Functional Evaluation and Nephrotoxicity Assessment of Human Renal Proximal Tubule Cells on a Chip
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Design of the Integrated Biomimetic Array Chip
2.2. Cells Culture
2.3. Construction of Human Renal Proximal Tubules on the iBAC
2.4. TEER Assay
2.5. Measurement of Paracellular Permeability
2.6. Morphological Studies
2.7. Quantitative Real-Time PCR
2.8. Analysis of Cell Viability on the iBAC
2.9. Data Analysis and Quantification
3. Results
3.1. Characteristics of Human Renal Proximal Tubule Model on the iBAC
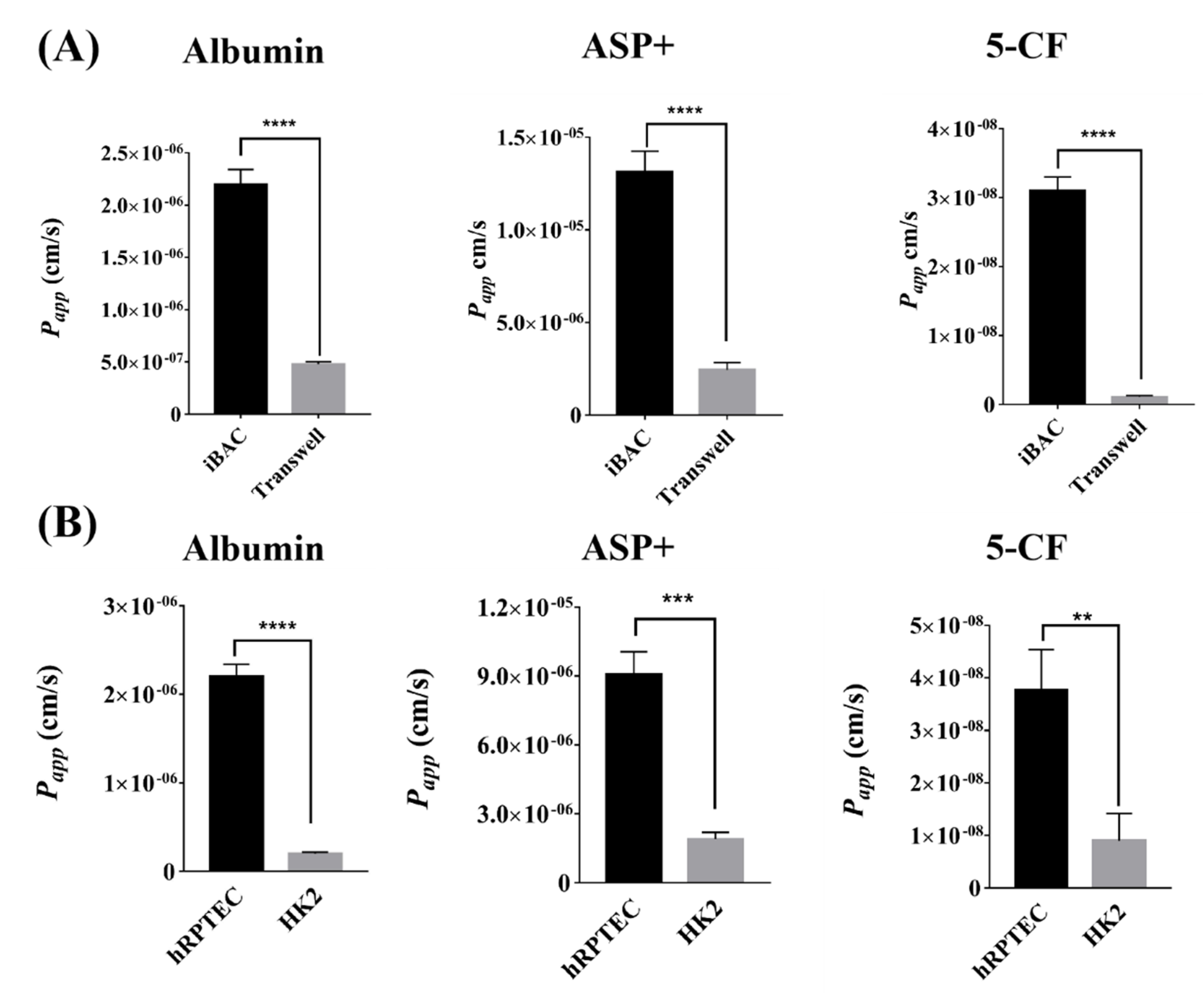
3.2. The Barrier Function of Human Renal Proximal Tubule Model on the iBAC
3.3. The Active Absorption Function of Human Renal Proximal Tubule Model on the iBAC
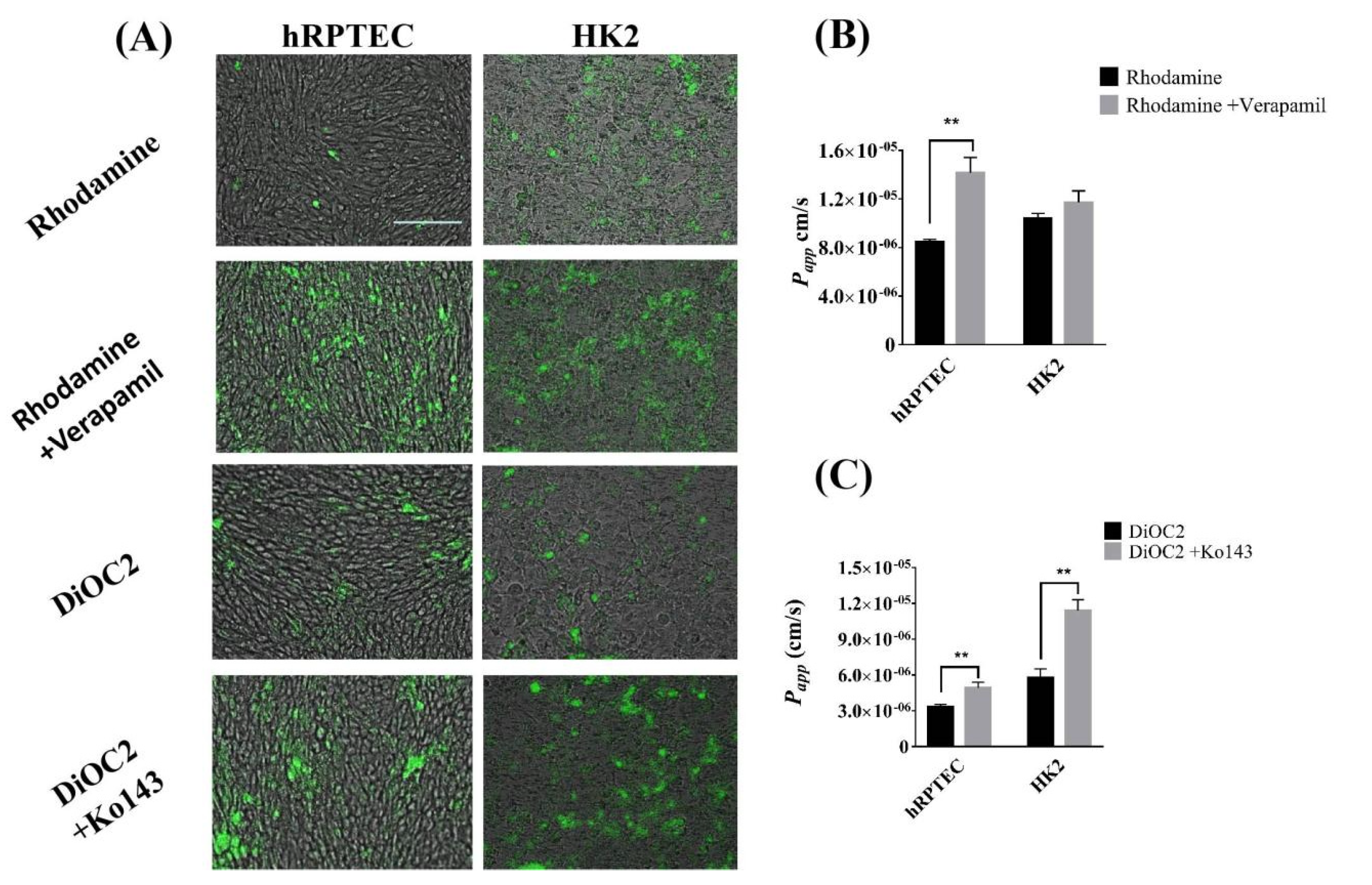
3.4. The Efflux Transporter Function of Human Renal Proximal Tubule Model on the iBAC
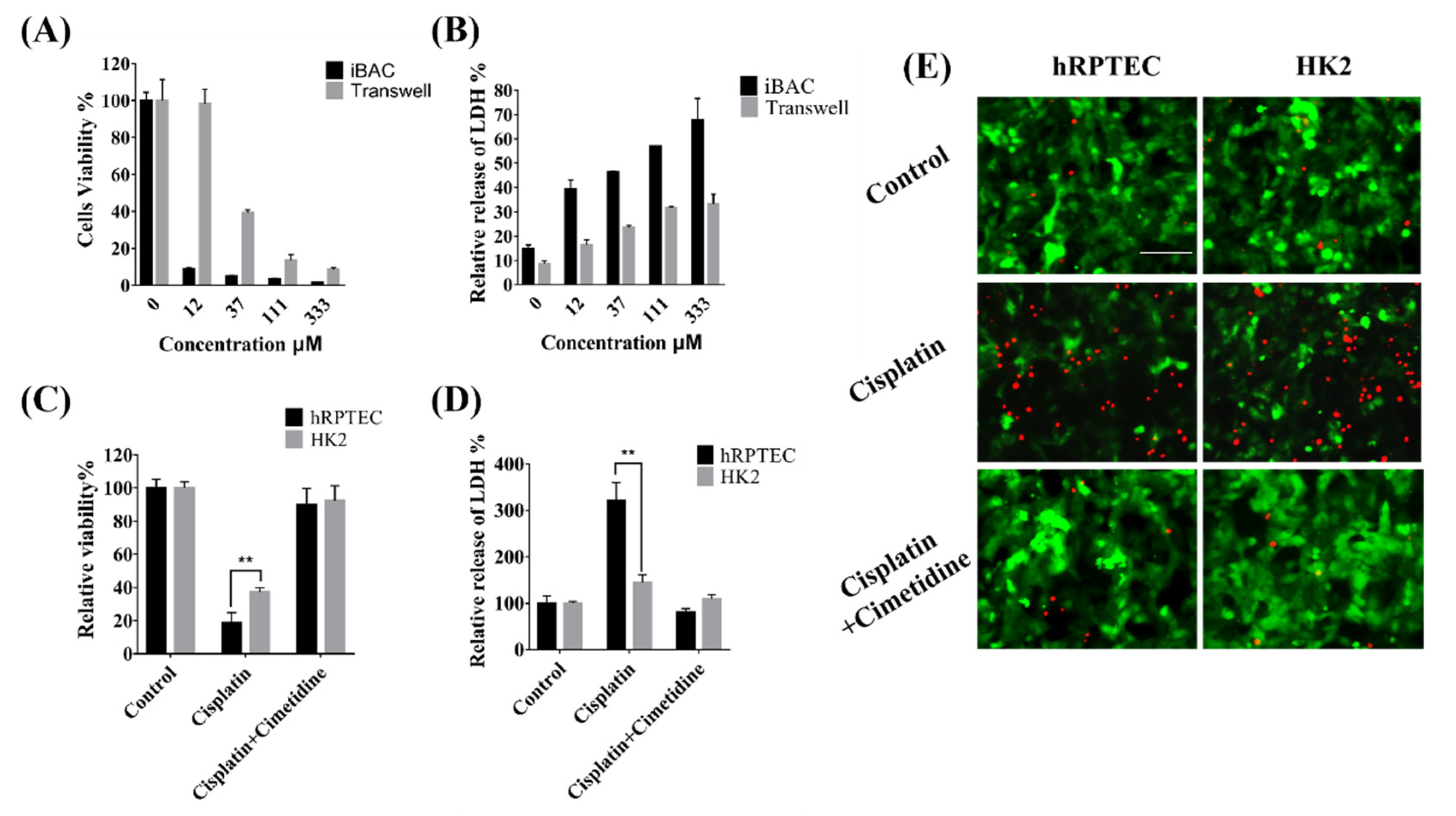
3.5. Nephrotoxicity of Cisplatin Mediated by OCT2 on the iBAC
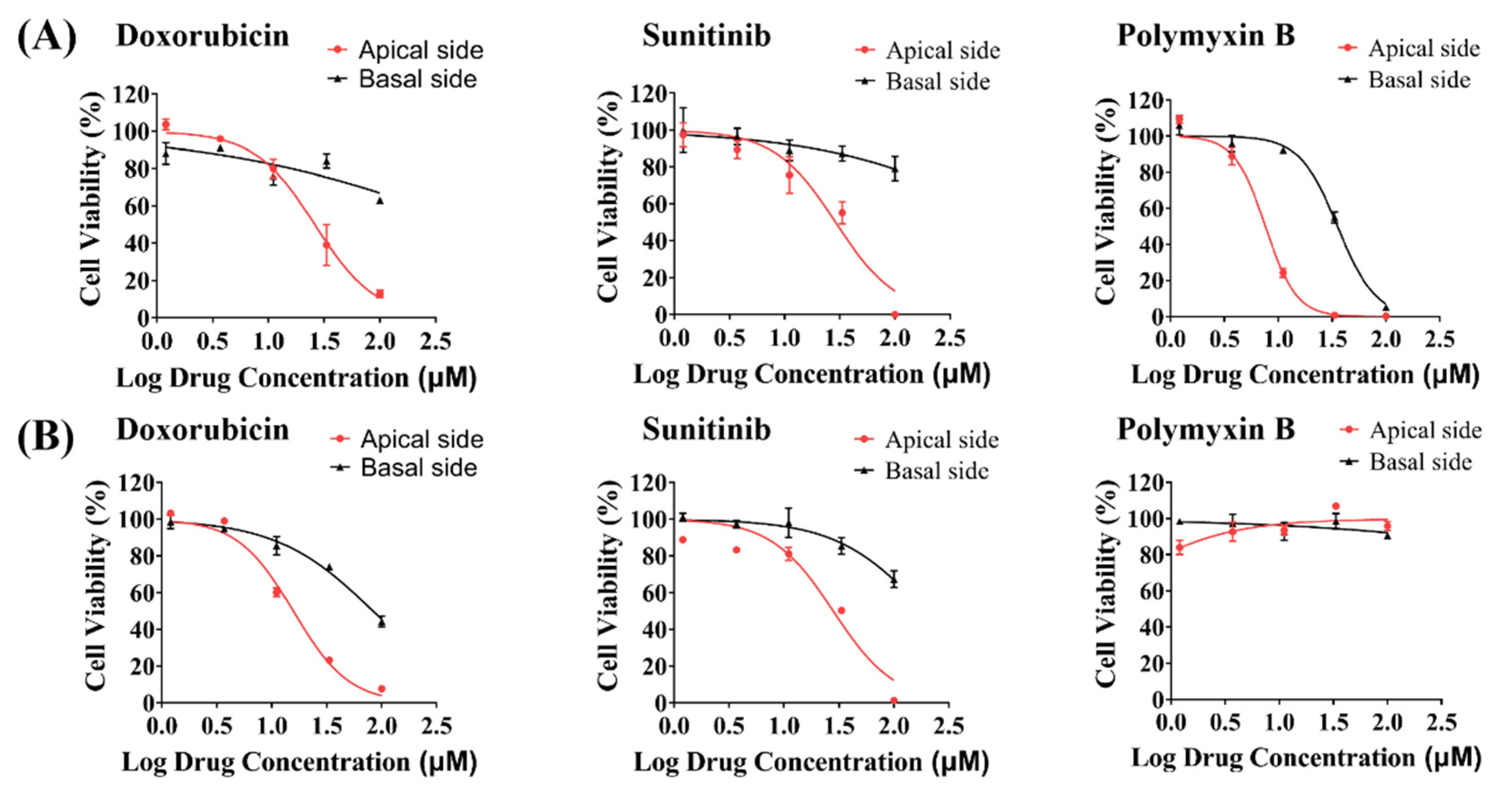
3.6. Toxicity Evaluation of Model Drugs on the Apical and Basal Side of hRPTECs on the iBAC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuk, A.; Bonventre, J.V. Acute Kidney Injury. Annu. Rev. Med. 2016, 67, 293–307. [Google Scholar] [CrossRef]
- Diekjurgen, D.; Grainger, D.W. Drug transporter expression profiling in a three-dimensional kidney proximal tubule in vitro nephrotoxicity model. Pflug. Arch. 2018, 470, 1311–1323. [Google Scholar] [CrossRef]
- Morrissey, K.M.; Stocker, S.L.; Wittwer, M.B.; Xu, L.; Giacomini, K.M. Renal transporters in drug development. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 503–529. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Evangelista, E.A.; Yang, J.; Kelly, E.J.; Yeung, C.K. Kidney Organoid and Microphysiological Kidney Chip Models to Accelerate Drug Development and Reduce Animal Testing. Front. Pharmacol. 2021, 12, 695920. [Google Scholar] [CrossRef] [PubMed]
- Mossoba, M.E.; Vohra, S.; Toomer, H.; Pugh-Bishop, S.; Keltner, Z.; Topping, V.; Black, T.; Olejnik, N.; Depina, A.; Belgrave, K.; et al. Comparison of diglycolic acid exposure to human proximal tubule cells in vitro and rat kidneys in vivo. Toxicol. Rep. 2017, 4, 342–347. [Google Scholar] [CrossRef]
- Diekjurgen, D.; Grainger, D.W. A murine ex vivo 3D kidney proximal tubule model predicts clinical drug-induced nephrotoxicity. Arch. Toxicol. 2019, 93, 1349–1364. [Google Scholar] [CrossRef]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2020, 20, 345–361. [Google Scholar] [CrossRef]
- Li, Z.; Su, W.; Zhu, Y.; Tao, T.; Li, D.; Peng, X.; Qin, J. Drug absorption related nephrotoxicity assessment on an intestine-kidney chip. Biomicrofluidics 2017, 11, 034114. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Wesseling-Perry, K.; Hasan, A.; Elkhammas, E.; Zhang, Y.S. Kidney-on-a-chip: Untapped opportunities. Kidney Int. 2018, 94, 1073–1086. [Google Scholar] [CrossRef]
- Kishi, S.; Matsumoto, T.; Ichimura, T.; Brooks, C.R. Human reconstructed kidney models. In Vitro Cell. Dev. Biol. Anim. 2021, 57, 133–147. [Google Scholar] [CrossRef]
- Ai, X.; Zhao, L.; Lu, Y.; Hou, Y.; Lv, T.; Jiang, Y.; Tu, P.; Guo, X. Integrated Array Chip for High-Throughput Screening of Species Differences in Metabolism. Anal. Chem. 2020, 92, 11696–11704. [Google Scholar] [CrossRef]
- Hou, Y.; Ai, X.; Zhao, L.; Gao, Z.; Wang, Y.; Lu, Y.; Tu, P.; Jiang, Y. An integrated biomimetic array chip for high-throughput co-culture of liver and tumor microtissues for advanced anticancer bioactivity screening. Lab Chip 2020, 20, 2482–2494. [Google Scholar] [CrossRef]
- Cong, Y.; Han, X.; Wang, Y.; Chen, Z.; Lu, Y.; Liu, T.; Wu, Z.; Jin, Y.; Luo, Y.; Zhang, X. Drug Toxicity Evaluation Based on Organ-on-a-chip Technology: A Review. Micromachines 2020, 11, 381. [Google Scholar] [CrossRef]
- Herland, A.; Maoz, B.M.; Das, D.; Somayaji, M.R.; Prantil-Baun, R.; Novak, R.; Cronce, M.; Huffstater, T.; Jeanty, S.S.F.; Ingram, M.; et al. Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Nat. Biomed. Eng. 2020, 4, 421–436. [Google Scholar] [CrossRef]
- Nieskens, T.T.; Wilmer, M.J. Kidney-on-a-chip technology for renal proximal tubule tissue reconstruction. Eur. J. Pharmacol. 2016, 790, 46–56. [Google Scholar] [CrossRef]
- Ferrell, N.; Sandoval, R.M.; Molitoris, B.A.; Brakeman, P.; Roy, S.; Fissell, W.H. Application of physiological shear stress to renal tubular epithelial cells. Methods Cell Biol. 2019, 153, 43–67. [Google Scholar] [CrossRef]
- Wilmer, M.J.; Ng, C.P.; Lanz, H.L.; Vulto, P.; Suter-Dick, L.; Masereeuw, R. Kidney-on-a-Chip Technology for Drug-Induced Nephrotoxicity Screening. Trends Biotechnol. 2016, 34, 156–170. [Google Scholar] [CrossRef]
- Nieskens, T.T.G.; Persson, M.; Kelly, E.J.; Sjogren, A.K. A Multicompartment Human Kidney Proximal Tubule-on-a-Chip Replicates Cell Polarization-Dependent Cisplatin Toxicity. Drug Metab. Dispos. 2020, 48, 1303–1311. [Google Scholar] [CrossRef]
- Vriend, J.; Peters, J.G.P.; Nieskens, T.T.G.; Skovronova, R.; Blaimschein, N.; Schmidts, M.; Roepman, R.; Schirris, T.J.J.; Russel, F.G.M.; Masereeuw, R.; et al. Flow stimulates drug transport in a human kidney proximal tubule-on-a-chip independent of primary cilia. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129433. [Google Scholar] [CrossRef]
- Jang, K.J.; Mehr, A.P.; Hamilton, G.A.; McPartlin, L.A.; Chung, S.; Suh, K.Y.; Ingber, D.E. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Biol. 2013, 5, 1119–1129. [Google Scholar] [CrossRef]
- Weber, E.J.; Lidberg, K.A.; Wang, L.; Bammler, T.K.; MacDonald, J.W.; Li, M.J.; Redhair, M.; Atkins, W.M.; Tran, C.; Hines, K.M.; et al. Human kidney on a chip assessment of polymyxin antibiotic nephrotoxicity. JCI Insight 2018, 3, e123673. [Google Scholar] [CrossRef]
- Bajaj, P.; Chung, G.; Pye, K.; Yukawa, T.; Imanishi, A.; Takai, Y.; Brown, C.; Wagoner, M.P. Freshly isolated primary human proximal tubule cells as an in vitro model for the detection of renal tubular toxicity. Toxicology 2020, 442, 152535. [Google Scholar] [CrossRef]
- Shen, J.X.; Youhanna, S.; Zandi Shafagh, R.; Kele, J.; Lauschke, V.M. Organotypic and Microphysiological Models of Liver, Gut, and Kidney for Studies of Drug Metabolism, Pharmacokinetics, and Toxicity. Chem. Res. Toxicol. 2020, 33, 38–60. [Google Scholar] [CrossRef]
- Ryan, M.J.; Johnson, G.; Kirk, J.; Fuerstenberg, S.M.; Zager, R.A.; Torok-Storb, B. HK-2: An immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994, 45, 48–57. [Google Scholar] [CrossRef]
- Jenkinson, S.E.; Chung, G.W.; van Loon, E.; Bakar, N.S.; Dalzell, A.M.; Brown, C.D. The limitations of renal epithelial cell line HK-2 as a model of drug transporter expression and function in the proximal tubule. Pflug. Arch. 2012, 464, 601–611. [Google Scholar] [CrossRef]
- Xu, Y.; Qin, S.; Niu, Y.; Gong, T.; Zhang, Z.; Fu, Y. Effect of fluid shear stress on the internalization of kidney-targeted delivery systems in renal tubular epithelial cells. Acta Pharm. Sin. B 2020, 10, 680–692. [Google Scholar] [CrossRef]
- Ingber, D.E. Is it Time for Reviewer 3 to Request Human Organ Chip Experiments Instead of Animal Validation Studies? Adv. Sci. 2020, 7, 2002030. [Google Scholar] [CrossRef]
- Khundmiri, S.J.; Chen, L.; Lederer, E.D.; Yang, C.R.; Knepper, M.A. Transcriptomes of Major Proximal Tubule Cell Culture Models. J. Am. Soc. Nephrol. 2021, 32, 86–97. [Google Scholar] [CrossRef]
- Rayner, S.G.; Phong, K.T.; Xue, J.; Lih, D.; Shankland, S.J.; Kelly, E.J.; Himmelfarb, J.; Zheng, Y. Reconstructing the Human Renal Vascular-Tubular Unit In Vitro. Adv. Healthc Mater. 2018, 7, e1801120. [Google Scholar] [CrossRef]
- Vriend, J.; Nieskens, T.T.G.; Vormann, M.K.; van den Berge, B.T.; van den Heuvel, A.; Russel, F.G.M.; Suter-Dick, L.; Lanz, H.L.; Vulto, P.; Masereeuw, R.; et al. Screening of Drug-Transporter Interactions in a 3D Microfluidic Renal Proximal Tubule on a Chip. AAPS J. 2018, 20, 87. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.X.; Kaeslin, G.; Ranall, M.V.; Blaskovich, M.A.; Becker, B.; Butler, M.S.; Little, M.H.; Lash, L.H.; Cooper, M.A. Evaluation of biomarkers for in vitro prediction of drug-induced nephrotoxicity: Comparison of HK-2, immortalized human proximal tubule epithelial, and primary cultures of human proximal tubular cells. Pharmacol. Res. Perspect. 2015, 3, e00148. [Google Scholar] [CrossRef]
- Li, S.; Zhao, J.; Huang, R.; Steiner, T.; Bourner, M.; Mitchell, M.; Thompson, D.C.; Zhao, B.; Xia, M. Development and Application of Human Renal Proximal Tubule Epithelial Cells for Assessment of Compound Toxicity. Curr. Chem. Genom. Transl. Med. 2017, 11, 19–30. [Google Scholar] [CrossRef]
- Pabla, N.; Murphy, R.F.; Liu, K.B.; Dong, Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am. J. Physiol.-Ren. Physiol. 2009, 296, F505–F511. [Google Scholar] [CrossRef]
- Ciarimboli, G. Membrane Transporters as Mediators of Cisplatin Side-effects. Anticancer Res. 2014, 34, 547–550. [Google Scholar] [CrossRef]
- Ramm, S.; Adler, M.; Vaidya, V.S. A High-Throughput Screening Assay to Identify Kidney Toxic Compounds. Curr. Protoc. Toxicol. 2016, 69, 9–10. [Google Scholar] [CrossRef]
- Perazella, M.A. Drug-induced acute kidney injury: Diverse mechanisms of tubular injury. Curr. Opin. Crit. Care 2019, 25, 550–557. [Google Scholar] [CrossRef]
- Lin, Z.W.; Will, Y. Evaluation of Drugs with Specific Organ Toxicities in Organ-Specific Cell Lines. Toxicol. Sci. 2012, 126, 114–127. [Google Scholar] [CrossRef]
- Mossoba, M.E.; Sprando, R.L. In Vitro to In Vivo Concordance of Toxicity Using the Human Proximal Tubule Cell Line HK-2. Int. J. Toxicol. 2020, 39, 452–464. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K. Toxicity of polymyxins: A systematic review of the evidence from old and recent studies. Crit. Care 2006, 10, R27. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, B.; Yan, L.; Li, J.; Luo, P.; Ai, X.; Tu, P. Functional Evaluation and Nephrotoxicity Assessment of Human Renal Proximal Tubule Cells on a Chip. Biosensors 2022, 12, 718. https://doi.org/10.3390/bios12090718
Jing B, Yan L, Li J, Luo P, Ai X, Tu P. Functional Evaluation and Nephrotoxicity Assessment of Human Renal Proximal Tubule Cells on a Chip. Biosensors. 2022; 12(9):718. https://doi.org/10.3390/bios12090718
Chicago/Turabian StyleJing, Bolin, Lei Yan, Jiajia Li, Piaopiao Luo, Xiaoni Ai, and Pengfei Tu. 2022. "Functional Evaluation and Nephrotoxicity Assessment of Human Renal Proximal Tubule Cells on a Chip" Biosensors 12, no. 9: 718. https://doi.org/10.3390/bios12090718
APA StyleJing, B., Yan, L., Li, J., Luo, P., Ai, X., & Tu, P. (2022). Functional Evaluation and Nephrotoxicity Assessment of Human Renal Proximal Tubule Cells on a Chip. Biosensors, 12(9), 718. https://doi.org/10.3390/bios12090718




