Abstract
Conjugated polymer (CP)-based organic field-effect transistors (OFETs) have been considered a potential sensor platform for detecting gas molecules because they can amplify sensing signals by controlling the gate voltage. However, these sensors exhibit significantly poorer oxidizing gas sensing performance than their inorganic counterparts. This paper presents a high-performance nitric oxide (NO) OFET sensor consisting of a poly(3-hexylthiophene) (P3HT) film with an ultrathin nanoporous structure. The ultrathin nonporous structure of the P3HT film was created via deposition through the shear-coating-assisted phase separation of polymer blends and selective solvent etching. The ultrathin nonporous structure of the P3HT film enhanced NO gas diffusion, adsorption, and desorption, resulting in the ultrathin nanoporous P3HT-film-based OFET gas sensor exhibiting significantly better sensing performance than pristine P3HT-film-based OFET sensors. Additionally, upon exposure to 10 ppm NO at room temperature, the nanoporous P3HT-film-based OFET gas sensor exhibited significantly better sensing performance (i.e., responsivity ≈ 42%, sensitivity ≈ 4.7% ppm−1, limit of detection ≈ 0.5 ppm, and response/recovery times ≈ 6.6/8.0 min) than the pristine P3HT-film-based OFET sensors.
1. Introduction
Nitrogen oxides (NOx) are generated as by-products of the combustion of nitrogen-containing fossil fuels, and they generally refer to compounds such as nitric dioxide (NO2), nitrous oxide (N2O), and nitric oxide (NO) [1,2]. In 1990, NOx was classified as one of the criterion pollutants under the Clean Air Act regulations, because it is a hazardous gas that can cause serious damage to human health and the environment [1,3]. Many studies have been conducted to develop gas sensors that can detect and monitor NOx. However, most studies have focused on the detection of NO2 gas because NO2 molecules have a stronger electron affinity than other NOx molecules (N2O and NO2) and can thus be more easily detected using an electrochemical gas sensor [1,4,5]. NO gas is one of the major components of NOx, which is generally known to cause acid rain, photochemical smog, and ozone depletion. Additionally, NO rapidly oxidizes to NO2, resulting in adverse effects on human health and the environment [6,7,8,9]. For example, NO gas substantially causes cyanosis, asthma, itching, diabetes, pulmonary inflammation, and fatality [10,11,12]. Hence, NO detection has become an important concern in industrial processing and pollution control.
A typical example of a commercial gas sensor is a metal oxide gas sensor that operates on the principle of resistance change due to analyte–active layer reduction/oxidation (redox) reactions at high temperatures [13,14]. However, metal oxide gas sensors have the disadvantage of requiring additional energy to achieve high gas sensing performance [15,16]. In this regard, conjugated polymer (CP)-based organic field-effect transistor (OFET) sensors are considerably interesting because of their various advantages, such as low cost, low power, and room temperature operation [1,17,18]. When CP-based OFET sensors are exposed to a target gas environment, their active layer undergoes changes in its density of states based on the redox properties of the gas molecules, thereby modulating the OFET channel current [1]. This sensing mechanism of metal oxide sensors is typically based on direct redox reactions (e.g., the Brønsted redox reaction), which require a high-temperature reaction environment. Conversely, OFET gas sensors can detect gas molecules at low power levels and room temperature via interactions such as Van der Waals forces, dipole–dipole interactions, and dipole-induced dipole interactions [1,19,20]. However, OFET-based sensors exhibit relatively low gas sensing performance because their sensing mechanism results in a weaker sensing signal than that generated by the sensing mechanism of direct redox [1].
Recently, several studies have been conducted to enhance the sensing properties of OFET-based sensors by optimizing the active layer morphology to increase interactions between the analyte and active layer and by minimizing the active layer thickness to improve the diffusion of analytes into the layer of the materials [21,22,23]. For example, some methods, such as colloidal templating [24], the breath figure method [1,25], and lithography [26], have been developed to fabricate porous CP films. However, these methods have limitations because of their complexity, high cost, and scale-up problems. Additionally, they generate nonuniform CP films with microporous structures [27,28].
Herein, we report a high-performance NO OFET sensor consisting of a poly(3-hexylthiophene) (P3HT) film with an ultrathin nanoporous structure that was fabricated via the shear-assisted phase separation (SAPS) of polymer blends, followed by selective solvent etching. In this study, P3HT was chosen as the CP model because of its good solubility in organic solvents and acceptable charge carrier mobility [21,29,30]. P3HT served as the active layer that recognizes NO molecules and transports its detection signal. Importantly, the ultrathin nanoporous structure of the P3HT film facilitated enhanced adsorption/desorption and diffusion of NO molecules, resulting in highly improved sensing performance. In particular, the nanoporous P3HT (N-P3HT) active-layer-based OFET NO sensor exhibited better sensing properties (i.e., responsivity ≈ 42%, sensitivity ≈ 4.7% ppm−1, limit of detection ≈ 0.5 ppm, and response/recovery times ≈ 6.6/8.0 min) than pristine P3HT (P-P3HT)-based OFET sensors.
2. Materials and Methods
2.1. Chemicals
Regioregular P3HT (regioregularity ≈ 96%; MW ≈ 69 kDa) was obtained from Rieke Metals Inc. Polystyrene (PS) (MW ≈ 192 kDa), chloroform (anhydrous grade), and 1,4-dioxane (anhydrous, 99.8%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All the materials were used without further purification.
2.2. CP-Based OFET Gas Sensor Fabrication
The FET structure was constructed on a highly doped Si/SiO2 wafer substrate. Source and drain Au/Cr electrodes were photolithographically defined to have a channel length of 50 µm and a width of 2 mm and were deposited on this Si/SiO2 wafer substrate using a thermal evaporator (JVMS-23M151S, the Energy Convergence Core Facility, Chonnam National University, Republic of Korea). The deposited Au electrodes consisted of 50 nm contacts on a 3 nm Cr adhesion layer. A previously reported procedure was used to deposit the CP active layers by shear coating (4 mm s−1 of shearing speed) the polymer blend solution onto a precleaned FET substrate under ambient conditions [21]. For the shear coating, the FET substrate and a cover glass were placed on each vacuum chuck attached to a software-controlled microstage (Zaber X-LHM200A-E03), as shown in Figure 1a. The cover glass was positioned above the FET substrate and tilted at an angle of 6° relative to it, and the gap between them was fixed at 200 μm. The gap between the cover glass and the FET substrate was filled with approximately 20 μL of a P3HT/PS blend solution using a pipette. To prepare this P3HT/PS blend solution, P3HT and PS were each dissolved in chloroform at a concentration of 5 mg mL−1 and stirred with a magnetic stirrer at ~55 °C for at least 30 min. Thereafter, the P3HT and PS solutions were cooled to room temperature (22 °C) and then combined using a P3HT:PS mass ratio of 7:3 [21]. Subsequently, the P3HT/PS film was deposited on the FET substrate by utilizing a stepper motor to drag the substrate while the cover glass remained fixed on the chuck. The prepared OFET devices were annealed in a vacuum oven at 55 °C for over 12 h to remove any residual solvent in the deposited polymer film.
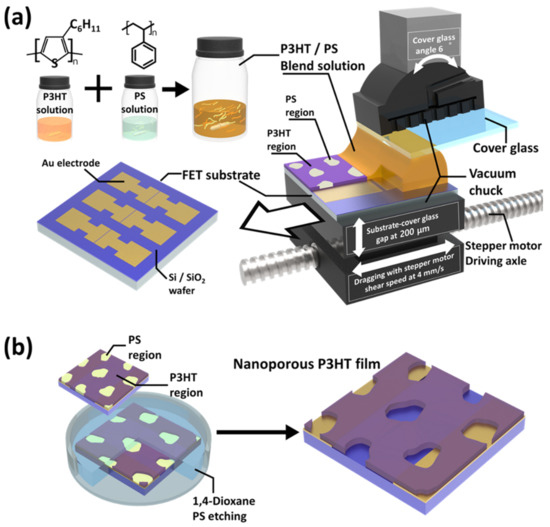
Figure 1.
Schematic illustration of (a) the preparation of the poly(3-hexylthiophene) (P3HT)/polystyrene (PS) blend film (weight ratio = 70:30) and (b) the creation of the nanoporous P3HT (N-P3HT) film using the shear-assisted phase separation (SAPS) method and selective solvent etching.
The phase-separated PS regions were removed from the P3HT/PS film that was deposited on the OFET substrate to generate a porous structure in the film (Figure 1b). The as-prepared OFET devices were immersed in a sufficient amount of 1,4-dioxane for more than 12 h. Subsequently, the OFET devices were removed from the 1,4-dioxane and dried with compressed air for a few seconds. The dried OFET devices were stored in a vacuum chamber for at least 12 h to remove any residual solvent.
2.3. Gas Sensing Evaluation and Characterization of the OFET Devices
A semiconductor analyzer (Keithley 4200-SCS) was used to measure the electrical properties of the OFET devices in an argon-filled glove box at room temperature. The charge carrier mobility was calculated from the saturation region (drain–source voltage (VDS) = −80 V) as follows:
where IDS is the drain–source current, μ is the charge carrier mobility, Ci is the capacitance per unit area of the dielectric layer of the OFET device, W and L are the channel width and length, respectively, and VGS and Vth are the gate–source and threshold voltages, respectively [31].
The NO sensor was tested using a homemade sensing chamber (volume: ~5 cm3) equipped with volumetric flow controllers and a data acquisition system. A certain concentration of NO gas and synthetic dry air (80% N2 and 20% O2) was introduced into the chamber through a volumetric flowmeter at a flow rate of 100 mL min−1. Standard NO gas (1000 ppm in N2, blending tolerance ±2%, Rigas Co. Ltd., Daejeon, Republic of Korea) was diluted with synthetic dry air to generate a gas mixture with various concentrations. The semiconductor analyzer was used as the data acquisition system to record real-time variations in the IDS value of the OFET sensor (VGS = 20 V and VDS = −80 V) at 3 min intervals. The responsivity of the sensor was calculated as follows:
where R (%) is the responsivity, and INO and Iair represent the initial currents of the sensor under NO gas and synthetic air conditions, respectively. The sensitivity of the gas sensor is defined as S = R/CNO (% ppm−1), where CNO is the NO concentration (ppm) [21]. The response time is the time required for the responsivity to increase to 90%, while the recovery time is the time required for the responsivity to decrease to 10% from its maximum value upon exposure to NO and synthetic air at 10 min intervals [21]. The film thickness and pore size of the active layer of the OFET sensor were observed using atomic force microscopy (AFM; NX20, Park Systems, Suwon, Republic of Korea).
3. Results and Discussion
Generally, the phase separation of P3HT and PS occurs owing to their low miscibility caused by their different solubility parameters (δP3HT = 14.8 ± 0.2 MPa1/2 and δPS = 17.9 ± 0.2 MPa1/2) [32]. To prepare the N-P3HT films, the P3HT/PS weight ratio of the blend solution was fixed at P3HT:PS = 7:3, and the shear speed was fixed at 4 mm s−1 during the SAPS process, according to a previous study [21]. Generally, the film thickness changes as the shearing speed changes during deposition using solution shear coating. Three coating regimes are basically defined based on the shear coating speed: the evaporation regime, transition regime, and Landau–Levich regime [21,33]. Polymer films with large thicknesses and pores are created both in the evaporation regime (ca. 0.5–2.0 mm s−1) and Landau–Levich regime (ca. 8.0–40 mm s−1), owing to their slow and fast shear speeds, respectively [21]. Thus, an intermediate shear speed (i.e., 4.0 mm s−1) was selected from the shear speed range belonging to the transition regime to fabricate P3HT films with the smallest possible thickness and nanopores. After the blend films were deposited on the FET substrate using the SAPS method, the PS regions of the P3HT/PS blend films were removed via selective solvent etching using 1,4-dioxane, which was used because it acts as a bad solvent for P3HT but a good one for PS [34,35]. The resulting N-P3HT film had a film thickness and pore size of approximately 8.1 nm and 0.017 µm2, respectively (Figure 2a,b; Table 1). The P-P3HT film had a film thickness of approximately 7.8 nm, indicating that both films had similar thicknesses. The N-P3HT film exhibited a higher surface area ratio (defined as the surface area/geometric area) of 1.15% than the P-P3HT film (0.06%), owing to their pores (Table 1). These results indicate that the N-P3HT film has a larger surface area than the P-P3HT film [21].
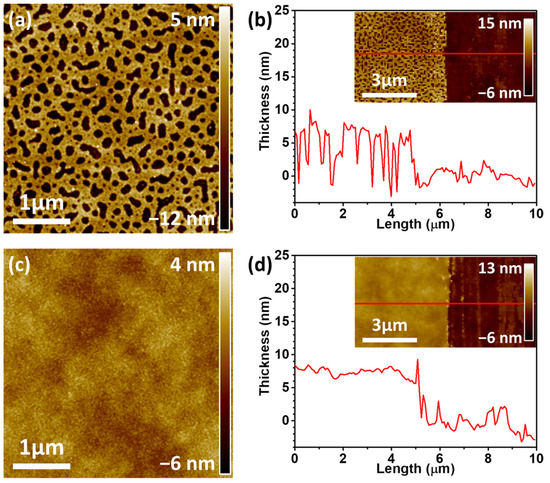
Figure 2.
AFM topology images and corresponding film thickness profiles (measured along the red line) of the (a,b) N-P3HT film and (c,d) pristine P3HT (P-P3HT) film.

Table 1.
Morphological properties of the P-P3HT and N-P3HT films prepared using the SAPS method at a shear speed of 4 mm s−1 and a solute/solvent ratio of 5 mg/mL.
The N-P3HT OFET sensor was tested using a homemade sensing chamber connected to volumetric controllers and a data acquisition system to evaluate its electrical properties and sensing performance (Figure 3a). A standard NO gas concentration (1000 ppm) was diluted with synthetic dry air using a volume flowmeter to create gas/air mixtures with different NO concentrations (0.5, 1, 2, 3, 4, 5, 10, 15, 20, and 30 ppm). A NO gas/air mixture containing a certain NO concentration and synthetic dry air (i.e., set as 0 ppm of the target gas) was selectively injected into the homemade sensing chamber through a two-way valve. Note that the oxidation of NO gas was not considered in this study, as it was mixed and injected into the sensing chamber within seconds [36]. As the NO gas was injected into the gas chamber, the NO molecules interacted with the P3HT active layer of the OFET gas sensor. Consequently, the channel current of the P3HT OFET gas sensor increased owing to the electron-withdrawing properties of the NO molecules [37], which is similar to the hole-doping mechanism of p-type semiconductors (Figure 3b,c) [1,38,39].
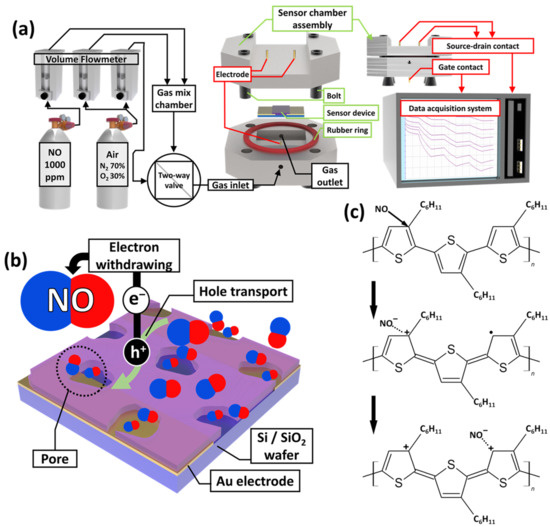
Figure 3.
Schematic illustration of (a) the NO gas flow, gas chamber, and data acquisition system. (b) Gas sensing mechanism of the N-P3HT-based OFET NO gas sensor for detecting NO molecules. (c) P3HT doping mechanism by adsorption of NO molecules.
As shown in Figure 4a,b, a semiconductor analyzer system was used to record the real-time variation in the electrical properties as the NO concentration changed. Generally, gas analytes are detected based on variations in the output current, threshold voltage, or charge carrier mobility of OFET sensors [21,40,41]. When the P-P3HT and N-P3HT OFET sensors were exposed to 30 ppm NO gas, their maximum current variation (29,960% and 42,975%, respectively) became more prominent than their threshold voltage variation (373% and 294%, respectively) and charge carrier mobility variation (116% and 42%, respectively) (Figure 4c–e and Figure S1). It should be noted that the maximum current variation value calculated based on the initial current (I0) recorded by the first exposure of synthetic air differs from the responsivity calculated based on the current (Iair) obtained by consecutive synthetic air exposure. This result indicates that measuring the output current variation of OFET sensors is more effective for detecting NO gas molecules than measuring their threshold voltage and charge carrier mobility. Additionally, selecting the optimum gate voltage of OFET sensors is crucial for amplifying their detection signal. Therefore, the I–V curves of the P-P3HT OFET and N-P3HT OFET sensors were obtained over various NO concentrations, as shown in Figure 4f and Figure S1a. The optimum gate voltage for both OFET sensors ranged between 10 and 30 V. Based on this result, a gate voltage of 20 V was selected and fixed for the NO sensing test over various NO concentrations. It should be noted that the N-P3HT OFET sensors had a higher charge carrier mobility than the P-P3HT OFET sensors (Figure 4e and Table S1). The high mobility of the N-P3HT OFET sensors is believed to be due to the PS chains that existed in the P3HT/PS blend films before PS removal. PS chains are known to facilitate the self-assembly of P3HT chains during the formation of P3HT/PS blend films (Figure S2), thereby improving the charge mobility of the blend films [42,43,44].
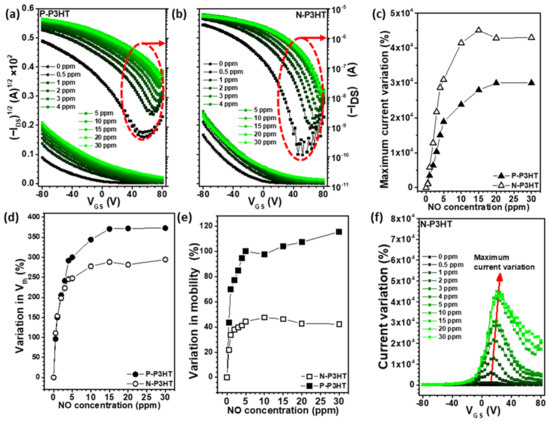
Figure 4.
Transfer curves of the OFETs based on the (a) P-P3HT and (b) N-P3HT active layers. The variation in the (c) maximum current, (d) threshold voltage, and (e) charge carrier mobility of the N-P3HT OFET and P-P3HT OFET sensors upon exposure to various NO concentrations. (f) Current variation ((ION − I0)/I0, %) over the gate voltage of the N-P3HT film.
Figure 5 shows the real-time responsivity and sensitivity of the P-P3HT and N-P3HT OFET sensors to various NO concentrations. Both sensors clearly exhibited baseline shifts (Figure 5a) and responsivity saturation (Figure 5b) as the NO concentration increased. The baseline shift and sensitivity saturation are related to the incomplete desorption of NO from the P3HT active layer of the sensor devices [21,45,46]. Consequently, the N-P3HT OFET sensor had a stronger response to NO concentrations than the P-P3HT OFET sensor (Figure 5a,b; Table 2). Specifically, the responsivity of the P-P3HT and N-P3HT OFET sensors increased from 5.1% to 28.1% and 10.1% to 42.5%, respectively, as the NO concentration increased from 0 to 10 ppm. However, the responsivity was nearly saturated when the NO concentration was further increased to 30 ppm, implying that the active sites of the P3HT layer were almost occupied with NO molecules [21]. Similarly, the sensitivity was calculated from the slope of the responsivity—NO concentration plots within a range of 0.5–10 ppm of NO [20,21], and the N-P3HT OFET sensor was found to have a higher sensitivity (4.71% ppm−1) than the P-P3HT OFET sensor (3.10% ppm−1) (Figure 5b). The excellent sensing behavior of the N-P3HT OFET sensor is attributed to its relatively large surface area (active site) and fast analyte diffusion caused by the nanoporous structure of the N-P3HT films. It should be noted that the responsivity of the P-P3HT and N-P3HT OFET sensors was not recorded at NO gas concentrations below 0.5, indicating that the limit of detection of both sensors was ~0.5 ppm.
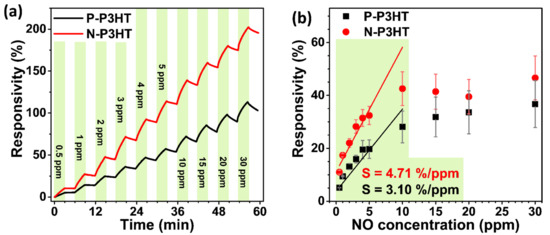
Figure 5.
(a) Real-time responsivity and (b) responsivity vs. NO concentration plots of the P-P3HT and N-P3HT OFET sensors.

Table 2.
Responsivity (%) of the P-P3HT-based and N-P3HT-based OFET sensors to various NO gas concentrations (on/off intervals of 3 min; VDS = −80 V and VGS = 20 V).
The reproducibility, response time, and recovery time of the N-P3HT OFET sensors, which are important parameters for practical application, were investigated by introducing 10 ppm NO gas and bare synthetic dry air alternately with a 10 min interval for 5 cycles, as shown in Figure 6 and Table S2. It should be noted that in general, the sensor signal of P3HT-based OFET sensors is not fully recovered due to the partial desorption of target gas molecules from the active layer of P3HT-based sensors, which also causes a corresponding baseline drift, as shown in Figure 6a [45,46,47,48,49]. As shown in Figure 6a, the N-P3HT OFET sensors exhibited a more stable response with a higher responsivity than the P-P3HT OFET sensors over the repeated cycles, demonstrating their good reproducibility and thereby practicability as gas sensors. Moreover, the N-P3HT OFET sensor exhibited better response and recovery times (6.6 and 8.0 min, respectively) than the P-P3HT OFET sensor (7.9 and 8.3 min, respectively) (Figure 6b and Table S2). The N-P3HT OFET sensor exhibited better sensing performance than the P-P3HT OFET sensor because of its superior morphological feature (i.e., its nanoporous structure), which can provide abundant active sites and facilitate rapid analyte adsorption, desorption, and diffusion [21]. Note that the sensing performance (i.e., responsivity, response/recovery time, and LOD) of the N-P3HT OFET sensors is comparable or superior to that of previously reported other types of gas sensors (e.g., inorganic and organic resistor type sensor) operated at room temperature (Table S3) [50,51,52,53]. Moreover, the sensing performance of the N-P3HT OFET sensors is better due to the easy control of pore size and the realization of smaller pore sizes, compared to that in previously reported studies on CP-based OFET NO sensors with a similar structure [1].
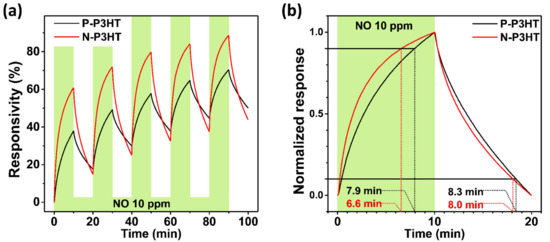
Figure 6.
(a) Time-dependent and (b) normalized responsivities of the P-P3HT OFET and N-P3HT OFET sensors.
4. Conclusions
Herein, we report highly improved NO gas sensing using an OFET sensor based on an ultrathin N-P3HT film. The resulting N-P3HT OFET sensor exhibited better NO gas sensing performance (responsivity ≈ ~42%, sensitivity ≈ ~4.7% ppm−1, response time ≈ 6.6 min, and recovery time ≈ 8.0 min) than the P-P3HT OFET sensor (28%, 3.1% ppm−1, 7.9min, and 8.3 min, respectively) at room temperature. This remarkable improvement was attributed to the nanoporous structure of the N-P3HT film, which (1) provided numerous active sites for NO gas molecules to interact with charge carriers in the P3HT film, resulting in improved responsivity, and (2) enabled rapid analyte diffusion, adsorption, and desorption, thereby improving the response and recovery times. Moreover, the N-P3HT OFET sensor showed excellent reproducibility with a stable response for five sensing cycles, demonstrating that it can be used for gas sensing. The N-P3HT OFET sensor presented herein could be a promising platform for detecting and monitoring gases that are harmful to human health and the environment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bios13010132/s1, Figure S1: (a) Current variation ((ION − I0)/I0) over the gate voltage of the P-P3HT film. (b) Threshold voltage and (c) charge carrier mobility of the corresponding P-P3HT and N-P3HT OFETs based on variations in the NO concentration; Figure S2: Normalized ultraviolet–visible absorption spectra of the P-P3HT and N-P3HT films. The P-P3HT film was fabricated using the shear coating method, and the N-P3HT film was fabricated using the SAPS method. The shear speed was set at 4 mm s−1; Table S1: Electrical properties of the P-P3HT-based and N-P3HT-based OFET devices under various NO concentrations; Table S2: Responsivity (%) and response/recovery times (min) of the P-P3HT-based and N-P3HT-based OFET NO sensors in relation to a 10 ppm NO concentration for five cycles (on/off intervals of 10 min); Table S3: Comparison of the gas sensing characteristics of various nitric oxide gas sensors.
Author Contributions
Conceptualization, G.J. and M.C.; methodology, G.J., S.Y.S. and P.K.; software, M.W. and H.J.C.; validation, S.Y.S. and P.K.; data curation, H.J.C. and E.W.; writing—original draft preparation, G.J. and S.Y.S.; writing—review and editing, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Korean Ministry of Education (NRF-2020R1I1A306849711), as well as the Basic Science Research Capacity Enhancement Project through the Korean Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Korean Ministry of Education (grant number: 2019R1A6C1010024).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and/or analyzed during this study are available upon reasonable request from the corresponding author.
Acknowledgments
The authors are grateful to the Center for Research Facilities at Chonnam National University for assisting with the AFM and GIXRD analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yu, S.H.; Girma, H.G.; Sim, K.M.; Yoon, S.; Park, J.M.; Kong, H.; Chung, D.S. Polymer-based flexible NO x sensors with ppb-level detection at room temperature using breath-figure molding. Nanoscale 2019, 11, 17709–17717. [Google Scholar] [CrossRef] [PubMed]
- Petruci, J.F.d.S.; Tütüncü, E.; Cardoso, A.A.; Mizaikoff, B. Real-Time and Simultaneous Monitoring of NO, NO2, and N2O Using Substrate–Integrated Hollow Waveguides Coupled to a Compact Fourier Transform Infrared (FT-IR) Spectrometer. Appl. Spectrosc. 2019, 73, 98–103. [Google Scholar] [PubMed]
- Lee, B. Highlights of the clean air act amendments off 1990. J. Air Waste Manag. Assoc. 1991, 41, 16–19. [Google Scholar] [CrossRef]
- Mirza, M.; Wang, J.; Wang, L.; He, J.; Jiang, C. Response enhancement mechanism of NO2 gas sensing in ultrathin pentacene field-effect transistors. Org. Electron. 2015, 24, 96–100. [Google Scholar] [CrossRef]
- Mane, A.; Moholkar, A. Effect of solution concentration on physicochemical and NO2 gas sensing properties of sprayed MoO3 nanobelts. Thin Solid Films 2018, 648, 50–61. [Google Scholar] [CrossRef]
- Suceska, M.; Tumara, B.S.; Skrlec, V.; Stankovic, S. Prediction of concentration of toxic gases produced by detonation of commercial explosives by thermochemical equilibrium calculations. Def. Technol. 2021, 18, 2181–2189. [Google Scholar] [CrossRef]
- Wu, S.; Yu, X.; Zhang, J.; Zhang, Y.; Zhu, Y.; Zhu, M. Construction of BiOCl/CuBi2O4 S-scheme heterojunction with oxygen vacancy for enhanced photocatalytic diclofenac degradation and nitric oxide removal. Chem. Eng. J. 2021, 411, 128555. [Google Scholar] [CrossRef]
- Hamed, A.M.; Wahab, B.I. Measuring the Acid Rain in Heet City of Iraq. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Raipur, India, 26–30 June 2022; p. 012022. [Google Scholar]
- Panigrahi, T.H.; Sahoo, S.R.; Murmu, G.; Maity, D.; Saha, S. Current challenges and developments of inorganic/organic materials for the abatement of toxic nitrogen oxides (NOx)–A critical review. Prog. Solid. State Chem. 2022, 68, 100380. [Google Scholar] [CrossRef]
- García-Morin, M.; Manrique-Martin, G.; Ropero, P.; Bardón-Cancho, E.; García-Rovés, R.Á.; Beléndez, C.; Cela, E. Hb M-Saskatoon: An unusual cause of cyanosis in a Spanish child. Pediatr. Hematol. Oncol. J. 2019, 4, 23–26. [Google Scholar] [CrossRef]
- Porrini, C.; Ramarao, N.; Tran, S.-L. Dr. NO and Mr. Toxic–the versatile role of nitric oxide. Biol. Chem. 2020, 401, 547–572. [Google Scholar] [CrossRef]
- Khot, S.; Phalake, S.; Mahadik, S.; Baragale, M.; Jagadale, S.; Burungale, V.; Navale, Y.; Patil, V.; Patil, V.; Patil, P. Synthesis of CuO thin film sensors by spray pyrolysis method for NO2 gas detection. Mater. Today Proc. 2021, 43, 2694–2697. [Google Scholar] [CrossRef]
- Urasinska-Wojcik, B.; Vincent, T.A.; Chowdhury, M.F.; Gardner, J.W. Ultrasensitive WO3 gas sensors for NO2 detection in air and low oxygen environment. Sens. Actuators B Chem. 2017, 239, 1051–1059. [Google Scholar] [CrossRef]
- Shendage, S.; Patil, V.; Vanalakar, S.; Patil, S.; Harale, N.; Bhosale, J.; Kim, J.; Patil, P. Sensitive and selective NO2 gas sensor based on WO3 nanoplates. Sens. Actuators B Chem. 2017, 240, 426–433. [Google Scholar] [CrossRef]
- Chatterjee, S.G.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sens. Actuators B Chem. 2015, 221, 1170–1181. [Google Scholar] [CrossRef]
- Long, H.; Harley-Trochimczyk, A.; Pham, T.; Tang, Z.; Shi, T.; Zettl, A.; Carraro, C.; Worsley, M.A.; Maboudian, R. High surface area MoS2/graphene hybrid aerogel for ultrasensitive NO2 detection. Adv. Funct. Mater. 2016, 26, 5158–5165. [Google Scholar] [CrossRef]
- Li, S.; Diao, Y.; Yang, Z.; He, J.; Wang, J.; Liu, C.; Liu, F.; Lu, H.; Yan, X.; Sun, P. Enhanced room temperature gas sensor based on Au-loaded mesoporous In2O3 nanospheres@ polyaniline core-shell nanohybrid assembled on flexible PET substrate for NH3 detection. Sens. Actuators B Chem. 2018, 276, 526–533. [Google Scholar] [CrossRef]
- Jian, Y.; Hu, W.; Zhao, Z.; Cheng, P.; Haick, H.; Yao, M.; Wu, W. Gas sensors based on chemi-resistive hybrid functional nanomaterials. Nano-Micro Lett. 2020, 12, 71. [Google Scholar] [CrossRef]
- Zhang, F.; Qu, G.; Mohammadi, E.; Mei, J.; Diao, Y. Solution-processed nanoporous organic semiconductor thin films: Toward health and environmental monitoring of volatile markers. Adv. Funct. Mater. 2017, 27, 1701117. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, L.; Zhu, X.; Zhou, X.; Chi, L. An ultrasensitive organic semiconductor NO2 sensor based on crystalline TIPS-Pentacene films. Adv. Mater. 2017, 29, 1703192. [Google Scholar] [CrossRef]
- Tran, V.V.; Jeong, G.; Kim, K.S.; Kim, J.; Jung, H.-R.; Park, B.; Park, J.-J.; Chang, M. Facile Strategy for Modulating the Nanoporous Structure of Ultrathin π-Conjugated Polymer Films for High-Performance Gas Sensors. ACS Sens. 2021, 7, 175–185. [Google Scholar] [CrossRef]
- Darshan, V.; Rajeev, V.; Unni, K.N. Enhanced performance of room temperature ammonia sensors using morphology-controlled organic field-effect transistors. Org. Electron. 2021, 98, 106280. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, S.; Huang, J. OFET chemical sensors: Chemical sensors based on ultrathin organic field-effect transistors. Polym. Int. 2021, 70, 414–425. [Google Scholar] [CrossRef]
- Pernites, R.B.; Foster, E.L.; Felipe, M.J.L.; Robinson, M.; Advincula, R.C. Patterned surfaces combining polymer brushes and conducting polymer via colloidal template electropolymerization. Adv. Mater. 2011, 23, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Bai, H.; Li, L. Breath figure: A nature-inspired preparation method for ordered porous films. Chem. Rev. 2015, 115, 9801–9868. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro-and nanoscale patterning. Nat. Protoc. 2010, 5, 491. [Google Scholar] [CrossRef]
- Guo, X.; Liu, L.; Zhuang, Z.; Chen, X.; Ni, M.; Li, Y.; Cui, Y.; Zhan, P.; Yuan, C.; Ge, H. A new strategy of lithography based on phase separation of polymer blends. Sci. Rep. 2015, 5, 15947. [Google Scholar] [CrossRef]
- Hulkkonen, H.H.; Salminen, T.; Niemi, T. Block copolymer patterning for creating porous silicon thin films with tunable refractive indices. ACS Appl. Mater. Interfaces 2017, 9, 31260–31265. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.; Song, J.; Jang, H.-J.; Dailey, J.; Yu, J.; Katz, H.E. Chemical and biomolecule sensing with organic field-effect transistors. Chem. Rev. 2018, 119, 3–35. [Google Scholar] [CrossRef]
- Chang, M.; Lee, J.; Kleinhenz, N.; Fu, B.; Reichmanis, E. Photoinduced anisotropic supramolecular assembly and enhanced charge transport of poly (3-hexylthiophene) thin films. Adv. Funct. Mater. 2014, 24, 4457–4465. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, H.; Liu, A.; Zhu, H.; Li, B.; Minari, T.; Balestra, F.; Ghibaudo, G.; Noh, Y.Y. Essential effects on the mobility extraction reliability for organic transistors. Adv. Funct. Mater. 2018, 28, 1803907. [Google Scholar] [CrossRef]
- Emerson, J.A.; Toolan, D.T.; Howse, J.R.; Furst, E.M.; Epps, T.H., III. Determination of solvent–polymer and polymer–polymer Flory–Huggins interaction parameters for poly (3-hexylthiophene) via solvent vapor swelling. Macromolecules 2013, 46, 6533–6540. [Google Scholar] [CrossRef]
- Niu, X.; Li, N.; Chen, Q.; Zhou, H. Insights into large-scale fabrication methods in perovskite photovoltaics. Adv. Energy Sustain. Res. 2021, 2, 2000046. [Google Scholar] [CrossRef]
- Heo, K.; Miesch, C.; Na, J.-H.; Emrick, T.; Hayward, R.C. Assembly of P3HT/CdSe nanowire networks in an insulating polymer host. Soft Matter 2018, 14, 5327–5332. [Google Scholar] [CrossRef]
- Otulakowski, L.; Dworak, A.; Forys, A.; Gadzinowski, M.; Slomkowski, S.; Basinska, T.; Trzebicka, B. Micellization of polystyrene-b-polyglycidol in dioxane and water/dioxane solutions. Polymers 2020, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, B.; Laskin, D.L.; Heck, D.E.; Laskin, J.D. The toxicology of inhaled nitric oxide. Toxicol. Sci. 2001, 59, 5–16. [Google Scholar] [CrossRef]
- Yoo, R.; Lee, H.-S.; Kim, W.; Park, Y.; Koo, A.; Jin, S.-H.; Pham, T.V.; Kim, M.J.; Maeng, S.; Lee, W. Selective detection of nitrogen-containing compound gases. Sensors 2019, 19, 3565. [Google Scholar] [CrossRef]
- Kumar, R.; Jenjeti, R.N.; Choutipalli, V.S.K.; Subramanian, V.; Sampath, S. Conductometric NOx sensor based on exfoliated two-dimensional layered MnPSe3. Sens. Actuators B Chem. 2021, 347, 130633. [Google Scholar] [CrossRef]
- Kwon, E.H.; An, H.; Park, M.B.; Kim, M.; Park, Y.D. Conjugated polymer–zeolite hybrids for robust gas sensors: Effect of zeolite surface area on NO2 sensing ability. Chem. Eng. J. 2021, 420, 129588. [Google Scholar] [CrossRef]
- Yu, J.; Yu, X.; Zhang, L.; Zeng, H. Ammonia gas sensor based on pentacene organic field-effect transistor. Sens. Actuators B Chem. 2012, 173, 133–138. [Google Scholar] [CrossRef]
- Verma, A.; Sahu, P.K.; Chaudhary, V.; Singh, A.K.; Mishra, V.; Prakash, R. Fabrication and Characterization of P3HT/MoS₂ Thin-Film Based Ammonia Sensor Operated at Room Temperature. IEEE Sens. J. 2022, 22, 10361–10369. [Google Scholar] [CrossRef]
- Jeong, G.; Choi, S.; Jang, M.; Chang, M. Thermal annealing effects on the morphology and charge transport of polymer semiconductor nanowires aligned in an insulating polymer matrix. Dye. Pigment. 2021, 185, 108962. [Google Scholar] [CrossRef]
- Choi, S.; Jeong, J.W.; Jo, G.; Ma, B.C.; Chang, M. Conjugated polymer/paraffin blends for organic field-effect transistors with high environmental stability. Nanoscale 2019, 11, 10004–10016. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Choi, D.; Wang, G.; Kleinhenz, N.; Persson, N.; Park, B.; Reichmanis, E. Photoinduced anisotropic assembly of conjugated polymers in insulating polymer blends. ACS Appl. Mater. Interfaces 2015, 7, 14095–14103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, B.; Huang, L.; Huang, W.; Wang, Z.; Zhu, W.; Chen, Y.; Mao, Y.; Facchetti, A.; Marks, T.J. Breath figure–derived porous semiconducting films for organic electronics. Sci. Adv. 2020, 6, eaaz1042. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yang, Z.; Li, Z.; Zhuang, X.; Akinwande, D.; Yu, J. Improved room temperature NO2 sensing performance of organic field-effect transistor by directly blending a hole-transporting/electron-blocking polymer into the active layer. ACS Appl. Mater. Interfaces 2018, 10, 38280–38286. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.J.; Shin, S.Y.; Van Tran, V.; Park, B.; Yoon, H.; Chang, M. Preparation of conjugated polymer/reduced graphene oxide nanocomposites for high-performance volatile organic compound sensors. Chem. Eng. J. 2021, 425, 131424. [Google Scholar] [CrossRef]
- Han, S.; Zhuang, X.; Shi, W.; Yang, X.; Li, L.; Yu, J. Poly (3-hexylthiophene)/polystyrene (P3HT/PS) blends based organic field-effect transistor ammonia gas sensor. Sens. Actuators B Chem. 2016, 225, 10–15. [Google Scholar] [CrossRef]
- Hou, S.; Yu, J.; Zhuang, X.; Li, D.; Liu, Y.; Gao, Z.; Sun, T.; Wang, F.; Yu, X. Phase separation of P3HT/PMMA blend film for forming semiconducting and dielectric layers in organic thin-film transistors for high-sensitivity NO2 detection. ACS Appl. Mater. Interfaces 2019, 11, 44521–44527. [Google Scholar] [CrossRef]
- Yeh, Y.-M.; Chang, S.-J.; Wang, P.-H.; Hsueh, T.-J. A TSV-Structured Room Temperature p-Type TiO2 Nitric Oxide Gas Sensor. Appl. Sci. 2022, 12, 9946. [Google Scholar] [CrossRef]
- Gupta Chatterjee, S.; Dey, S.; Samanta, D.; Santra, S.; Chatterjee, S.; Guha, P.; Chakraborty, A.K. Near room temperature sensing of nitric oxide using SnO2/Ni-decorated natural cellulosic graphene nanohybrid film. J. Mater. Sci. Mater. Electron. 2018, 29, 20162–20171. [Google Scholar] [CrossRef]
- Khasim, S.; Pasha, A.; Badi, N.; Imran, M.; Al-Ghamdi, S. Development of high-performance flexible and stretchable sensor based on secondary doped PEDOT–PSS: TiO2 nanocomposite for room-temperature detection of nitric oxide. J. Mater. Sci. Mater. Electron. 2021, 32, 7491–7508. [Google Scholar] [CrossRef]
- Gusain, A.; Joshi, N.J.; Varde, P.; Aswal, D. Flexible NO gas sensor based on conducting polymer poly [N-9′-heptadecanyl-2, 7-carbazole-alt-5, 5-(4′, 7′-di-2-thienyl-2′, 1′, 3′-benzothiadiazole)](PCDTBT). Sens. Actuators B Chem. 2017, 239, 734–745. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).