| a-NP | a-naphthyl phosphate | LBL | Layer-by-layer |
| ATCI | acetylthiocholine iodide | LC-FLD | liquid chromatography coupled with fluorescence detection |
| AuAgNRs | Au–Ag heterogeneous nanorods | MAb | monoclonal antibody |
| AuE | gold electrode | MB | methylene blue |
| AuNPs | gold nanoparticle | MHCS | mesoporous hollow carbon spheres |
| AuNPs/G/PhNO2 | AuNPs-dotted 4-nitrophenylazo-functionalised graphene | MNPs | Magnetic nanoparticles |
| AuNRs | gold nanorods | MOCPs | metal organic coordination polymers |
| AuPtNPs | gold-platinum alloy nanoparticles | MOF | Metal–organic framework |
| BChE | butylcholinesterase | MoS2 | Molybdenum disulfide |
| black phosphorusNSs | black phosphorus nanosheets | MPS | (3-mercaptopropyl)-trimethoxysilane |
| BP | black phosphene | MS | mass spectrometry detection |
| BSA | bovine serum albumin | Nb. BbvCI | nicking endonuclease |
| CA | cysteamine | N-Cu-MOF | nitrogen-doped copper metal–organic backbone |
| CB | carbamate | NH2 | amino groups |
| CdS-G | CdS-decorated graphene | Ni | nickel |
| CFME | carbon fiber microelectrodes | Ni-MOF | nickel-based metal–organic framework |
| c-MWCNT | carboxylated multi-walled carbon nanotubes | NMOF | nano-MOF |
| CNTs | carbon nanotubes | OP | organophosphorus |
| Co | cobalt | OTA | ochratoxin A |
| COFs | covalent organic frameworks | P | phosphorus |
| Co-MOF | cobalt-based metal–organic framework | PABA | 4-aminobenzoic acid |
| COOH | carboxyl groups | PANI | polymer polyaniline |
| CS | complementary strand | PAT | patulin |
| Cu-MOF | copper-based metal–organic framework | PDMA | poly (2,5-dimethoxyaniline) |
| DAD | diode-array detection | PdNPs | Palladium nanoparticles |
| DON | deoxynivalenol | PEC | photoelectrochemistry |
| DPV | differential pulse voltammetry | PEI | polyethylenimine |
| ECL | electrochemiluminescence | PI | polyimide |
| EIS | electrochemical impedance spectroscopy | PtNi | PtNi nanoclusters |
| ErGO | electrochemically reduced GO | PtNPs | platinum nanoparticles |
| FAO | Food and Agriculture Organization | QDs | quantum dots |
| FBThF | 4,7-bis (furan−2-yl) benzo [c] [1,2,5] thiadiazole | rGO | reduced graphene oxide |
| Fc | ferrocene | rMoS2 | reduced MoS2 |
| FC6S | 6-(Ferrocenyl) hexanethiol | SPA | Staphylococcal protein A |
| Fe | iron | SPCE | screen printed carbon electrodes |
| Fe3O4 | iron oxides | ssDNA | signal strand DNA |
| Fe3O4NP | iron oxide nanoparticles | SWCNT | single-walled carbon nanotubes |
| Fe3O4NRs | Fe3O4 nanorods | Tb | Toluidine blue |
| Fe-MOF | iron-based metal–organic framework | Thi | thionine |
| FGO | Carboxyl-functionalized graphene oxide | TLC | thin-layer chromatography |
| FM | fumonisin | TMDs | transition metal dichalcogenides |
| f-MNP | functionalized magnetic nanoparticle | TTBO | 5,6-bis(octyloxy)-4,7-bis (thiopheno [3] [3,2-b] thiophene-2-yl) benzo [c] [1,2,5] oxadiazole |
| FTO | fluorine-doped tin-oxide electrode | VNSWCNTs | vertical nitrogen-doped single-walled carbon nanotubes |
| GA | Glutaraldehyde | WHO | World Health Organization |
| GC | gas chromatography | ZEN | zearalenone |
| g-CNNS | 2D graphite-like carbon nitride nanosheet | ZnONRs | Zinc oxide nanorods |
| GO | graphene oxide | Zr-MOF | zirconium-based metal–organic framework |
| GQD | graphene quantum dots | 2-ABA | 2-aminobenzylamine |
| GS | graphene sheets | 2D | two-dimensional |
References
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2020, 283, 124657. [Google Scholar] [CrossRef]
- George, J.; Shukla, Y. Pesticides and cancer: Insights into toxicoproteomic-based findings. J. Proteom. 2011, 74, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.A.; Iorhemen, O.T.; Tay, J.H. Occurrence, impacts and removal of emerging substances of concern from wastewater. Environ. Technol. Innov. 2016, 5, 161–175. [Google Scholar] [CrossRef]
- Pope, C.; Karanth, S.; Liu, J. Pharmacology and toxicology of cholinesterase inhibitors: Uses and misuses of a common mechanism of action. Environ. Toxicol. Pharmacol. 2005, 19, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Cheung, C.-T.; DeLisio, M.; Rosenberg, J.; Tsai, R.; Kagiwada, R.; Rutledge, D. A single chip two-stage W-band grid amplifier. IEEE MTT-S Int. Microw. Symp. Dig. 2004, 1, 79–82. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Zhao, Z.; Huang, C.; Zhang, M.; Wang, H.; Wang, X. Homogeneous liquid-liquid extraction combined with high performance liquid chromatography-fluorescence detection for determination of polycyclic aromatic hydrocarbons in vegetables. J. Sep. Sci. 2009, 32, 2051–2057. [Google Scholar] [CrossRef]
- Chen, F.; Luan, C.; Wang, L.; Wang, S.; Shao, L. Simultaneous determination of six mycotoxins in peanut by high-performance liquid chromatography with a fluorescence detector. J. Sci. Food Agric. 2016, 97, 1805–1810. [Google Scholar] [CrossRef]
- Tuzimski, T.; Rejczak, T. Application of HPLC–DAD after SPE/QuEChERS with ZrO 2 -based sorbent in d-SPE clean-up step for pesticide analysis in edible oils. Food Chem. 2016, 190, 71–79. [Google Scholar] [CrossRef]
- Abdallah, O.I.; Alamer, S.S.; Alrasheed, A.M. Monitoring pesticide residues in dates marketed in Al-Qassim, Saudi Arabia using a QuEChERS methodology and liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2018, 32, e4199. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Moltó, J.C.; Mañes, J.; Berrada, H. Exposure assessment approach through mycotoxin/creatinine ratio evaluation in urine by GC–MS/MS. Food Chem. Toxicol. 2014, 72, 69–75. [Google Scholar] [CrossRef]
- Zhang, H.; Watts, S.; Philix, M.C.; Snyder, S.A.; Ong, C.N. Occurrence and distribution of pesticides in precipitation as revealed by targeted screening through GC-MS/MS. Chemosphere 2018, 211, 210–217. [Google Scholar] [CrossRef]
- Chatzimitakos, T.G.; Anderson, J.L.; Stalikas, C.D. Matrix solid-phase dispersion based on magnetic ionic liquids: An alternative sample preparation approach for the extraction of pesticides from vegetables. J. Chromatogr. A 2018, 1581–1582, 168–172. [Google Scholar] [CrossRef]
- Kang, Y.; Wu, T.; Chen, W.; Li, L.; Du, Y. A novel metastable state nanoparticle-enhanced Raman spectroscopy coupled with thin layer chromatography for determination of multiple pesticides. Food Chem. 2018, 270, 494–501. [Google Scholar] [CrossRef]
- Fang, L.; Liao, X.; Jia, B.; Shi, L.; Kang, L.; Zhou, L.; Kong, W. Recent progress in immunosensors for pesticides. Biosens. Bioelectron. 2020, 164, 112255. [Google Scholar] [CrossRef]
- Zamora-Sequeira, R.; Starbird-Pérez, R.; Rojas-Carillo, O.; Vargas-Villalobos, S. What are the Main Sensor Methods for Quantifying Pesticides in Agricultural Activities? A Review. Molecules 2019, 24, 2659. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Srivastava, S.; Ali, A.; Singh, C. Nanomaterial-Based Biosensors for Food Toxin Detection. Appl. Biochem. Biotechnol. 2014, 174, 880–896. [Google Scholar] [CrossRef]
- Verma, N.; Bhardwaj, A. Biosensor Technology for Pesticides—A review. Appl. Biochem. Biotechnol. 2015, 175, 3093–3119. [Google Scholar] [CrossRef]
- Bilal, S.; Hassan, M.M.; Rehman, M.F.U.; Nasir, M.; Sami, A.J.; Hayat, A. An insect acetylcholinesterase biosensor utilizing WO3/g-C3N4 nanocomposite modified pencil graphite electrode for phosmet detection in stored grains. Food Chem. 2021, 346, 128894. [Google Scholar] [CrossRef]
- Aćimović, S.S.; Ortega, M.A.; Sanz, V.; Berthelot, J.; Garcia-Cordero, J.L.; Renger, J.; Maerkl, S.J.; Kreuzer, M.P.; Quidant, R. LSPR Chip for Parallel, Rapid, and Sensitive Detection of Cancer Markers in Serum. Nano Lett. 2014, 14, 2636–2641. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Santella, R. The Role of Aflatoxins in Hepatocellular Carcinoma. Hepat. Mon. 2012, 12, e7238. [Google Scholar] [CrossRef] [PubMed]
- De Girolamo, A.; McKeague, M.; Miller, J.D.; DeRosa, M.C.; Visconti, A. Determination of ochratoxin A in wheat after clean-up through a DNA aptamer-based solid phase extraction column. Food Chem. 2011, 127, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Cortinovis, C.; Pizzo, F.; Spicer, L.J.; Caloni, F. Fusarium mycotoxins: Effects on reproductive function in domestic animals—A review. Theriogenology 2013, 80, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.; Bracarense, A.-P.F.L.; Schwartz, H.E.; Lucioli, J.; Cossalter, A.-M.; Moll, W.-D.; Schatzmayr, G.; Oswald, I.P. Biotransformation Approaches To Alleviate the Effects Induced by Fusarium Mycotoxins in Swine. J. Agric. Food Chem. 2013, 61, 6711–6719. [Google Scholar] [CrossRef]
- Kadir, M.K.A.; Tothill, I.E. Development of an Electrochemical Immunosensor for Fumonisins Detection in Foods. Toxins 2010, 2, 382–398. [Google Scholar] [CrossRef]
- Wang, S.; Quan, Y.; Lee, N.; Kennedy, I.R. Rapid Determination of Fumonisin B1 in Food Samples by Enzyme-Linked Immunosorbent Assay and Colloidal Gold Immunoassay. J. Agric. Food Chem. 2006, 54, 2491–2495. [Google Scholar] [CrossRef]
- Sadrabadi, N.R.; Ensafi, A.A.; Heydari-Bafrooei, E.; Fazilati, M. Screening of Food Samples for Zearalenone Toxin Using an Electrochemical Bioassay Based on DNA–Zearalenone Interaction. Food Anal. Methods 2016, 9, 2463–2470. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, J.; Li, F.; Li, J.; Shen, Z. A Calibration Curve Implanted Enzyme-Linked Immunosorbent Assay for Simultaneously Quantitative Determination of Multiplex Mycotoxins in Cereal Samples, Soybean and Peanut. Toxins 2020, 12, 718. [Google Scholar] [CrossRef]
- Gerez, J.R.; Desto, S.S.; Bracarense, A.P.F.R.L. Deoxynivalenol induces toxic effects in the ovaries of pigs: An ex vivo approach. Theriogenology 2016, 90, 94–100. [Google Scholar] [CrossRef]
- De Girolamo, A.; Ciasca, B.; Pascale, M.; Lattanzio, V.M. Determination of Zearalenone and Trichothecenes, Including Deoxynivalenol and Its Acetylated Derivatives, Nivalenol, T-2 and HT-2 Toxins, in Wheat and Wheat Products by LC-MS/MS: A Collaborative Study. Toxins 2020, 12, 786. [Google Scholar] [CrossRef]
- Ramalingam, S.; Bahuguna, A.; Kim, M. The effects of mycotoxin patulin on cells and cellular components. Trends Food Sci. Technol. 2019, 83, 99–113. [Google Scholar] [CrossRef]
- Barreira, M.J.; Alvito, P.C.; Almeida, C.M. Occurrence of patulin in apple-based-foods in Portugal. Food Chem. 2010, 121, 653–658. [Google Scholar] [CrossRef]
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of Mycotoxins and Their Consequences on Human Health. Toxicol. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective Colorimetric Detection of Polynucleotides Based on the Distance-Dependent Optical Properties of Gold Nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Spectral Properties and Relaxation Dynamics of Surface Plasmon Electronic Oscillations in Gold and Silver Nanodots and Nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef]
- Geng, P.; Fu, Y.; Yang, M.; Sun, Q.; Liu, K.; Zhang, X.; Xu, Z.; Zhang, W. Amplified Electrochemical Immunosensor for Calmodulin Detection Based on Gold-Silver-Graphene Hybrid Nanomaterials and Enhanced Gold Nanorods Labels. Electroanalysis 2014, 26, 2002–2009. [Google Scholar] [CrossRef]
- Talan, A.; Mishra, A.; Eremin, S.A.; Narang, J.; Kumar, A.; Gandhi, S. Ultrasensitive electrochemical immuno-sensing platform based on gold nanoparticles triggering chlorpyrifos detection in fruits and vegetables. Biosens. Bioelectron. 2018, 105, 14–21. [Google Scholar] [CrossRef]
- Song, Y.; Chen, J.; Sun, M.; Gong, C.; Shen, Y.; Song, Y.; Wang, L. A simple electrochemical biosensor based on AuNPs/MPS/Au electrode sensing layer for monitoring carbamate pesticides in real samples. J. Hazard. Mater. 2016, 304, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Grace, R.; Sundar, K.; Mohan, N.; Selvakumar, D.; Kumar, N. Fabrication of an Enzymatic Biosensor Based on Gold Nanoparticles Modified Electrochemical Transducer for the Detection of Organophosphorus Compounds. Nano Hybrids Compos. 2016, 12, 67–73. [Google Scholar] [CrossRef]
- Li, M.; Singh, R.; Marques, C.; Zhang, B.; Kumar, S. 2D material assisted SMF-MCF-MMF-SMF based LSPR sensor for creatinine detection. Opt. Express 2021, 29, 38150. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Agrawal, N.; Singh, R.; Kumar, S.; Zhang, B.; Saha, C.; Kumar, C. A novel periodically tapered structure-based gold nanoparticles and graphene oxide—Immobilized optical fiber sensor to detect ascorbic acid. Opt. Laser Technol. 2020, 127, 106156. [Google Scholar] [CrossRef]
- Singh, L.; Singh, R.; Zhang, B.; Cheng, S.; Kaushik, B.K.; Kumar, S. LSPR based uric acid sensor using graphene oxide and gold nanoparticles functionalized tapered fiber. Opt. Fiber Technol. 2019, 53, 102043. [Google Scholar] [CrossRef]
- Lv, J.; He, B.; Wang, N.; Li, M.; Lin, Y. A gold nanoparticle based colorimetric and fluorescent dual-channel probe for acetylcholinesterase detection and inhibitor screening. RSC Adv. 2018, 8, 32893–32898. [Google Scholar] [CrossRef]
- Unser, S.; Holcomb, S.; Cary, R.; Sagle, L. Collagen-Gold Nanoparticle Conjugates for Versatile Biosensing. Sensors 2017, 17, 378. [Google Scholar] [CrossRef]
- Song, M.-J.; Hwang, S.W.; Whang, D. Amperometric hydrogen peroxide biosensor based on a modified gold electrode with silver nanowires. J. Appl. Electrochem. 2010, 40, 2099–2105. [Google Scholar] [CrossRef]
- Lang, Q.; Han, L.; Hou, C.; Wang, F.; Liu, A. A sensitive acetylcholinesterase biosensor based on gold nanorods modified electrode for detection of organophosphate pesticide. Talanta 2016, 156–157, 34–41. [Google Scholar] [CrossRef]
- Andreescu, S.; Marty, J.-L. Twenty years research in cholinesterase biosensors: From basic research to practical applications. Biomol. Eng. 2006, 23, 1–15. [Google Scholar] [CrossRef]
- Turan, J.; Kesik, M.; Soylemez, S.; Goker, S.; Coskun, S.; Unalan, H.E.; Toppare, L. An effective surface design based on a conjugated polymer and silver nanowires for the detection of paraoxon in tap water and milk. Sens. Actuators B Chem. 2016, 228, 278–286. [Google Scholar] [CrossRef]
- Chauhan, N.; Pundir, C.S. An amperometric biosensor based on acetylcholinesterase immobilized onto iron oxide nanoparticles/multi-walled carbon nanotubes modified gold electrode for measurement of organophosphorus insecticides. Anal. Chim. Acta 2011, 701, 66–74. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Zhang, Q.; Zheng, Z.; Wang, L.-S.; Peng, D.-L. Facile synthesis of Fe3O4/C composites for broadband microwave absorption properties. Appl. Surf. Sci. 2018, 445, 82–88. [Google Scholar] [CrossRef]
- Luo, R.; Feng, Z.; Shen, G.; Xiu, Y.; Zhou, Y.; Niu, X.; Wang, H. Acetylcholinesterase Biosensor Based On Mesoporous Hollow Carbon Spheres/Core-Shell Magnetic Nanoparticles-Modified Electrode for the Detection of Organophosphorus Pesticides. Sensors 2018, 18, 4429. [Google Scholar] [CrossRef]
- Cancar, H.D.; Soylemez, S.; Akpinar, Y.; Kesik, M.; Göker, S.; Gunbas, G.; Volkan, M.; Toppare, L. A Novel Acetylcholinesterase Biosensor: Core–Shell Magnetic Nanoparticles Incorporating a Conjugated Polymer for the Detection of Organophosphorus Pesticides. ACS Appl. Mater. Interfaces 2016, 8, 8058–8067. [Google Scholar] [CrossRef]
- Morozan, A.; Jaouen, F. Metal organic frameworks for electrochemical applications. Energy Environ. Sci. 2012, 5, 9269–9290. [Google Scholar] [CrossRef]
- Dang, W.; Sun, Y.; Jiao, H.; Xu, L.; Lin, M. AuNPs-NH2/Cu-MOF modified glassy carbon electrode as enzyme-free electrochemical sensor detecting H2O2. J. Electroanal. Chem. 2020, 856, 113592. [Google Scholar] [CrossRef]
- Gaoab, F.; Tua, X.; Maa, X.; Xiea, Y.; Zoua, J.; Huanga, X.; Qub, F.; Yua, Y.; Lua, L. NiO@Ni-MOF nanoarrays modified Ti mesh as ultrasensitive electrochemical sensing platform for luteolin detection. Talanta 2020, 215, 120891. [Google Scholar] [CrossRef]
- Deep, A.; Bhardwaj, S.K.; Paul, A.; Kim, K.-H.; Kumar, P. Surface assembly of nano-metal organic framework on amine functionalized indium tin oxide substrate for impedimetric sensing of parathion. Biosens. Bioelectron. 2015, 65, 226–231. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, J.; Wang, C.; Zhai, T.-T.; Bao, W.-J.; Xu, J.-J.; Xia, X.-H.; Chen, H.-Y. Hot Electron of Au Nanorods Activates the Electrocatalysis of Hydrogen Evolution on MoS2 Nanosheets. J. Am. Chem. Soc. 2015, 137, 7365–7370. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, H.; Liu, T. In Situ Thermal Preparation of Polyimide Nanocomposite Films Containing Functionalized Graphene Sheets. ACS Appl. Mater. Interfaces 2010, 2, 3702–3708. [Google Scholar] [CrossRef] [PubMed]
- Longun, J.; Iroh, J. Nano-graphene/polyimide composites with extremely high rubbery plateau modulus. Carbon 2012, 50, 1823–1832. [Google Scholar] [CrossRef]
- Jia, L.; Zhou, Y.; Wu, K.; Feng, Q.; Wang, C.; He, P. Acetylcholinesterase modified AuNPs-MoS2-rGO/PI flexible film biosensor: Towards efficient fabrication and application in paraoxon detection. Bioelectrochemistry 2019, 131, 107392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yao, Y.; Li, X.; Lan, L.; Jiang, C.; Ping, J. Metallic Transition Metal Dichalcogenide Nanosheets as an Effective and Biocompatible Transducer for Electrochemical Detection of Pesticide. Anal. Chem. 2018, 90, 11658–11664. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, M. Analytical applications of carbon nanotubes: A review. TrAC Trends Anal. Chem. 2006, 25, 480–489. [Google Scholar] [CrossRef]
- Jha, N.; Ramaprabhu, S. Development of Au nanoparticles dispersed carbon nanotube-based biosensor for the detection of paraoxon. Nanoscale 2010, 2, 806–810. [Google Scholar] [CrossRef]
- Dhull, V. A Nafion/AChE-cSWCNT/MWCNT/Au-based amperometric biosensor for the determination of organophosphorous compounds. Environ. Technol. 2018, 41, 566–576. [Google Scholar] [CrossRef]
- Silva, R.M.; Fernandes, A.J.; Ferro, M.C.; Pinna, N.; Silva, R.F. Vertically aligned N-doped CNTs growth using Taguchi experimental design. Appl. Surf. Sci. 2015, 344, 57–64. [Google Scholar] [CrossRef]
- Xu, M.; Jiang, S.; Jiang, B.; Zheng, J. Organophosphorus pesticides detection using acetylcholinesterase biosensor based on gold nanoparticles constructed by electroless plating on vertical nitrogen-doped single-walled carbon nanotubes. Int. J. Environ. Anal. Chem. 2019, 99, 913–927. [Google Scholar] [CrossRef]
- Varghese, S.S.; Lonkar, S.; Singh, K.K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Recent advances in graphene-based biosensors. Biosens. Bioelectron. 2011, 26, 4637–4648. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, X.; Chen, P. ChemInform Abstract: Biological and Chemical Sensors Based on Graphene Materials. Cheminform 2012, 43, 2283–2307. [Google Scholar] [CrossRef]
- Zhou, D.; Cui, Y.; Han, B. Graphene-based hybrid materials and their applications in energy storage and conversion. Chin. Sci. Bull. 2012, 57, 2983–2994. [Google Scholar] [CrossRef]
- Zeng, Q.; Cheng, J.; Tang, L.; Liu, X.; Liu, Y.; Li, J.; Jiang, J. Self-Assembled Graphene-Enzyme Hierarchical Nanostructures for Electrochemical Biosensing. Adv. Funct. Mater. 2010, 20, 3366–3372. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Q.; Dai, L.; Yan, J.; Ju, C.; Qiu, B.; Wu, X. A highly sensitive and rapid organophosphate biosensor based on enhancement of CdS–decorated graphene nanocomposite. Anal. Chim. Acta 2011, 695, 84–88. [Google Scholar] [CrossRef]
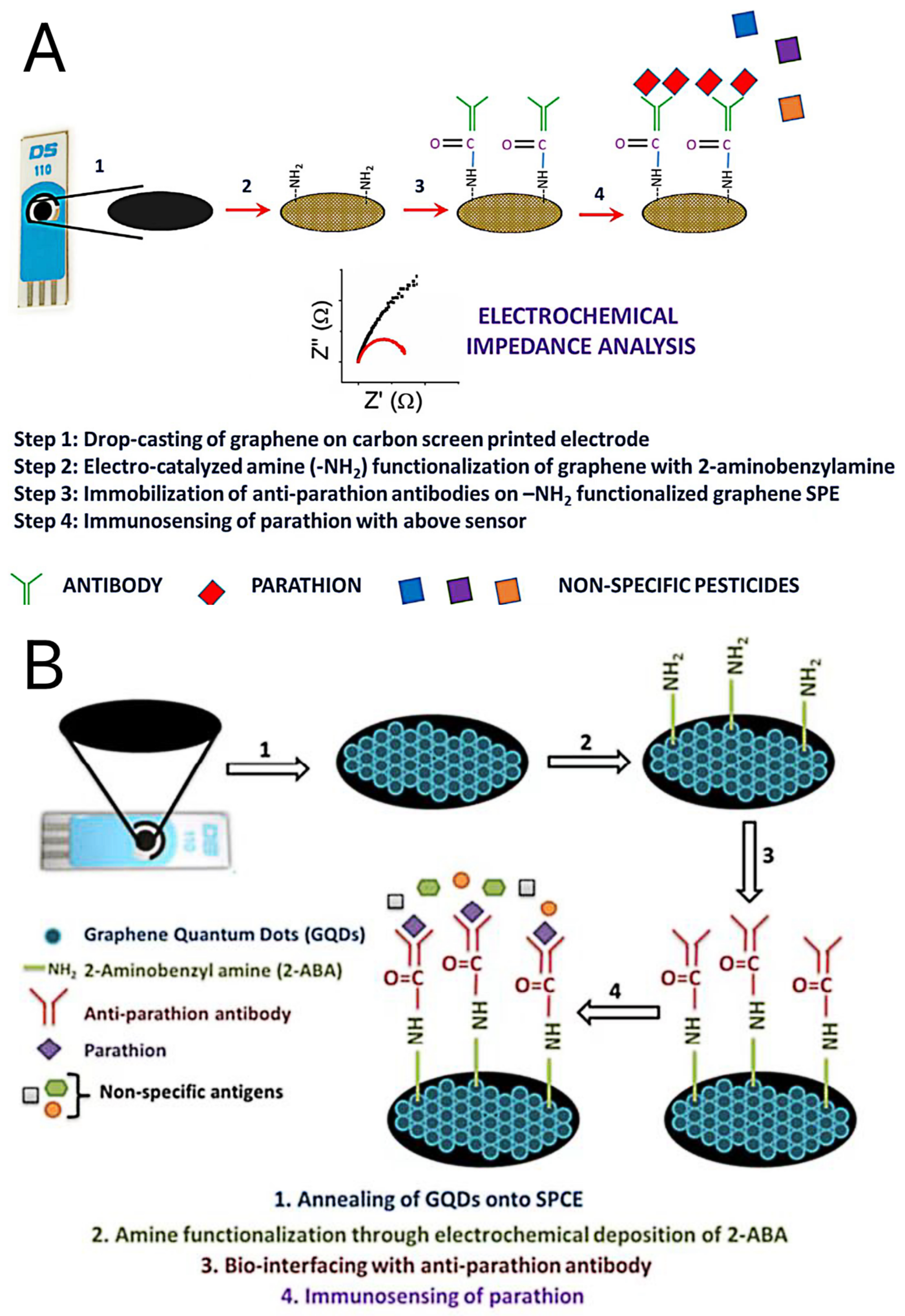
- Mehta, J.; Vinayak, P.; Tuteja, S.K.; Chhabra, V.A.; Bhardwaj, N.; Paul, A.K.; Kim, K.-H.; Deep, A. Graphene modified screen printed immunosensor for highly sensitive detection of parathion. Biosens. Bioelectron. 2016, 83, 339–346. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Tuteja, S.K.; Vinayak, P.; Paul, A.K.; Kim, K.-H.; Deep, A. Graphene quantum dot modified screen printed immunosensor for the determination of parathion. Anal. Biochem. 2017, 523, 1–9. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Kadam, U.S.; Trinh, K.H.; Kumar, V.; Lee, K.W.; Cho, Y.; Can, M.-H.T.; Lee, H.; Kim, Y.; Kim, S.; Kang, J.; et al. Identification and structural analysis of novel malathion-specific DNA aptameric sensors designed for food testing. Biomaterials 2022, 287, 121617. [Google Scholar] [CrossRef]
- Selvolini, G.; Băjan, I.; Hosu, O.; Cristea, C.; Săndulescu, R.; Marrazza, G. DNA-Based Sensor for the Detection of an Organophosphorus Pesticide: Profenofos. Sensors 2018, 18, 2035. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, X.; Li, Y.; Li, C.; Yang, L.; Ma, K.; Zhang, Z.; Huang, H. Electrochemical aptasensor based on Mo2C/Mo2N and gold nanoparticles for determination of chlorpyrifos. Microchim. Acta 2021, 188, 170. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Hou, J.; Zhao, Y.; Bao, J.; Yang, M.; Fa, H.; Yang, Y.; Li, L.; Huo, D.; Hou, C. Dual-signal aptamer sensor based on polydopamine-gold nanoparticles and exonuclease I for ultrasensitive malathion detection. Sens. Actuators B Chem. 2019, 287, 428–436. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Z.; Wu, X.; Jiang, H. Immune-biosensor for aflatoxin B1 based bio-electrocatalytic reaction on micro-comb electrode. Biochem. Eng. J. 2006, 32, 211–217. [Google Scholar] [CrossRef]
- Sunday, C.E.; Masikini, M.; Wilson, L.; Rassie, C.; Waryo, T.; Baker, P.G.L.; Iwuoha, E.I. Application on Gold Nanoparticles-Dotted 4-Nitrophenylazo Graphene in a Label-Free Impedimetric Deoxynivalenol Immunosensor. Sensors 2015, 15, 3854–3871. [Google Scholar] [CrossRef] [PubMed]
- Jalalian, S.H.; Ramezani, M.; Danesh, N.M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. A novel electrochemical aptasensor for detection of aflatoxin M1 based on target-induced immobilization of gold nanoparticles on the surface of electrode. Biosens. Bioelectron. 2018, 117, 487–492. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, M.; Li, Z.; Liao, W.; Chen, B.; Yang, T.; Hu, R.; Yang, Y.; Meng, S. Highly sensitive and convenient aptasensor based on Au NPs@Ce-TpBpy COF for quantitative determination of zearalenone. RSC Adv. 2022, 12, 17312–17320. [Google Scholar] [CrossRef]
- Subak, H.; Selvolini, G.; Macchiagodena, M.; Ozkan-Ariksoysal, D.; Pagliai, M.; Procacci, P.; Marrazza, G. Mycotoxins aptasensing: From molecular docking to electrochemical detection of deoxynivalenol. Bioelectrochemistry 2020, 138, 107691. [Google Scholar] [CrossRef]
- Lu, X.; Zhi, F.; Shang, H.; Wang, X.; Xue, Z. Investigation of the electrochemical behavior of multilayers film assembled porphyrin/gold nanoparticles on gold electrode. Electrochim. Acta 2010, 55, 3634–3642. [Google Scholar] [CrossRef]
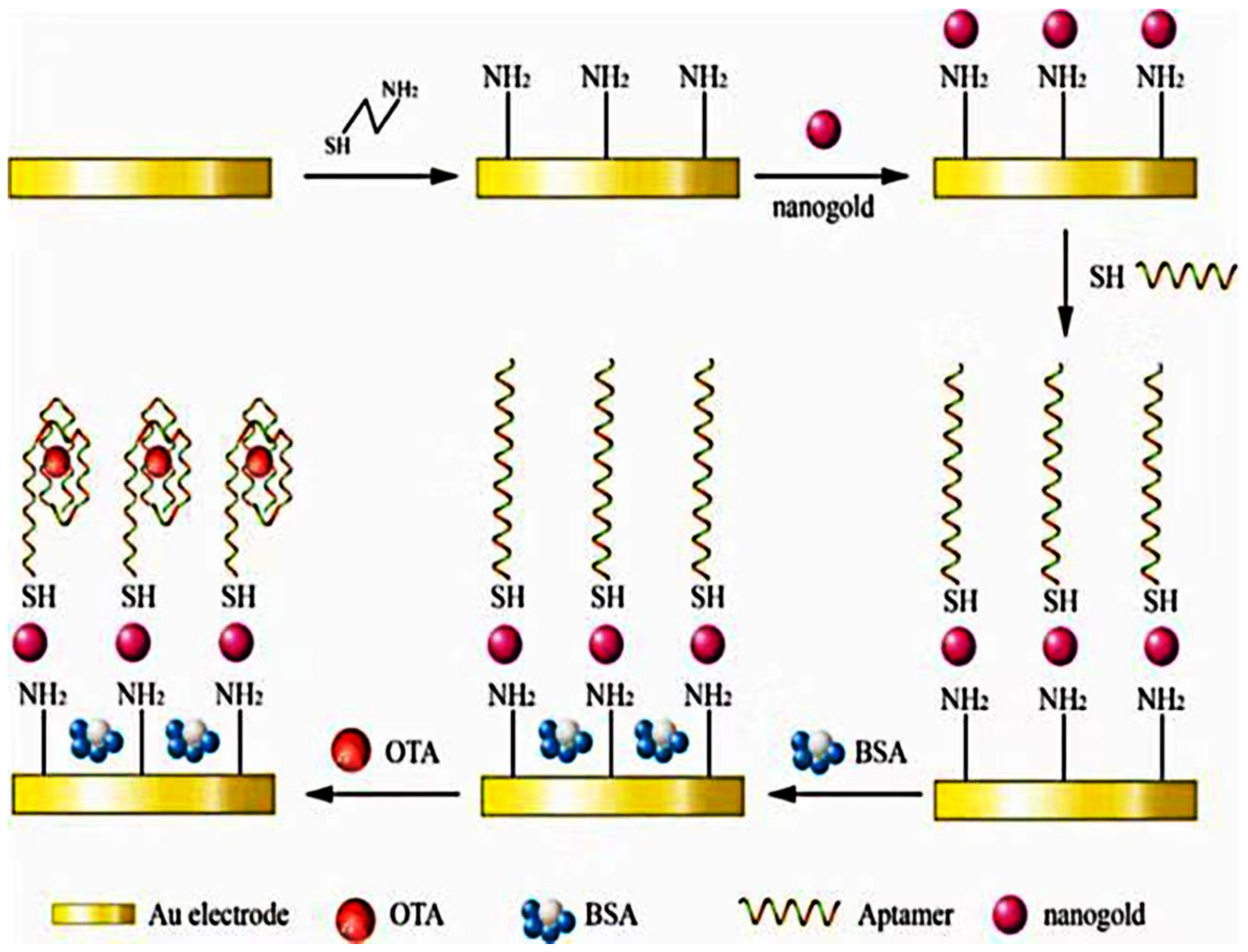
- Yang, C.; Wang, Y.; Marty, J.-L.; Yang, X. Aptamer-based colorimetric biosensing of Ochratoxin A using unmodified gold nanoparticles indicator. Biosens. Bioelectron. 2011, 26, 2724–2727. [Google Scholar] [CrossRef]
- Nan, M.; Bi, Y.; Xue, H.; Xue, S.; Long, H.; Pu, L.; Fu, G. Rapid Determination of Ochratoxin A in Grape and Its Commodities Based on a Label-Free Impedimetric Aptasensor Constructed by Layer-by-Layer Self-Assembly. Toxins 2019, 11, 71. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Wang, J.; Zhang, J.; Zhang, H.; Zhou, H.; Song, D. Preparation and application of novel nanocomposites of magnetic-Au nanorod in SPR biosensor. Biosens. Bioelectron. 2012, 34, 137–143. [Google Scholar] [CrossRef]
- Du, X.; Dai, L.; Jiang, D.; Li, H.; Hao, N.; You, T.; Mao, H.; Wang, K. Gold nanrods plasmon-enhanced photoelectrochemical aptasensing based on hematite/N-doped graphene films for ultrasensitive analysis of 17β-estradiol. Biosens. Bioelectron. 2017, 91, 706–713. [Google Scholar] [CrossRef]
- Wei, M.; Xin, L.; Feng, S.; Liu, Y. Simultaneous electrochemical determination of ochratoxin A and fumonisin B1 with an aptasensor based on the use of a Y-shaped DNA structure on gold nanorods. Microchim. Acta 2020, 187, 102. [Google Scholar] [CrossRef]
- Karimi, A.; Hayat, A.; Andreescu, S. Biomolecular detection at ssDNA-conjugated nanoparticles by nano-impact electrochemistry. Biosens. Bioelectron. 2017, 87, 501–507. [Google Scholar] [CrossRef]
- Chen, K.-J.; Lee, C.-F.; Rick, J.; Wang, S.-H.; Liu, C.-C.; Hwang, B.-J. Fabrication and application of amperometric glucose biosensor based on a novel PtPd bimetallic nanoparticle decorated multi-walled carbon nanotube catalyst. Biosens. Bioelectron. 2012, 33, 75–81. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, H.; Shi, W.; Liu, H.; Huang, Y. Fe–Co bimetallic alloy nanoparticles as a highly active peroxidase mimetic and its application in biosensing. Chem. Commun. 2013, 49, 5013–5015. [Google Scholar] [CrossRef]
- Deng, Y.-J.; Tian, N.; Zhou, Z.-Y.; Huang, R.; Liu, Z.-L.; Xiao, J.; Sun, S.-G. Alloy tetrahexahedral Pd–Pt catalysts: Enhancing significantly the catalytic activity by synergy effect of high-index facets and electronic structure. Chem. Sci. 2012, 3, 1157–1161. [Google Scholar] [CrossRef]
- Zhang, C.; He, J.; Zhang, Y.; Chen, J.; Zhao, Y.; Niu, Y.; Yu, C. Cerium dioxide-doped carboxyl fullerene as novel nanoprobe and catalyst in electrochemical biosensor for amperometric detection of the CYP2C19*2 allele in human serum. Biosens. Bioelectron. 2018, 102, 94–100. [Google Scholar] [CrossRef]
- Ji, X.; Yu, C.; Wen, Y.; Chen, J.; Yu, Y.; Zhang, C.; Gao, R.; Mu, X.; He, J. Fabrication of pioneering 3D sakura-shaped metal-organic coordination polymers Cu@L-Glu phenomenal for signal amplification in highly sensitive detection of zearalenone. Biosens. Bioelectron. 2019, 129, 139–146. [Google Scholar] [CrossRef]
- Safavi, A.; Farjami, F. Electrodeposition of gold–platinum alloy nanoparticles on ionic liquid–chitosan composite film and its application in fabricating an amperometric cholesterol biosensor. Biosens. Bioelectron. 2011, 26, 2547–2552. [Google Scholar] [CrossRef]
- Makaraviciute, A.; Ramanaviciene, A. Site-directed antibody immobilization techniques for immunosensors. Biosens. Bioelectron. 2013, 50, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, J. Oriented immobilization of proteins on solid supports for use in biosensors and biochips: A review. Microchim. Acta 2015, 183, 1–19. [Google Scholar] [CrossRef]
- Liu, N.; Nie, D.; Tan, Y.; Zhao, Z.; Liao, Y.; Wang, H.; Sun, C.; Wu, A. An ultrasensitive amperometric immunosensor for zearalenones based on oriented antibody immobilization on a glassy carbon electrode modified with MWCNTs and AuPt nanoparticles. Microchim. Acta 2016, 184, 147–153. [Google Scholar] [CrossRef]
- He, B.; Yan, X. A “signal-on” voltammetric aptasensor fabricated by hcPt@AuNFs/PEI-rGO and Fe3O4NRs/rGO for the detection of zearalenone. Sens. Actuators B Chem. 2019, 290, 477–483. [Google Scholar] [CrossRef]
- Wen, X.; Huang, Q.; Nie, D.; Zhao, X.; Cao, H.; Wu, W.; Han, Z. A Multifunctional N-Doped Cu–MOFs (N–Cu–MOF) Nanomaterial-Driven Electrochemical Aptasensor for Sensitive Detection of Deoxynivalenol. Molecules 2021, 26, 2243. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, R.; Liu, X.; Shan, G.; Chen, Y.; Tong, T.; Liu, Y. Laser-induced formation of Au/Pt nanorods with peroxidase mimicking and SERS enhancement properties for application to the colorimetric determination of H2O2. Microchim. Acta 2018, 185, 445. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, W.; Lv, S.; Han, J.; Xie, G.; Chen, S. A one-step structure-switching electrochemical sensor for transcription factor detection enhanced with synergistic catalysis of PtNi@MIL-101 and Exo III-assisted cycling amplification. Chem. Commun. 2018, 54, 11901–11904. [Google Scholar] [CrossRef]
- He, B.; Dong, X. Nb.BbvCI powered DNA walking machine-based Zr-MOFs-labeled electrochemical aptasensor using Pt@AuNRs/Fe-MOFs/PEI-rGO as electrode modification material for patulin detection. Chem. Eng. J. 2020, 405, 126642. [Google Scholar] [CrossRef]
- Cao, J.-T.; Yang, J.-J.; Zhao, L.-Z.; Wang, Y.-L.; Wang, H.; Liu, Y.-M.; Ma, S.-H. Graphene oxide@gold nanorods-based multiple-assisted electrochemiluminescence signal amplification strategy for sensitive detection of prostate specific antigen. Biosens. Bioelectron. 2018, 99, 92–98. [Google Scholar] [CrossRef]
- He, B.; Yan, X. Ultrasensitive electrochemical aptasensor based on CoSe2/AuNRs and 3D structured DNA-PtNi@Co-MOF networks for the detection of zearalenone. Sens. Actuators B Chem. 2019, 306, 127558. [Google Scholar] [CrossRef]
- Wu, S.; Zeng, Z.; He, Q.; Wang, Z.; Wang, S.J.; Du, Y.; Yin, Z.; Sun, X.; Chen, W.; Zhang, H. Electrochemically Reduced Single-Layer MoS2 Nanosheets: Characterization, Properties, and Sensing Applications. Small 2012, 8, 2264–2270. [Google Scholar] [CrossRef]
- Han, Z.; Tang, Z.; Jiang, K.; Huang, Q.; Meng, J.; Nie, D.; Zhao, Z. Dual-target electrochemical aptasensor based on co-reduced molybdenum disulfide and Au NPs (rMoS2-Au) for multiplex detection of mycotoxins. Biosens. Bioelectron. 2020, 150, 111894. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Ge, J.; He, Y. Ultrasensitive determination for flavin coenzyme by using a ZnO nanorod photoelectrode in a four-electrode system. Microchim. Acta 2017, 184, 2333–2339. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Liu, X.; Xian, Y.; Shi, G.; Jin, L. ZnO nanorods/Au hybrid nanocomposites for glucose biosensor. Biosens. Bioelectron. 2010, 26, 275–278. [Google Scholar] [CrossRef]
- Fang, L.; Huang, K.; Zhang, B.; Liu, Y.; Zhang, Q. A label-free electrochemistry biosensor based flower-like 3-dimensional ZnO superstructures for detection of DNA arrays. New J. Chem. 2014, 38, 5918–5924. [Google Scholar] [CrossRef]
- He, B.; Dong, X. Aptamer based voltammetric patulin assay based on the use of ZnO nanorods. Microchim. Acta 2018, 185, 462. [Google Scholar] [CrossRef]
- Di, J.; Peng, S.; Shen, C.; Gao, Y.; Tu, Y. One-step method embedding superoxide dismutase and gold nanoparticles in silica sol–gel network in the presence of cysteine for construction of third-generation biosensor. Biosens. Bioelectron. 2007, 23, 88–94. [Google Scholar] [CrossRef]
- Gomes, S.P.; Odložilíková, M.; Almeida, M.G.; Araújo, A.N.; Couto, C.M.; Montenegro, M.C.B. Application of lactate amperometric sol–gel biosensor to sequential injection determination of l-lactate. J. Pharm. Biomed. Anal. 2007, 43, 1376–1381. [Google Scholar] [CrossRef]
- Li, S.C.; Chen, J.H.; Cao, H.; Yao, D.S.; Liu, D.L. Amperometric biosensor for aflatoxin B1 based on aflatoxin-oxidase immobilized on multiwalled carbon nanotubes. Food Control. 2011, 22, 43–49. [Google Scholar] [CrossRef]
- Singh, C.; Srivastava, S.; Ali, A.; Gupta, T.K.; Sumana, G.; Srivastava, A.; Mathur, R.; Malhotra, B.D. Carboxylated multiwalled carbon nanotubes based biosensor for aflatoxin detection. Sens. Actuators B Chem. 2013, 185, 258–264. [Google Scholar] [CrossRef]
- Fujita, K.; Murata, K.; Masuda, M.; Nakamura, N.; Ohno, H. Ionic liquids designed for advanced applications in bioelectrochemistry. RSC Adv. 2012, 2, 4018–4030. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Zhang, Q.; Li, Y.; Zhang, W.; Ding, X. Molecular Characterization of Monoclonal Antibodies against Aflatoxins: A Possible Explanation for the Highest Sensitivity. Anal. Chem. 2012, 84, 5229–5235. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Hu, C.; Wu, H.; Yang, Y.; Huang, C.; Jia, N. Highly sensitive electrochemical impedance spectroscopy immunosensor for the detection of AFB1 in olive oil. Food Chem. 2015, 176, 22–26. [Google Scholar] [CrossRef]
- Masikini, M.; Williams, A.R.; Sunday, C.E.; Waryo, T.T.; Nxusani, E.; Wilson, L.; Qakala, S.; Bilibana, M.; Douman, S.; Jonnas, A.; et al. Label Free Poly(2,5-dimethoxyaniline)–Multi-Walled Carbon Nanotubes Impedimetric Immunosensor for Fumonisin B1 Detection. Materials 2016, 9, 273. [Google Scholar] [CrossRef]
- Choudhary, M.; Singh, A.; Kaur, S.; Arora, K. Enhancing Lung Cancer Diagnosis: Electrochemical Simultaneous Bianalyte Immunosensing Using Carbon Nanotubes–Chitosan Nanocomposite. Appl. Biochem. Biotechnol. 2014, 174, 1188–1200. [Google Scholar] [CrossRef]
- Najeeb, C.K.; Chang, J.; Lee, J.-H.; Lee, M.; Kim, J.-H. Preparation of semiconductor-enriched single-walled carbon nanotube dispersion using a neutral pH water soluble chitosan derivative. J. Colloid Interface Sci. 2011, 354, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, X.; Zhang, X.; Qing, Y.; Luo, M.; Liu, X.; Li, C.; Li, Y.; Xia, H.; Qiu, J. A Highly Sensitive Electrochemical Immunosensor for Fumonisin B1Detection in Corn Using Single-Walled Carbon Nanotubes/Chitosan. Electroanalysis 2015, 27, 2679–2687. [Google Scholar] [CrossRef]
- Abnous, K.; Danesh, N.M.; Alibolandi, M.; Ramezani, M.; Taghdisi, S.M. Amperometric aptasensor for ochratoxin A based on the use of a gold electrode modified with aptamer, complementary DNA, SWCNTs and the redox marker Methylene Blue. Microchim. Acta 2017, 184, 1151–1159. [Google Scholar] [CrossRef]
- Bai, Y.; Koh, C.G.; Boreman, M.; Juang, Y.-J.; Tang, I.-C.; Lee, L.J.; Yang, S.-T. Surface Modification for Enhancing Antibody Binding on Polymer-Based Microfluidic Device for Enzyme-Linked Immunosorbent Assay. Langmuir 2006, 22, 9458–9467. [Google Scholar] [CrossRef] [PubMed]
- Riberi, W.I.; Tarditto, L.V.; Zon, M.A.; Arévalo, F.J.; Fernández, H. Development of an electrochemical immunosensor to determine zearalenone in maize using carbon screen printed electrodes modified with multi-walled carbon nanotubes/polyethyleneimine dispersions. Sens. Actuators B Chem. 2018, 254, 1271–1277. [Google Scholar] [CrossRef]
- Bai, L.; Chai, Y.; Pu, X.; Yuan, R. A signal-on electrochemical aptasensor for ultrasensitive detection of endotoxin using three-way DNA junction-aided enzymatic recycling and graphene nanohybrid for amplification. Nanoscale 2013, 6, 2902–2908. [Google Scholar] [CrossRef]
- Ma, L.; Bai, L.; Zhao, M.; Zhou, J.; Chen, Y.; Mu, Z. An electrochemical aptasensor for highly sensitive detection of zearalenone based on PEI-MoS2-MWCNTs nanocomposite for signal enhancement. Anal. Chim. Acta 2019, 1060, 71–78. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef]
- Srivastava, S.; Kumar, V.; Ali, M.A.; Solanki, P.R.; Srivastava, A.; Sumana, G.; Saxena, P.S.; Joshi, A.G.; Malhotra, B.D. Electrophoretically deposited reduced graphene oxide platform for food toxin detection. Nanoscale 2013, 5, 3043–3051. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Z.; Yang, G.; Yang, H.; Zhao, F. A novel electrochemical immunosensor for aflatoxin B1 based on Au nanoparticles-poly 4-aminobenzoic acid supported graphene. Appl. Surf. Sci. 2020, 527, 146934. [Google Scholar] [CrossRef]
- Zhong, J.; Gao, S.; Xue, G.; Wang, B. Study on Enhancement Mechanism of Conductivity Induced by Graphene Oxide for Polypyrrole Nanocomposites. Macromolecules 2015, 48, 1592–1597. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Gunasekaran, S. Spectroscopic and microscopic investigation of gold nanoparticle nucleation and growth mechanisms using gelatin as a stabilizer. J. Nanoparticle Res. 2012, 14, 1200. [Google Scholar] [CrossRef]
- Lu, L.; Seenivasan, R.; Wang, Y.-C.; Yu, J.-H.; Gunasekaran, S. An Electrochemical Immunosensor for Rapid and Sensitive Detection of Mycotoxins Fumonisin B1 and Deoxynivalenol. Electrochim. Acta 2016, 213, 89–97. [Google Scholar] [CrossRef]
- Zhang, T.; Xing, B.; Han, Q.; Lei, Y.; Wu, D.; Ren, X.; Wei, Q. Electrochemical immunosensor for ochratoxin A detection based on Au octahedron plasmonic colloidosomes. Anal. Chim. Acta 2018, 1032, 114–121. [Google Scholar] [CrossRef]
- Song, X.; Wang, D.; Kim, M. Development of an immuno-electrochemical glass carbon electrode sensor based on graphene oxide/gold nanocomposite and antibody for the detection of patulin. Food Chem. 2020, 342, 128257. [Google Scholar] [CrossRef]
- Goud, K.Y.; Hayat, A.; Catanante, G.; Satyanarayana , M.; Gobi, K.V.; Mary, J.L. An electrochemical aptasensor based on functionalized graphene oxide assisted electrocatalytic signal amplification of methylene blue for aflatoxin B1 detection. Electrochim. Acta 2017, 244, 96–103. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Kandula, S.; Shanker, V. Fe-doped and -mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight. J. Mater. Chem. A 2014, 2, 6772. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. 2010, 8, 271–275. Nat. Mater. 2010, 8, 271–275. [Google Scholar] [CrossRef]
- Zhu, X.; Kou, F.; Xu, H.; Han, Y.; Yang, G.; Huang, X.; Chen, W.; Chi, Y.; Lin, Z. Label-free ochratoxin A electrochemical aptasensor based on target-induced noncovalent assembly of peroxidase-like graphitic carbon nitride nanosheet. Sens. Actuators B Chem. 2018, 270, 263–269. [Google Scholar] [CrossRef]
- Wang, D.; Gan, N.; Zhou, J.; Xiong, P.; Cao, Y.; Li, T.; Pan, D.; Jiang, S. Signal amplification for multianalyte electrochemical immunoassay with bidirectional stripping voltammetry using metal-enriched polymer nanolabels. Sens. Actuators B Chem. 2014, 197, 244–253. [Google Scholar] [CrossRef]
- Zeng, X.; Gao, H.; Pan, D.; Sun, Y.; Cao, J.; Wu, Z.; Pan, Z. Highly Sensitive Electrochemical Determination of Alfatoxin B1 Using Quantum Dots-Assembled Amplification Labels. Sensors 2015, 15, 20648–20658. [Google Scholar] [CrossRef]
- Xuan, Z.; Liu, H.; Ye, J.; Li, L.; Tian, W.; Wang, S. Reliable and disposable quantum dot–based electrochemical immunosensor for aflatoxin B1 simplified analysis with automated magneto-controlled pretreatment system. Anal. Bioanal. Chem. 2020, 412, 7615–7625. [Google Scholar] [CrossRef]
- Wang, C.; Qian, J.; An, K.; Huang, X.; Zhao, L.; Liu, Q.; Hao, N.; Wang, K. Magneto-controlled aptasensor for simultaneous electrochemical detection of dual mycotoxins in maize using metal sulfide quantum dots coated silica as labels. Biosens. Bioelectron. 2017, 89, 802–809. [Google Scholar] [CrossRef]
- Sun, C.; Liao, X.; Jia, B.; Shi, L.; Zhang, D.; Wang, R.; Zhou, L.; Kong, W. Development of a ZnCdS@ZnS quantum dots–based label-free electrochemiluminescence immunosensor for sensitive determination of aflatoxin B1 in lotus seed. Microchim. Acta 2020, 187, 236. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, T.; Li, Y.; Cao, W.; Pang, X.; Wu, D.; Wei, Q. A simple label-free photoelectrochemical immunosensor for highly sensitive detection of aflatoxin B1based on CdS–Fe3O4magnetic nanocomposites. RSC Adv. 2015, 5, 19581–19586. [Google Scholar] [CrossRef]
- Xu, J.; Qiao, X.; Wang, Y.; Sheng, Q.; Yue, T.; Zheng, J.; Zhou, M. Electrostatic assembly of gold nanoparticles on black phosphorus nanosheets for electrochemical aptasensing of patulin. Microchim. Acta 2019, 186, 238. [Google Scholar] [CrossRef]
- Keyes, R.W. The Electrical Properties of Black Phosphorus. Phys. Rev. 1953, 92, 580–584. [Google Scholar] [CrossRef]
- Tao, W.; Zhu, X.; Yu, X.; Zeng, X.; Xiao, Q.; Zhang, X.; Ji, X.; Wang, X.; Shi, J.; Zhang, H.; et al. Black Phosphorus Nanosheets as a Robust Delivery Platform for Cancer Theranostics. Adv. Mater. 2016, 29, 1603276. [Google Scholar] [CrossRef]
- Pumera, M. Phosphorene and black phosphorus for sensing and biosensing. TrAC Trends Anal. Chem. 2017, 93, 1–6. [Google Scholar] [CrossRef]
- Eswaraiah, V.; Zeng, Q.; Long, Y.; Liu, Z. Black Phosphorus Nanosheets: Synthesis, Characterization and Applications. Small 2016, 12, 3480–3502. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, S.; Wang, Z.; Yang, Z.; Liu, F.; Xue-Feng, Y.; Wang, J.; Yi, Y.; Zhang, H.; Liao, L.; et al. Metal-Ion-Modified Black Phosphorus with Enhanced Stability and Transistor Performance. Adv. Mater. 2017, 29, 1703811. [Google Scholar] [CrossRef]
- Xiang, Y.; Camarada, M.B.; Wen, Y.; Wu, H.; Chen, J.; Li, M.; Liao, X. Simple voltammetric analyses of ochratoxin A in food samples using highly-stable and anti-fouling black phosphorene nanosensor. Electrochim. Acta 2018, 282, 490–498. [Google Scholar] [CrossRef]
- Zhao, H.; Qiao, X.; Zhang, X.; Niu, C.; Yue, T.; Sheng, Q. Simultaneous electrochemical aptasensing of patulin and ochratoxin A in apple juice based on gold nanoparticles decorated black phosphorus nanomaterial. Anal. Bioanal. Chem. 2021, 413, 3131–3140. [Google Scholar] [CrossRef]
- Buiculescu, R.; Chaniotakis, N.A. The stabilization of Au NP–AChE nanocomposites by biosilica encapsulation for the development of a thiocholine biosensor. Bioelectrochemistry 2012, 86, 72–77. [Google Scholar] [CrossRef]
- Azizah, N.; Hashim, U.; Gopinath, S.C.B.; Nadzirah, S. Gold nanoparticle mediated method for spatially resolved deposition of DNA on nano-gapped interdigitated electrodes, and its application to the detection of the human Papillomavirus. Microchim. Acta 2016, 183, 3119–3126. [Google Scholar] [CrossRef]
- Oh, B.-K.; Kim, Y.-K.; Park, K.W.; Lee, W.H.; Choi, J.-W. Surface plasmon resonance immunosensor for the detection of Salmonella typhimurium. Biosens. Bioelectron. 2004, 19, 1497–1504. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.-Q.; Peng, L.-Z.; Lei, Y.-M.; Chai, Y.-Q.; Yuan, R.; Zhuo, Y. Strong Electrochemiluminescence from MOF Accelerator Enriched Quantum Dots for Enhanced Sensing of Trace cTnI. Anal. Chem. 2018, 90, 3995–4002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lv, S.; Lin, Z.; Tang, D. CdS:Mn quantum dot-functionalized g-C3N4 nanohybrids as signal-generation tags for photoelectrochemical immunoassay of prostate specific antigen coupling DNAzyme concatamer with enzymatic biocatalytic precipitation. Biosens. Bioelectron. 2017, 95, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, M.; Wei, D. Advances in gold nanoparticles for mycotoxin analysis. Anal. 2021, 146, 1793–1806. [Google Scholar] [CrossRef] [PubMed]
- Rhouati, A.; Bulbul, G.; Latif, U.; Hayat, A.; Li, Z.-H.; Marty, J.L. Nano-Aptasensing in Mycotoxin Analysis: Recent Updates and Progress. Toxins 2017, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Hu, H.; Wang, S.; Hu, S. Nanomaterials-based electrochemical sensors for nitric oxide. Microchim. Acta 2014, 182, 455–467. [Google Scholar] [CrossRef]
- Kadam, U.S.; Hong, J.C. Advances in aptameric biosensors designed to detect toxic contaminants from food, water, human fluids, and the environment. Trends Environ. Anal. Chem. 2022, 36, e00184. [Google Scholar] [CrossRef]
- Jara, M.D.L.; Alvarez, L.A.C.; Guimarães, M.C.C.; Antunes, P.W.P.; de Oliveira, J.P. Lateral flow assay applied to pesticides detection: Recent trends and progress. Environ. Sci. Pollut. Res. 2022, 29, 46487–46508. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Guo, Y. Recent Trends in Nanomaterial-Based Biosensors for Point-of-Care Testing. Front. Chem. 2020, 8, 586702. [Google Scholar] [CrossRef]



| Pesticides | Classification | Health Hazards | Reference |
|---|---|---|---|
| Carbamate (CB) | Insecticide | Diarrhea, respirator disorder, carcinogenic, and reproductive toxicity | [1] |
| Organophosphorus (OP) | Insecticide | Carcinogenic, poses potential risk to endocrine, metabolic, neurological, hepatorenal disorders, psychiatric manifestations, and neuritis. | [4] |
| Nanomaterial | Sample | Analyte | Stability | Linear Range | Limit of Detection | Ref. |
|---|---|---|---|---|---|---|
| AuNPs/Ab | Apple, pomegranate and cabbage | OP (chlorpyrifos) | 21 days (100%) | 1 fM−1 μM | 10 fM | [40] |
| AuNPs/MPS/AchE | Fruit | Carbamate | 28 days (88%) 7 days (100%) | 0.003–2.00 µM | 1.0 nM | [41] |
| AuNPs/AchE | Methyl parathion | OP (Methyl parathion) | / | 0.2–1 µg/L | 0.6 µg/L | [42] |
| AuNRs/AchE | River water | OP (Paraoxon; dimethoate) | 30 days (93%) | 1 nM−5 µM (Paraoxon); 5 nM−1 µM (dimethoate) | 0.7 nM (paraoxon); 3.9 nM (dimethoate) | [49] |
| AuAgNRs/AchE | River water | OP (Paraoxon; dimethoate) | / | 5 nM−1 µM (Paraoxon); 10 nM−5 µM (dimethoate) | 0.7 nmol/L | [49] |
| AgNWs/PTTBO/BchE | Tap water and milk | OP (paraoxon) | 15 days (100%) 25 days (slight decrease) | 10–120 µM and 0.5–8 µM | 0.212 µM | [51] |
| Fe3O4NPs/c-MWCNT/AchE | Milk and water | OP (malathion, chlorpyrifos, monocrotophos, endosulfan) | 60 days (50 uses) (75%) | 0.1–40 nM (malathion); 0.1–50 nM (chlorpyrifos); 1–50 nM (monocrotophos) 10–100 nM (endosulfan) | 0.1 nM (malathion and chlorpyrifos); 1 nM (monocrotophos); 10 nM (endosulfan) | [52] |
| Fe3O4NPs/MHCS/AchE | Practical pear | OP (malathion) | 30 days (79%) | 0.01–50 ppb; 50–600 ppb | 0.0182 ppb | [54] |
| Poly(FBThF)/f-MNPs (SiO2-Fe3O4NPs-COOH)/AchE | Tap water | OP (Paraoxon) (Trichlorfon) | 60 days (65%) 10 days (100%) | 0.05–5 µg/L (paraoxon) 5–9.28 µg/L (paraoxon); 0.05–4.1 µg/L (trichlorfon) 4.1–9 µg/L (trichlorfon) | 0.022 µg/L (Paraoxon); 0.037 µg/L (trichlorfon) | [55] |
| Cd-MOF/2-ABA/Ab | Rice | OP (parathion) | 25 days (75%) | 0.1–20 ng/mL | 0.1 ng/mL | [59] |
| rGO/MoS2/AuNPs/AchE | Spiked vegetable water | OP (paraoxon) | 7 days (96%) | 0.005–0.15 μg/mL | 0.0014 μg/mL | [63] |
| MoS2/AuNPs/AchE | Apple and pakchoi | OP (Paraoxon) | / | 1.0–1000 μg/L | 0.013 μg/L | [64] |
| Nanomaterial | Sample | Analyte | Stability | Linear Range | Limit of Detection | Ref. |
|---|---|---|---|---|---|---|
| Au/MWCNT/AChE | Paraoxon | OP (paraoxon) | / | 0.1–7 nM | 0.1 nM (0.025 ppb) | [66] |
| AuNPs/MWCNTs/c-SWCNTs/AChE/Nafion | Cabbage, onion, spinach | OP (Methyl Parathion, Monocrotophos, Chlorpyrifos and Endosulfan) | 60 days | 0.1–130 µM | 1.9 nM (Methyl Parathion) 2.3 nM (Monocrotophos) 2.2 nM (Chlorpyrifos) 2.5 nM (Endosulfan) | [67] |
| VNSWCNTs/AuNPs/AChE | Cabbage water sample, tap water, purified water, river water and lake water | OP (Malathion, Methyl parathion and Chlorpyrifos) | 28 days (95%) 7 days (99%) | 1.00 × 10−5–1.00 ppb (Malathion, Methyl parathion and Chlorpyrifos) | 1.96 × 10−6 ppb (Malathion) 3.04 × 10−6 ppb (Methyl parathion) 2.06 × 10−6 ppb (Chlorpyrifos) | [69] |
| CdS-G/Chitosan/AChE | OP | OP | 20 days (83%) | 2 ng/mL−2 μg/mL | 0.7 ng/mL | [75] |
| GS/2-ABA/Ab | Tomato and carrot sample | OP | 50 days (>95%) | 0.1–1000 ng/L | 52 pg/L | [76] |
| GQD/2-ABA/Ab | Parathion sample | OP | 60 days (constant) | 0.01–106 ng/L | 46 pg/L | [77] |
| Biosensor | Sample | Analyte | Stability | Linear Range | Limit of Detection | Ref. |
|---|---|---|---|---|---|---|
| 6-FAM/Apt (G4Q)/ThT | Cucumber and Chinese cabbage | OP (malathion) | / | / | 2.01 ppb | [79] |
| PANI/AuNPs/Apt | Pear juice | OP (profenofos) | / | 0.1–10 µM | 0.27 µM | [80] |
| Mo2C/Mo2N/AuNPs/Fc-CP/Apt | Apple and pakchoi | OP (chlorpyrifos) | 7 days (92.3%−94.7%) | 0.1–400 ng/mL | 0.036 ng/mL | [81] |
| PDA/AuNPs/Fc-CP/Tn-Apt/Exo I | Cauliflflower and cabbage | OP (malathion) | 5 days/once (four times RSD 4.48%) | 0.5–650 ng/L | 0.5 ng/L | [82] |
| Nanomaterial | Sample | Analyte | Stability | Linear Range | Limit of Detection | Ref. |
|---|---|---|---|---|---|---|
| HRP/Ab/AuNPs | AFB1 solution | AFB1 | 12 days (90%) | 0.5–10 ng/ml | 0.1 ng/ml | [83] |
| Nafion/G/AuNPs/PhNO2/Ab | Cereal | DON | 5 days (80.3%) | 6–30 ng/mL | 0.3 µg/mL | [84] |
| AFM1 (Apt)/AuNPs/CS | Milk and serum | AFM1 | 10 days (96%) | 2–600 ng/L | 0.9 ng/L | [85] |
| AuNPs/COF/Apt | Cornflour | ZEN | / | 0.001–10 ng/mL | 0.389 pg/mL | [86] |
| AuNPs–PANI/thiol-tethered-Apt/CS | Spiked maize flour | DON | / | 5–30 ng/mL | 3.2 ng/mL | [87] |
| Thi-Apt/AuNPs/CA | Grape and its commodities | OTA | / | 0.1–10 ng/mL | 0.03 ng/mL | [90] |
| CS/Apt1-Thi-AuNRs/Apt2-Fc-AuNRs | spiked beer | OTA and FB1 | 21 days (93.7% (Thi) and 91.4% (Fc)) | 0.001–100 ng/mL | 0.00047 ng/mL | [93] |
| AgNPs/Apt | OTA | OTA | / | 0.07–10 nM | 0.05 nM | [94] |
| MOCP/Pd-PtNPs/CS/Apt/Au-PANI-Au nanohybrid | Beer | ZEN | 28 days (93.5%) | 1 fg/mL−100 ng/mL | 0.45 fg/mL | [99] |
| PEI-MWCNTs/AuPtNPs/SPA-Ab | Corn flour and corn-based baby food | ZEN | 10 days (89.04%) | 0.005–50 ng/mL | 1.5 pg/mL | [103] |
| Fe3O4NRs/rGO/AuNPs/CS1/Apt/hcPtAuNFs/Thi/CS2/PEI-rGO | Maize | ZEN | 10 days (94.68%) | 0.5 pg/mL−50 ng/mL | 0.105 pg/mL | [104] |
| N-Cu-MOF/Apt | Spiked wheat | DON | / | 0.02–20 ng/mL | 0.008 ng/mL | [105] |
| PEI-rGO/Fe-MOF/PtAuNRs/MB-Zr-MOF/CS1/Apt/CS2 | Spiked apple juice and apple wine | PAT | 10 days (95.3%) | 5.0 × 10−5–5.0 × 10−1 ng/mL | 4.14 × 10−5 ng/mL | [108] |
| CoSe2/AuNRs/3dsDNA-PtNi/Co-MOF/Apt | Maize | ZEN | 21 days (93.1%) | 10.0 fg/mL−10.0 ng/mL | 1.37 fg/mL | [110] |
| rMoS2/AuNPs/CS1/Apt1/Thi MoS2/AuNPs/CS2/Apt2/FC6S | Maize | ZEN, FB1 | 14 days (90.2% (FB1)) 14 days (90.0% (ZEN)) | 0.001–10 ng/mL (ZEN); 0.001–100 ng/mL (FB1) | 0.0005 ng/mL | [112] |
| ZnONRs/chitosan/Thi-AuNPs/Apt | Spiked juice | PAT | 7 days (94.4%) | 50 ng/mL−0.5 pg/mL | 0.27 ng/mL | [116] |
| Nanomaterial | Sample | Analyte | Stability | Linear Range | Limit of Detection | Ref. |
|---|---|---|---|---|---|---|
| MWCNTs/sol-gel/AFO | AFB1 solution | AFB1 | 23 days (97.1%) 7 days (99.4%) | 3.2–721 nmol/L | 1.6 nmol/L | [119] |
| c-MWCNTs/Ab/BSA | AFB1 solution | AFB1 | 45 days (92%) | 0.25–1.375 ng/ml | 0.08 ng/ml | [120] |
| MWCNTs/RTIL/Ab | Olive oil | AFB1 | 60 days (87%) | 0.1–10 ng/mL | 0.03 ng/mL | [123] |
| PDMA-MWCNT/Ab | Certified Corn Reference Material | FB1 | 5 days (81%) | 7–49 ng/L | 3.8 pg/L | [124] |
| SWCNT/chitosan/FB1-BSA/Ab | Spiked corn | FB1 | 5 days (60%) | 0.01–1000 ng/mL | 2 pg/mL | [127] |
| CS/Apt/SWCNT/MB | Serum and grape juice | OTA | / | 134–58 pM | 52 pM | [128] |
| PEI-MWCNTs/AuNPs/Apt | Maize | ZEN | 5 days (88%) | 0.0001–0.1 ng/mL | 0.15 pg/mL | [130] |
| PEI-MoS2/MWCNTs/Tb/PtAuNPs/Apt | Beer | ZEN | 25 days (87.2%) 15 days (95.3%) | 0.5 pg/mL−50 ng/mL | 0.17 pg/mL | [132] |
| BSA/anti-AFB1/rGO | AFB1 solution | AFB1 | 45 days (no signicant decrease) | 0.125–1.5 ng/ml | 0.15 ng/ml | [134] |
| Au-Poly (PPABA)/rGO/Ab | vegetable oil | AFB1 | 10 days (96.3%) | 0.01–25 ng/mL | 0.001 ng/mL | [135] |
| ErGO-PPy/AuNPs/Ab | Spiked corn | DON | 12 days (96.6%) | 0.05–1 ppm (DON) 0.2–4.5 ppm (FB1) | 8.6 ppb (DON) 4.2 ppb (FB1) | [138] |
| GO/AuNPs/IgG | PAT | PAT | / | 5–200 µg/L | 5 µg/L | [140] |
| FGO/HMDA/Apt | Alcoholic beverage | AFB1 | / | 0.05 ng/mL | 0.05–6.0 ng/mL | [141] |
| g-CNNS/Apt | Red wines, juices, and corn | OTA | / | 0.2–500 nM | 0.073 nM | [144] |
| Nanomaterial | Sample | Analyte | Stability | Linear Range | Limit of Detection | Ref. |
|---|---|---|---|---|---|---|
| QDs (PbS)/mAb | Real peanut sample | AFB1 | / | 0.04–15 ng/mL | 0.018 ng/mL | [146] |
| BSA/mAb/IMB/QDs (CdTe) | Wheat, maize, husky rice, and peanut oil | AFB1 | / | 0.08–800μg/kg | 0.05μg/kg | [147] |
| MBs (Fe4-Au)/CS/Apt/QDs (CdTe)/SiO2 and MBs (Fe3O4-Au)/CS/Apt/QDs (PbS)/SiO2 | Maize sample | FB1; OTA | 21 days (98.7% Cd2+; 98.1% Pb2+) | 0.05–50 ng/mL (FB1) 0.01–10 ng/mL (OTA) | 20 pg/mL (FB1) 5 pg/mL (OTA) | [148] |
| QDs (ZnCdS/ZnS)/Au/Nafion composite film/Ab | Lotus seed | AFB1 | / | 0.05–100 ng/mL | 0.01 ng/mL | [149] |
| QDs (CdS)/Fe3O4/Ab | Corn sample | AFB1 | 12 days (91%) | 0.01–80 ng/mL | 5.0 pg/mL | [150] |
| Black phosphorus NSs/Apt | Spiked apple juice sample | PAT | / | 1 nM−1µM | 0.3 nM | [151] |
| Black phosphorus NSs/AuNPs/thiolated Apt | Spiked apple juice sample | PAT | / | 0.1 nM−10.0µM | 0.03 nM | [151] |
| Ag+-BP | Alcoholic beverage samples | AFB1 | / | 0.05 ng/mL | 0.05–6.0 ng/mL | [157] |
| AuNPs/BP/Fc-Apt (OTA)/Mb-Apt (PAT) | Apple juice | OTA, PAT | 21 days (92.0%) | 0.01 × 10−7 µg/mL −0.10 µg/mL | / | [158] |
| Nanomaterial | Merits | Demerits |
|---|---|---|
| Au nanomaterial | Easily decorated to increase the binding area, good electrical conductivity, easy to immobilize biomolecules. | Specific surface area is relatively small, poor detection stability of the sensor, easy to form irreversible aggregation, seriously affect by the environment. |
| Ag nanomaterial | Among all metals, has the highest electrical conductivity, among all metals, has the best thermal conductivity, among all metals, has the best reflectivity, almost completely harmless to the human body. | Specific surface area is relatively small, for AChE immobilization, has worse catalytic ability than Au, the stability of the sensor is low. |
| CNTs | High surface area, abundant reaction sites, excellent electrochemical stability, high thermal conductivity, good mechanical and chemical stability, the sensor has good repeatability. | Poor dispersion, poor biocompatibility. |
| G/GO/rGO/ErGO | Abundant reaction sites, higher surface area than CNT, better conductivity and thermal conductivity than CNT, rGO has better conductivity, better dispersion than GO, easy manufacturing and relatively low cost than GO, sensor has good stability and repeatability. | Expensive and difficult to produce on a large scale, G is unstable with oxygen and heat, large graphene sheets contain some toxicity and impurities, size and thickness of G sheets are difficult to control. |
| Bimetallic nanomaterials | Combine the advantages of two metal elements. | Poor detection stability of the sensor, contains the disadvantages of two metal elements. |
| MNPs | Superparamagnetic or ferromagnetic, large surface area, high charge transfer capacity, excellent renewability. | High reactivity, low stability, potential genotoxicity. |
| MOF | Good structural tenability, high surface area, | Poor electronic conductivity, poor water stability. |
| QDs | Unique photocatalytic properties, long fluorescence lifetime. | High biological toxicity, chemical properties are relatively unstable, high demand for synthesis conditions, poor water solubility. |
| Black phosphorus and black phosphene BP | Good biodegradability, low cytotoxicity. | Low stability, seriously affect by the environment, reacts highly with water and oxygen, little research in the field of electrochemical biosensors. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Z.; Huang, Y.; Hu, X.; Zhang, J.; Chen, Q.; Chen, H. Recent Progress in Electrochemical Nano-Biosensors for Detection of Pesticides and Mycotoxins in Foods. Biosensors 2023, 13, 140. https://doi.org/10.3390/bios13010140
Gong Z, Huang Y, Hu X, Zhang J, Chen Q, Chen H. Recent Progress in Electrochemical Nano-Biosensors for Detection of Pesticides and Mycotoxins in Foods. Biosensors. 2023; 13(1):140. https://doi.org/10.3390/bios13010140
Chicago/Turabian StyleGong, Zhaoyuan, Yueming Huang, Xianjing Hu, Jianye Zhang, Qilei Chen, and Hubiao Chen. 2023. "Recent Progress in Electrochemical Nano-Biosensors for Detection of Pesticides and Mycotoxins in Foods" Biosensors 13, no. 1: 140. https://doi.org/10.3390/bios13010140
APA StyleGong, Z., Huang, Y., Hu, X., Zhang, J., Chen, Q., & Chen, H. (2023). Recent Progress in Electrochemical Nano-Biosensors for Detection of Pesticides and Mycotoxins in Foods. Biosensors, 13(1), 140. https://doi.org/10.3390/bios13010140






