Gold Nanoparticle-Based Enzyme-Assisted Cyclic Amplification for the Highly-Sensitive Detection of miRNA-21
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Preparation of the Reagents
2.4. Synthesis of the AuNPs
2.5. Functionalization of the AuNPs
2.6. SIB Functionalization
2.7. Detection of Target miRNA-21
2.8. Testing of miRNA-21
3. Results and Discussion
3.1. Principle of miRNA Detection
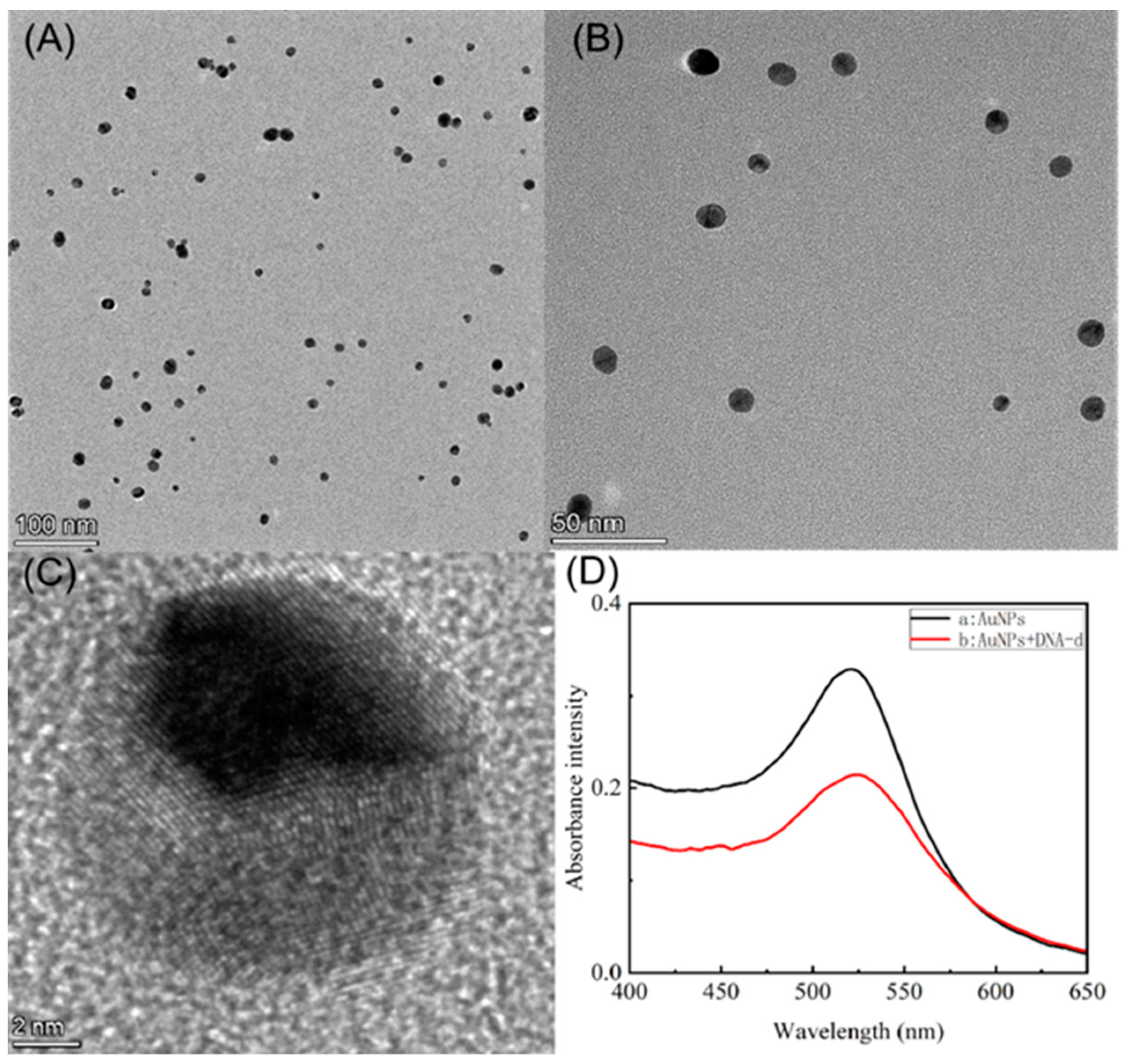
3.2. Characterization of the AuNPs
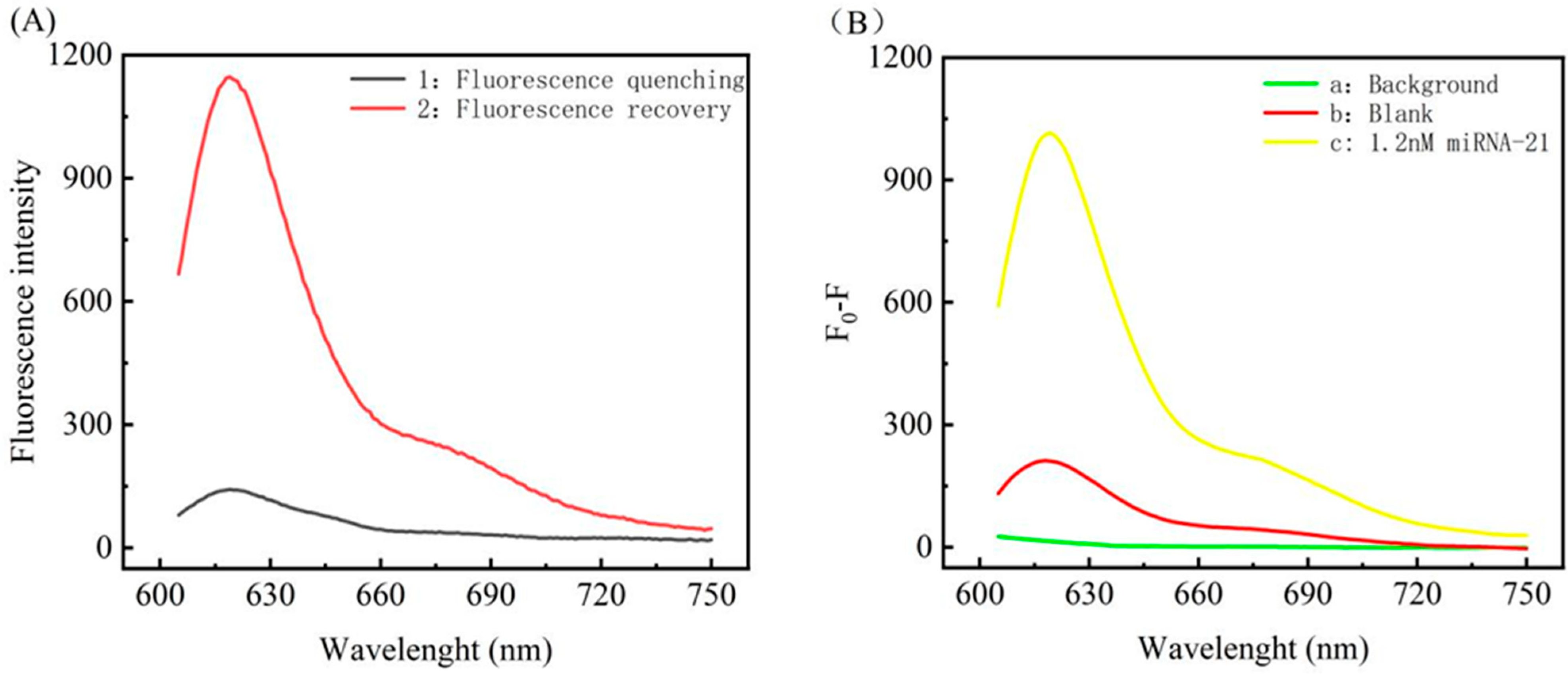
3.3. Feasibility Study of the miRNA Assay
3.4. Optimization of the Experimental Conditions
3.4.1. AuNPs–DNA Optimization
3.4.2. Exo III Optimization
3.4.3. Temperature Optimization
3.4.4. Time Optimization
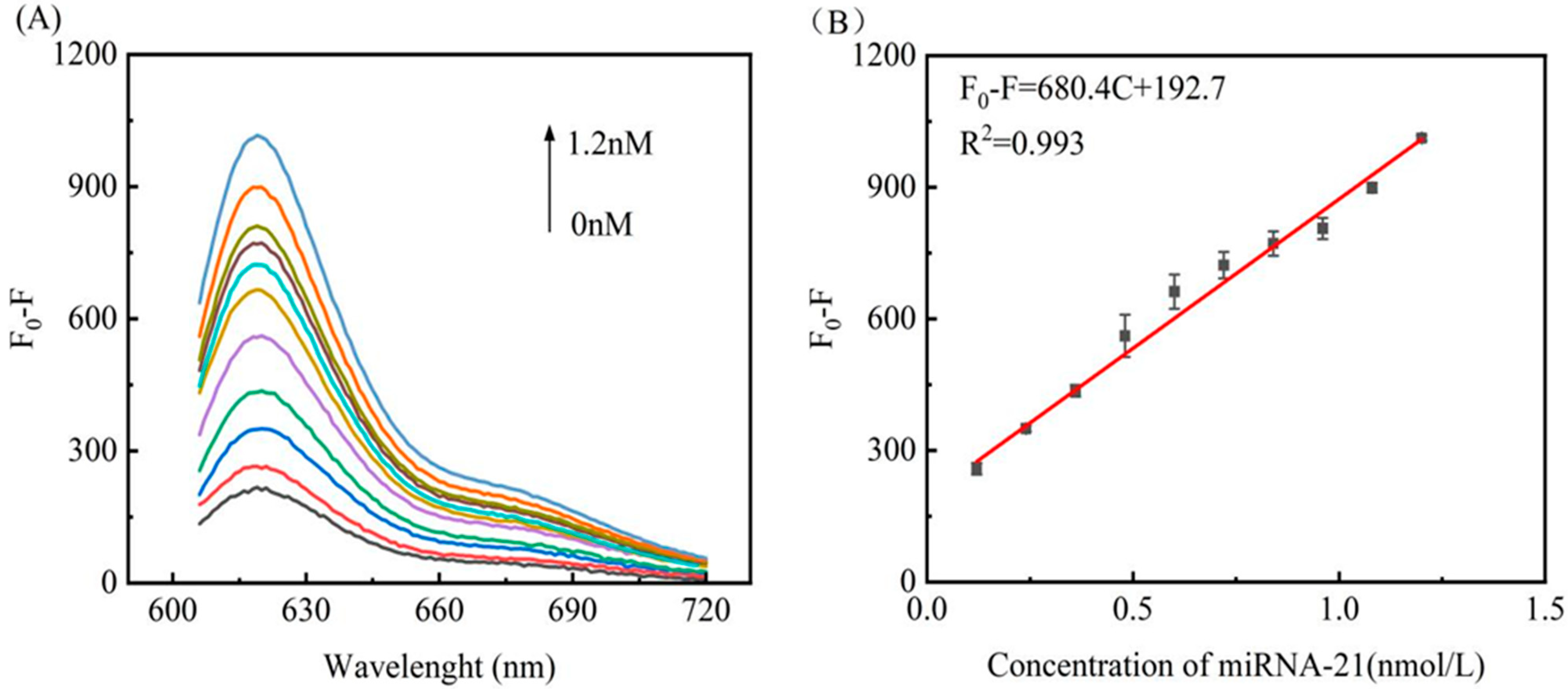
3.5. Assay Performance for miRNA-21 Detection
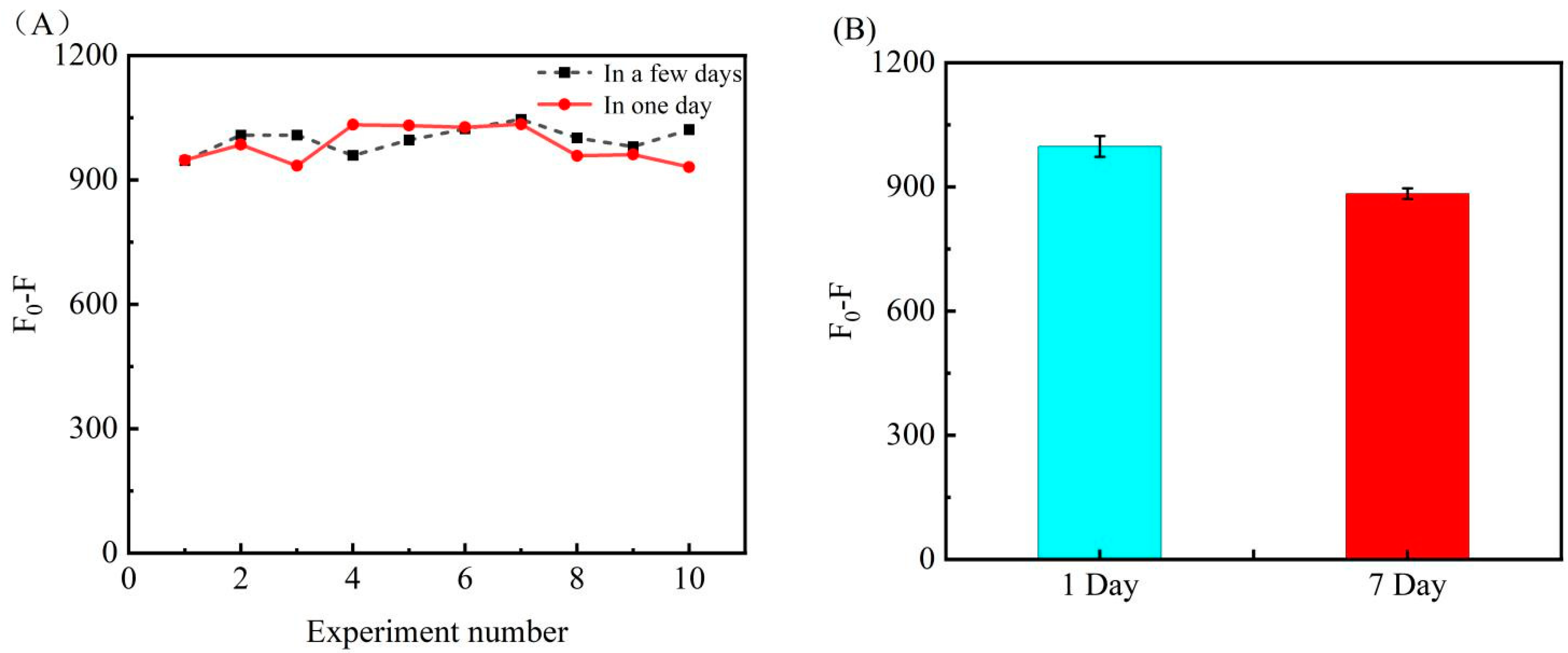
3.6. Repeatability and Longtime Stability of Biosensor
3.7. Specificity Analysis
3.8. Determination of Target DNA in Human Serum Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA Translation and Stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [PubMed]
- Gangaraju, V.K.; Lin, H. MicroRNAs: Key regulators of stem cells. Nat. Rev. Mol. Cell Biol. 2009, 10, 116–125. [Google Scholar] [PubMed]
- Cullen, B.R. Viral and cellular messenger RNA targets of viral microRNAs. Nature 2009, 457, 421–425. [Google Scholar] [PubMed]
- Nicoloso, M.S.; Spizzo, R.; Shimizu, M.; Rossi, S.; Calin, G.A. MicroRNAs—The micro steering wheel of tumour metastases. Nat. Rev. Cancer 2009, 9, 293–302. [Google Scholar]
- He, C.; Chen, S.; Zhao, J.; Tian, J.; Zhao, S. Ultrasensitive detection of microRNA-21 based on electrophoresis assisted cascade chemiluminescence signal amplification for the identification of cancer cells. Talanta 2020, 209, 120505. [Google Scholar]
- Li, S.; Yang, X.; Yang, J.; Zhen, J.; Zhang, D. Serum microRNA-21 as a potential diagnostic biomarker for breast cancer: A systematic review and meta-analysis. Clin. Exp. Med. 2016, 16, 29–35. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar]
- Saheb Sharif-Askari, N.; Saheb Sharif-Askari, F.; Guraya, S.Y.; Bendardaf, R.; Hamoudi, R. Integrative systematic review meta-analysis and bioinformatics identifies MicroRNA-21 and its target genes as biomarkers for colorectal adenocarcinoma. Int. J. Surg. 2020, 73, 113–122. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar]
- Hill, M.; Tran, N. Global miRNA to miRNA Interactions: Impacts for miRNA-21. Trends Cell Biol. 2021, 31, 3–5. [Google Scholar]
- Tabnak, P.; Masrouri, S.; Geraylow, K.R.; Zarei, M.; Esmailpoor, Z.H. Targeting miRNAs with anesthetics in cancer: Current understanding and future perspectives. Biomed. Pharmacother. 2021, 144, 112309. [Google Scholar] [PubMed]
- Huang, J.; Shangguan, J.; Guo, Q.; Ma, W.; Wang, H.; Jia, R.; Ye, Z.; He, X.; Wang, K. Colorimetric and fluorescent dual-mode detection of microRNA based on duplex-specific nuclease assisted gold nanoparticle amplification. Analyst 2019, 144, 4917–4924. [Google Scholar] [PubMed]
- Li, W.; Ruan, K. MicroRNA detection by microarray. Anal. Bioanal. Chem. 2009, 394, 1117–1124. [Google Scholar] [PubMed]
- Várallyay, É.; Burgyán, J.; Havelda, Z. MicroRNA detection by northern blotting using locked nucleic acid probes. Nat. Protoc. 2008, 3, 190–196. [Google Scholar] [PubMed]
- Biggar, K.K.; Kornfeld, S.F.; Storey, K.B. Amplification and sequencing of mature microRNAs in uncharacterized animal models using stem–loop reverse transcription–polymerase chain reaction. Anal. Biochem. 2011, 416, 231–233. [Google Scholar]
- Wang, Q.; Sun, H.; Wen, D.; Wang, L.; Li, L.; Kong, J.; Zhang, X. Ultrasensitive electrochemical detection of miRNA based on polymerization signal amplification. Talanta 2021, 235, 122744. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, X.; Huang, H.; Wu, J. Exosome based miRNA delivery strategy for disease treatment. Chin. Chem. Lett. 2022, 33, 1693–1704. [Google Scholar]
- Li, T.; Duan, R.; Duan, Z.; Huang, F.; Xia, F. Fluorescence Signal Amplification Strategies Based on DNA Nanotechnology for miRNA Detection. Chem. Res. Chin. Univ. 2020, 36, 194–202. [Google Scholar]
- Abdullah Al-maskri, A.A.; Ye, J.; Talap, J.; Hu, H.; Sun, L.; Yu, L.; Cai, S.; Zeng, S. Reverse transcription-based loop-mediated isothermal amplification strategy for real-time miRNA detection with phosphorothioated probes. Anal. Chim. Acta 2020, 1126, 1–6. [Google Scholar]
- Wang, R.; Lan, L.; Liu, L.; Cheng, L. Asymmetric polymerase chain reaction and loop-mediated isothermal amplification (AP-LAMP) for ultrasensitive detection of microRNAs. Chin. Chem. Lett. 2020, 31, 159–162. [Google Scholar]
- Mansourian, N.; Rahaie, M.; Hosseini, M. A Nanobiosensor Based on Fluorescent DNA-Hosted Silver Nanocluster and HCR Amplification for Detection of MicroRNA Involved in Progression of Multiple Sclerosis. J. Fluoresc. 2017, 27, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yang, K.; Feng, Z.; Xie, Y.; Han, W.; Chen, Q.; Li, S.; Zhang, Y.; Yu, Y.; Zou, G. Selective and sensitive detection of miRNA-198 using single polymeric microfiber waveguide platform with heterogeneous CHA amplification strategy. Talanta 2022, 240, 123218. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Fu, S.; Lu, J.; Hu, K.; Liang, X.; Li, Q.; Zhao, S.; Tian, J. Detection of microRNA using enzyme-assisted amplifying and DNA-templated silver nanoclusters signal-off fluorescence bioassay. Talanta 2020, 210, 120623. [Google Scholar] [CrossRef]
- Xu, L.; Shoaie, N.; Jahanpeyma, F.; Zhao, J.; Azimzadeh, M.; Al−Jamal, K.T. Optical, electrochemical and electrical (nano)biosensors for detection of exosomes: A comprehensive overview. Biosens. Bioelectron. 2020, 161, 112222. [Google Scholar] [CrossRef]
- Jannathul Firdhouse, M.; Lalitha, P. Biogenic green synthesis of gold nanoparticles and their applications—A review of promising properties. Inorg. Chem. Commun. 2022, 143, 109800. [Google Scholar] [CrossRef]
- Malathi, S.; Pakrudheen, I.; Kalkura, S.N.; Webster, T.J.; Balasubramanian, S. Disposable biosensors based on metal nanoparticles. Sens. Int. 2022, 3, 100169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, L.; Yu, Q.; Qiu, W.; Li, K.; Cheng, L.; Zhang, T.; Qian, L.; Zhang, X.; Liu, G. Gold-platinum nanoflowers as colored and catalytic labels for ultrasensitive lateral flow MicroRNA-21 assay. Sens. Actuators B Chem. 2021, 344, 130325. [Google Scholar] [CrossRef]
- He, W.; Li, S.; Wang, L.; Zhu, L.; Zhang, Y.; Luo, Y.; Huang, K.; Xu, W. AuNPs-DNAzyme molecular motor biosensor mediated by neighborhood click chemistry reactions for the ultrasensitive detection of microRNA-155. Sens. Actuators B Chem. 2019, 290, 503–511. [Google Scholar] [CrossRef]
- Yang, W.; Tian, J.; Ma, Y.; Wang, L.; Zhao, Y.; Zhao, S. A label-free fluorescent probe based on DNA-templated silver nanoclusters and exonuclease III-assisted recycling amplification detection of nucleic acid. Anal. Chim. Acta 2015, 900, 90–96. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H.; Amini, B. A fluorescence Nano-biosensors immobilization on Iron (MNPs) and gold (AuNPs) nanoparticles for detection of Shigella spp. Mater. Sci. Eng. C 2019, 105, 110113. [Google Scholar] [CrossRef]
- Shan, S.; Zhong, Z.; Lai, W.; Xiong, Y.; Cui, X.; Liu, D. Immunomagnetic nanobeads based on a streptavidin-biotin system for the highly efficient and specific separation of Listeria monocytogenes. Food Control 2014, 45, 138–142. [Google Scholar] [CrossRef]
- Shi, L.; Buhler, E.; Boué, F.; Carn, F. How does the size of gold nanoparticles depend on citrate to gold ratio in Turkevich synthesis? Final answer to a debated question. J. Colloid Interface Sci. 2017, 492, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Gu, Y.; Lu, H.; Dong, H.; Zhang, K.; Dai, W.; Meng, X.; Yang, F.; Zhang, X. Highly-sensitive microRNA detection based on bio-bar-code assay and catalytic hairpin assembly two-stage amplification. Anal. Chim. Acta 2018, 1004, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, L.; Xu, X.; Jiang, W. An enzyme-free and label-free fluorescence biosensor for microRNA detection based on cascade amplification of DNAzyme-powered three-dimensional DNA walker and hybridization chain reaction. Sens. Actuators B Chem. 2018, 268, 287–292. [Google Scholar] [CrossRef]



| Sample | Added (nM) | Found (nM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 0.24 | 0.218 0.221 0.244 | 90.8 95.4 95.5 | 6.4 |
| 2 | 0.54 | 0.538 0.525 0.525 | 97.9 95.5 95.5 | 1.4 |
| 3 | 0.75 | 0.747 0.753 0.750 | 99.6 100.4 100.0 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qing, Y.; Yang, Y.; Ouyang, P.; Fang, C.; Fang, H.; Liao, Y.; Li, H.; Wang, Z.; Du, J. Gold Nanoparticle-Based Enzyme-Assisted Cyclic Amplification for the Highly-Sensitive Detection of miRNA-21. Biosensors 2022, 12, 724. https://doi.org/10.3390/bios12090724
Qing Y, Yang Y, Ouyang P, Fang C, Fang H, Liao Y, Li H, Wang Z, Du J. Gold Nanoparticle-Based Enzyme-Assisted Cyclic Amplification for the Highly-Sensitive Detection of miRNA-21. Biosensors. 2022; 12(9):724. https://doi.org/10.3390/bios12090724
Chicago/Turabian StyleQing, Yang, Yuxing Yang, Ping Ouyang, Chenxin Fang, Haobin Fang, Yazhen Liao, Haiyu Li, Zhencui Wang, and Jie Du. 2022. "Gold Nanoparticle-Based Enzyme-Assisted Cyclic Amplification for the Highly-Sensitive Detection of miRNA-21" Biosensors 12, no. 9: 724. https://doi.org/10.3390/bios12090724
APA StyleQing, Y., Yang, Y., Ouyang, P., Fang, C., Fang, H., Liao, Y., Li, H., Wang, Z., & Du, J. (2022). Gold Nanoparticle-Based Enzyme-Assisted Cyclic Amplification for the Highly-Sensitive Detection of miRNA-21. Biosensors, 12(9), 724. https://doi.org/10.3390/bios12090724





