A Review: Research Progress of Neural Probes for Brain Research and Brain–Computer Interface
Abstract
:1. Introduction
2. Microelectrodes
2.1. Rigid Microelectrodes
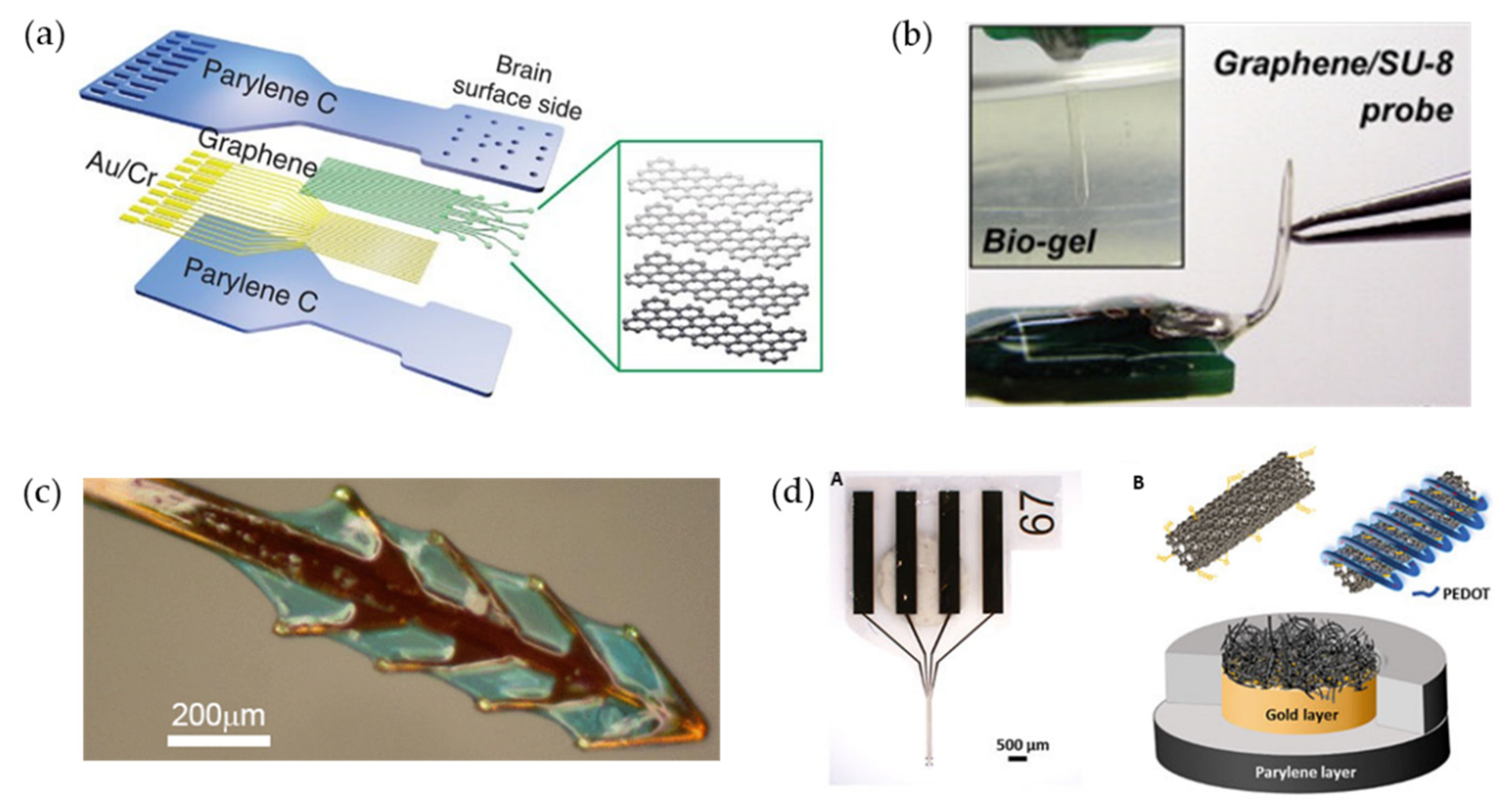
2.2. Strategies for Microelectrode Flexibility
2.3. Methods of Flexible Microelectrode Insertion
3. Optoprobes
3.1. Optogenetics
3.2. Optoprobes
3.2.1. Multifunctional Optoprobes
3.2.2. Artifact-Free Optoprobes
3.2.3. Three-Dimensional Drivable Optoprobes
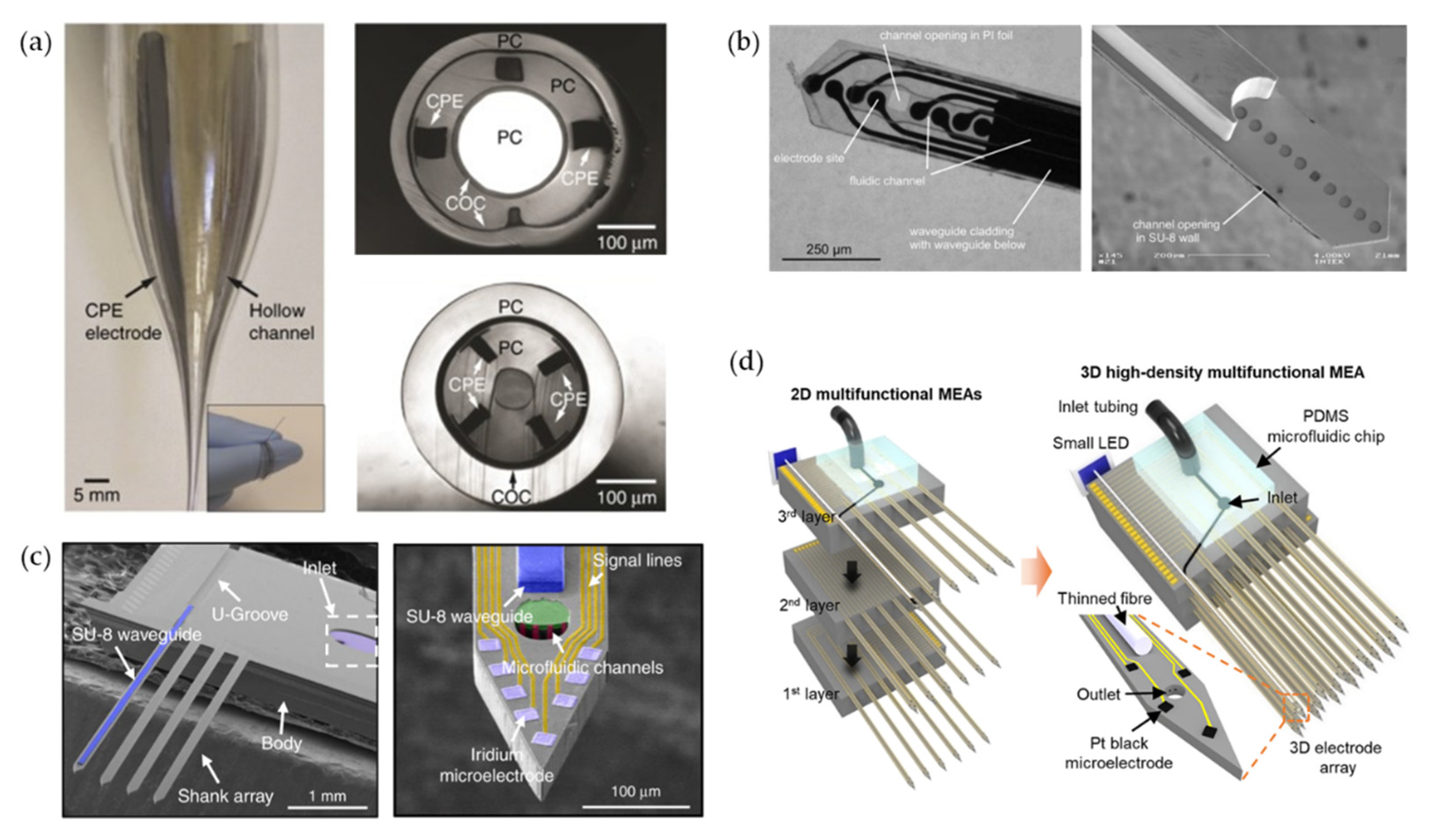
3.2.4. Flexible Optoprobes
4. Magnetrodes
4.1. Magnetophysiology
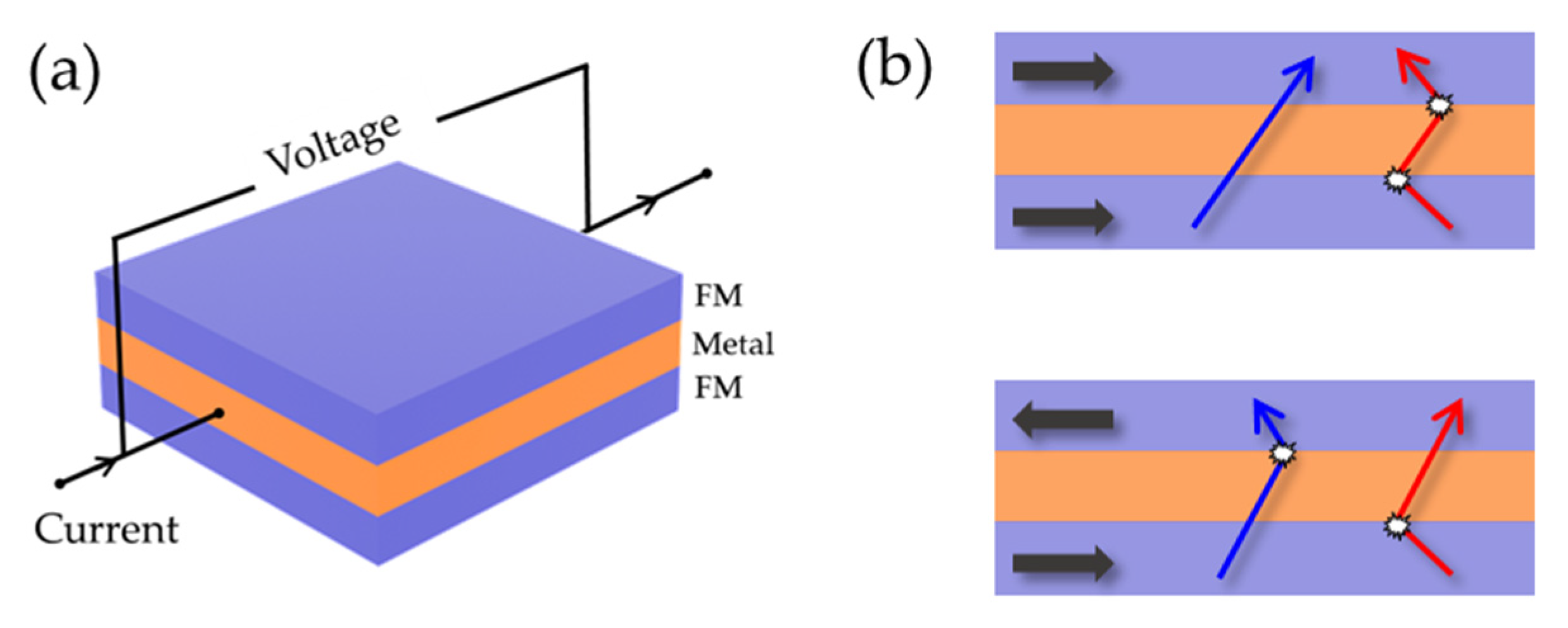
4.2. MR Sensors
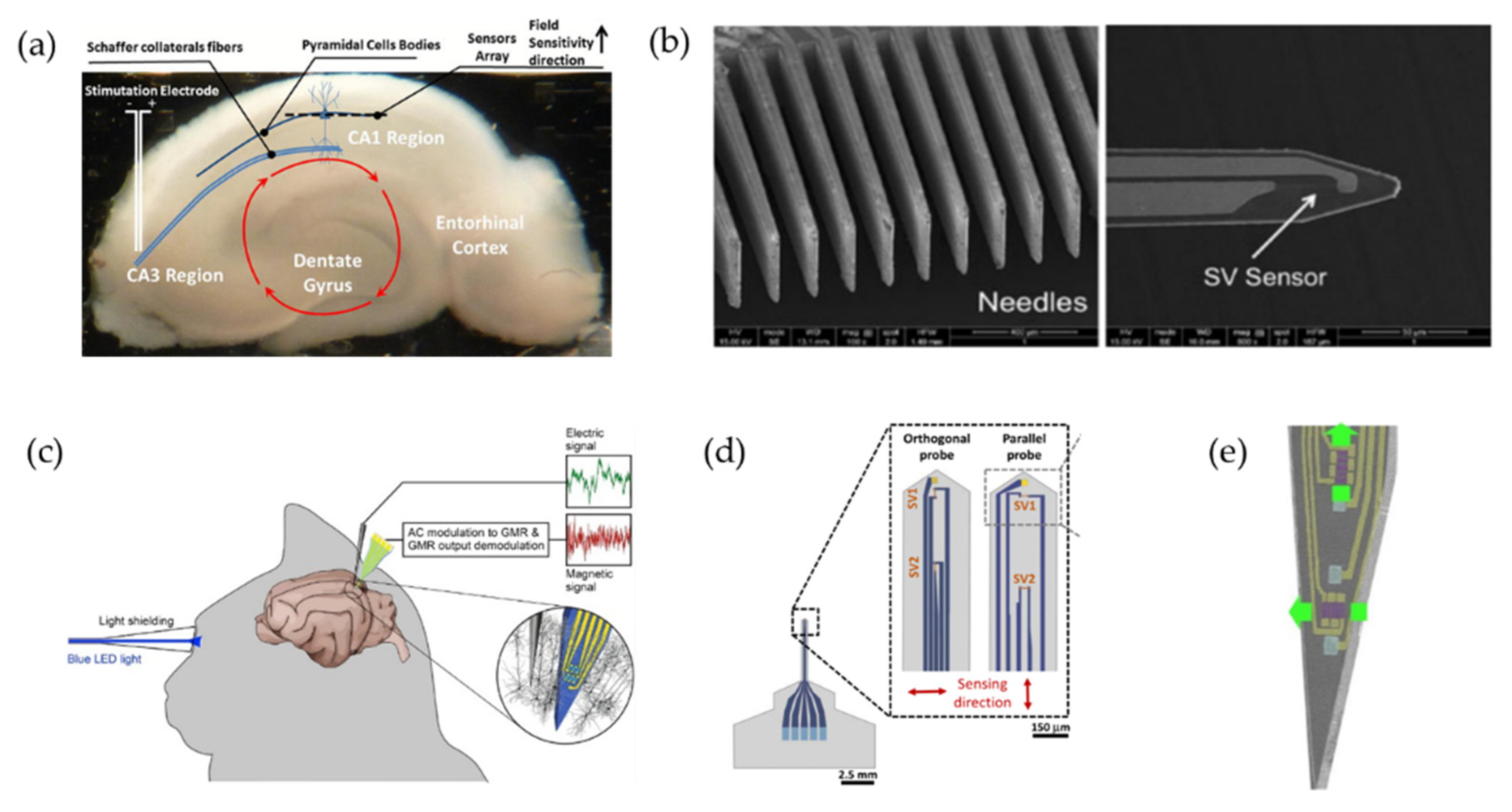
4.3. Magnetrodes
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMR | anisotropic magnetoresistance |
| BCI | brain–computer interface |
| CNTs | carbon nanotubes |
| CPE | composite-conductive polyethylene |
| DOS | density of states |
| ERF | event-related field |
| FDA | Food and Drug Administration |
| GaN | gallium nitride |
| GRIN | gradient-index |
| MEG | magnetoencephalography |
| MR | magnetoresistive |
| NM | non-magnetic |
| PC | polycarbonate |
| PEC | photoelectrochemical |
| PEG | poly (ethylene glycol) |
| PI | polyimide |
| SNR | signal-to-noise ratio |
| TMR | tunneling magnetoresistance |
| UEA | Utah electrode array |
| AC | alternating current |
| CIP | current-in-plane |
| COC | cyclic olefin copolymer |
| CPs | conductive polymers |
| EEG | electroencephalogram |
| EMI | electromagnetic interference |
| FM | ferromagnetic |
| fNIRS | functional near-infrared spectroscopy |
| GMR | giant magnetoresistance |
| MEAs | microelectrode arrays |
| MEMS | microelectromechanical systems |
| MTJ | magnetic tunnel junction |
| nT/pT/fT | nano/pico/femto tesla |
| PDMS | polydimethylsiloxane |
| PEDOT | Poly (3,4-ethylenedioxythiophene) |
| PLGA | poly (lactic-co-glycolic acid) |
| PV | photovoltaic |
| SV | spin-valve |
| TTX | tetrodotoxin |
References
- Azevedo, F.A.C.; Carvalho, L.R.B.; Grinberg, L.T.; Farfel, J.M.; Ferretti, R.E.L.; Leite, R.E.P.; Jacob, W.; Lent, R.; Herculano-Houzel, S. Equal Numbers of Neuronal and Nonneuronal Cells Make the Human Brain an Isometrically Scaled-Up Primate Brain. J. Comp. Neurol. 2009, 513, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Fetz, E.E. Restoring motor function with bidirectional neural interfaces. Prog. Brain Res. 2015, 218, 241–252. [Google Scholar] [PubMed]
- Bell, C.J.; Shenoy, P.; Chalodhorn, R.; Rao, R.P.N. Control of a humanoid robot by a noninvasive brain-computer interface in humans. J. Neural Eng. 2008, 5, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Kansaku, K.; Hata, N.; Takano, K. My thoughts through a robot’s eyes: An augmented reality-brain-machine interface. Neurosci. Res. 2010, 66, 219–222. [Google Scholar] [CrossRef]
- Vázquez-Guardado, A.; Yang, Y.; Bandodkar, A.J.; Rogers, J.A. Recent advances in neurotechnologies with broad potential for neuroscience research. Nat. Neurosci. 2020, 23, 1522–1536. [Google Scholar] [CrossRef]
- Millán Jdel, R.; Renkens, F.; Mouriño, J.; Gerstner, W. Noninvasive brain-actuated control of a mobile robot by human EEG. IEEE Trans. Biomed. Eng. 2004, 51, 1026–1033. [Google Scholar] [CrossRef] [Green Version]
- Rebsamen, B.; Guan, C.; Zhang, H.; Wang, C.; Teo, C.; Ang, M.H., Jr.; Burdet, E. A brain controlled wheelchair to navigate in familiar environments. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 590–598. [Google Scholar] [CrossRef] [Green Version]
- Müller, K.R.; Tangermann, M.; Dornhege, G.; Krauledat, M.; Curio, G.; Blankertz, B. Machine learning for real-time single-trial EEG-analysis: From brain-computer interfacing to mental state monitoring. J. Neurosci. Methods 2008, 167, 82–90. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; da Silva, F.H.L. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Fazli, S.; Mehnert, J.; Steinbrink, J.; Curio, G.; Villringer, A.; Müller, K.R.; Blankertz, B. Enhanced performance by a hybrid NIRS-EEG brain computer interface. Neuroimage 2012, 59, 519–529. [Google Scholar] [CrossRef]
- Coyle, S.M.; Ward, T.E.; Markham, C.M. Brain-computer interface using a simplified functional near-infrared spectroscopy system. J. Neural Eng. 2007, 4, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Kauhanen, L.; Nykopp, T.; Lehtonen, J.; Jylänki, P.; Heikkonen, J.; Rantanen, P.; Alaranta, H.; Sams, M. EEG and MEG brain-computer interface for tetraplegic patients. IEEE Trans. Neural Syst. Rehabil. Eng. 2006, 14, 190–193. [Google Scholar] [CrossRef]
- Yeom, H.G.; Kim, J.S.; Chung, C.K. Estimation of the velocity and trajectory of three-dimensional reaching movements from non-invasive magnetoencephalography signals. J. Neural Eng. 2013, 10, 026006. [Google Scholar] [CrossRef]
- Gross, J. Magnetoencephalography in Cognitive Neuroscience: A Primer. Neuron 2019, 104, 189–204. [Google Scholar] [CrossRef]
- Hamalainen, M.; Hari, R.; Ilmoniemi, R.J.; Knuutila, J.; Lounasmaa, O.V. Magnetoencephalography—Theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev. Mod. Phys. 1993, 65, 413–497. [Google Scholar] [CrossRef] [Green Version]
- Seymour, J.P.; Wu, F.; Wise, K.D.; Yoon, E. State-of-the-art MEMS and microsystem tools for brain research. Microsyst. Nanoeng. 2017, 3, 16066. [Google Scholar] [CrossRef]
- Choi, J.-R.; Kim, S.-M.; Ryu, R.-H.; Kim, S.-P.; Sohn, J.-W. Implantable Neural Probes for Brain-Machine Interfaces? Current Developments and Future Prospects. Exp. Neurobiol. 2018, 27, 453–471. [Google Scholar] [CrossRef]
- Cogan, S.F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [Green Version]
- Viswam, V.; Obien, M.E.J.; Franke, F.; Frey, U.; Hierlemann, A. Optimal Electrode Size for Multi-Scale Extracellular-Potential Recording From Neuronal Assemblies. Front. Neurosci. 2019, 13, 385. [Google Scholar] [CrossRef] [Green Version]
- Alt, M.T.; Fiedler, E.; Rudmann, L.; Ordonez, J.S.; Ruther, P.; Stieglitz, T. Let There Be Light—Optoprobes for Neural Implants. Proc. IEEE 2017, 105, 101–138. [Google Scholar] [CrossRef]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Caruso, L.; Wunderle, T.; Lewis, C.M.; Valadeiro, J.; Trauchessec, V.; Trejo Rosillo, J.; Amaral, J.P.; Ni, J.; Jendritza, P.; Fermon, C.; et al. In Vivo Magnetic Recording of Neuronal Activity. Neuron 2017, 95, 1283–1291.e1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, T. Fabrication of extremely fine glass micropipette electrodes. J. Phys. E Sci. Instrum. 1969, 2, 1087. [Google Scholar] [CrossRef] [PubMed]
- Simons, D.J.; Land, P.W. A reliable technique for marking the location of extracellular recording sites using glass micropipettes. Neurosci. Lett. 1987, 81, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Pine, J. Recording action potentials from cultured neurons with extracellular microcircuit electrodes. J. Neurosci. Methods 1980, 2, 19–31. [Google Scholar] [CrossRef]
- Palmer, C. A microwire technique for recording single neurons in unrestrained animals. Brain Res. Bull. 1978, 3, 285–289. [Google Scholar] [CrossRef]
- Lehew, G.; Nicolelis, M.A.L. State-of-the-Art Microwire Array Design for Chronic Neural Recordings in Behaving Animals, 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2008. [Google Scholar]
- Verloop, A.J.; Holsheimer, J. A simple method for the construction of electrode arrays. J. Neurosci. Methods 1984, 11, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Fekete, Z. Recent advances in silicon-based neural microelectrodes and microsystems: A review. Sens. Actuators B Chem. 2015, 215, 300–315. [Google Scholar] [CrossRef]
- Barrese, J.C.; Rao, N.; Paroo, K.; Triebwasser, C.; Vargas-Irwin, C.; Franquemont, L.; Donoghue, J.P. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J. Neural Eng. 2013, 10, 066014. [Google Scholar] [CrossRef]
- Ghane-Motlagh, B.; Sawan, M. Design and implementation challenges of microelectrode arrays: A review. Mater. Sci. Appl. 2013, 4, 483. [Google Scholar]
- Bretag, A.H. The glass micropipette electrode: A history of its inventors and users to 1950. J. Gen. Physiol. 2017, 149, 417–430. [Google Scholar] [CrossRef]
- Strumwasser, F. Long-Term Recording from Single Neurons in Brain of Unrestrained Mammals. Science 1958, 127, 469–470. [Google Scholar] [CrossRef]
- Nicolelis, M.A.L.; Dimitrov, D.; Carmena, J.M.; Crist, R.; Lehew, G.; Kralik, J.D.; Wise, S.P. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc. Natl. Acad. Sci. USA 2003, 100, 11041–11046. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.E.; Campbell, P.K.; Normann, R.A. A glass/silicon composite intracortical electrode array. Ann. Biomed. Eng. 1992, 20, 423–437. [Google Scholar] [CrossRef]
- Campbell, P.K.; Jones, K.E.; Huber, R.J.; Horch, K.W.; Normann, R.A. A silicon-based, three-dimensional neural interface: Manufacturing processes for an intracortical electrode array. IEEE Trans. Biomed. Eng. 1991, 38, 758–768. [Google Scholar] [CrossRef]
- Scholvin, J.; Kinney, J.P.; Bernstein, J.G.; Moore-Kochlacs, C.; Kopell, N.; Fonstad, C.G.; Boyden, E.S. Close-Packed Silicon Microelectrodes for Scalable Spatially Oversampled Neural Recording. IEEE Trans. Biomed. Eng. 2016, 63, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, R.; Negi, S.; Solzbacher, F. Wafer-scale fabrication of penetrating neural microelectrode arrays. Biomed. Microdevices 2010, 12, 797–807. [Google Scholar] [CrossRef]
- Hochberg, L.R.; Serruya, M.D.; Friehs, G.M.; Mukand, J.A.; Saleh, M.; Caplan, A.H.; Branner, A.; Chen, D.; Penn, R.D.; Donoghue, J.P. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 2006, 442, 164–171. [Google Scholar] [CrossRef]
- Chaudhary, U.; Vlachos, I.; Zimmermann, J.B.; Espinosa, A.; Tonin, A.; Jaramillo-Gonzalez, A.; Khalili-Ardali, M.; Topka, H.; Lehmberg, J.; Friehs, G.M.; et al. Spelling interface using intracortical signals in a completely locked-in patient enabled via auditory neurofeedback training. Nat. Commun. 2022, 13, 1236. [Google Scholar] [CrossRef]
- Kim, S.; Callier, T.; Tabot, G.A.; Gaunt, R.A.; Tenore, F.V.; Bensmaia, S.J. Behavioral assessment of sensitivity to intracortical microstimulation of primate somatosensory cortex. Proc Natl Acad Sci USA 2015, 112, 15202–15207. [Google Scholar] [CrossRef] [Green Version]
- Flesher, S.N.; Downey, J.E.; Weiss, J.M.; Hughes, C.L.; Herrera, A.J.; Tyler-Kabara, E.C.; Boninger, M.L.; Collinger, J.L.; Gaunt, R.A. A brain-computer interface that evokes tactile sensations improves robotic arm control. Science 2021, 372, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Branner, A.; Normann, R.A. A multielectrode array for intrafascicular recording and stimulation in sciatic nerve of cats. Brain Res. Bull. 2000, 51, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Wise, K.D.; Anderson, D.J. A high-yield microassembly structure for three-dimensional microelectrode arrays. IEEE Trans. Biomed. Eng. 2000, 47, 281–289. [Google Scholar] [PubMed]
- Wise, K.D.; Angell, J.B.; Starr, A. An integrated-circuit approach to extracellular microelectrodes. IEEE Trans. Biomed. Eng. 1970, 17, 238–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barz, F.; Livi, A.; Lanzilotto, M.; Maranesi, M.; Bonini, L.; Paul, O.; Ruther, P. Versatile, modular 3D microelectrode arrays for neuronal ensemble recordings: From design to fabrication, assembly, and functional validation in non-human primates. J. Neural Eng. 2017, 14, 036010. [Google Scholar] [CrossRef]
- Wang, X.; Gu, Y.; Xiong, Z.; Cui, Z.; Zhang, T. Silk-Molded Flexible, Ultrasensitive, and Highly Stable Electronic Skin for Monitoring Human Physiological Signals. Adv. Mater. 2014, 26, 1336–1342. [Google Scholar] [CrossRef]
- Baek, J.Y.; An, J.H.; Choi, J.M.; Park, K.S.; Lee, S.H. Flexible polymeric dry electrodes for the long-term monitoring of ECG. Sens. Actuators A Phys. 2008, 143, 423–429. [Google Scholar] [CrossRef]
- Tang, J.; Guo, H.; Zhao, M.; Yang, J.; Tsoukalas, D.; Binzhen, Z.; Liu, J.; Xue, C.; Zhang, W. Highly Stretchable Electrodes on Wrinkled Polydimethylsiloxane Substrates. Sci. Rep. 2015, 5, 16527. [Google Scholar] [CrossRef] [Green Version]
- Rousche, P.J.; Pellinen, D.S.; Pivin, D.P., Jr.; Williams, J.C.; Vetter, R.J.; Kipke, D.R. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans. Biomed. Eng. 2001, 48, 361–371. [Google Scholar] [CrossRef]
- Moon, J.H.; Baek, D.H.; Choi, Y.Y.; Lee, K.H.; Kim, H.C.; Lee, S.H. Wearable polyimide-PDMS electrodes for intrabody communication. J. Micromech. Microeng. 2010, 20, 025032. [Google Scholar] [CrossRef]
- Park, D.-W.; Schendel, A.A.; Mikael, S.; Brodnick, S.K.; Richner, T.J.; Ness, J.P.; Hayat, M.R.; Atry, F.; Frye, S.T.; Pashaie, R.; et al. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 2014, 5, 5258. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Rodger, D.C.; Pinto, A.; Meng, E.; Weiland, J.D.; Humayun, M.S.; Tai, Y.-C. Parylene-based integrated wireless single-channel neurostimulator. Sens. Actuators A Phys. 2011, 166, 193–200. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lin, C.-T.; Hsu, W.-L.; Chang, Y.-C.; Yeh, S.-R.; Li, L.-J.; Yao, D.-J. A flexible hydrophilic-modified graphene microprobe for neural and cardiac recording. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 600–604. [Google Scholar] [CrossRef]
- Wu, F.; Im, M.; Yoon, E. A flexible fish-bone-shaped neural probe strengthened by biodegradable silk coating for enhanced biocompatibility. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 966–969. [Google Scholar]
- Ansaldo, A.; Castagnola, E.; Maggiolini, E.; Fadiga, L.; Ricci, D. Superior electrochemical performance of carbon nanotubes directly grown on sharp microelectrodes. ACS Nano 2011, 5, 2206–2214. [Google Scholar] [CrossRef]
- Sridharan, A.; Muthuswamy, J. Soft, Conductive, Brain-Like, Coatings at Tips of Microelectrodes Improve Electrical Stability under Chronic, In Vivo Conditions. Micromachines 2021, 12, 761. [Google Scholar] [CrossRef]
- Shoval, A.; Adams, C.; David-Pur, M.; Shein, M.; Hanein, Y.; Sernagor, E. Carbon nanotube electrodes for effective interfacing with retinal tissue. Front. Neuroeng. 2009, 2, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, L.H.; Jansen, M.; Maybeck, V.; Hauf, M.V.; Seifert, M.; Stutzmann, M.; Sharp, I.D.; Offenhäusser, A.; Garrido, J.A. Graphene transistor arrays for recording action potentials from electrogenic cells. Adv. Mater. 2011, 23, 5045–5049. [Google Scholar] [CrossRef] [PubMed]
- Zhan, B.; Li, C.; Yang, J.; Jenkins, G.; Huang, W.; Dong, X. Graphene field-effect transistor and its application for electronic sensing. Small 2014, 10, 4042–4065. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.-Z.; Li, W.; Ai, W.; Tai, Q.; Xie, L.-H.; Cao, Y.; Liu, J.-Q.; Yi, M.-D.; Ling, H.-F.; Li, Z.-H.; et al. Chemoselective reduction of graphene oxide and its application in nonvolatile organic transistor memory devices. RSC Adv. 2013, 3, 25788–25791. [Google Scholar] [CrossRef]
- Green, R.; Abidian, M.R. Conducting Polymers for Neural Prosthetic and Neural Interface Applications. Adv. Mater. 2015, 27, 7620–7637. [Google Scholar] [CrossRef]
- Maziz, A.; Özgür, E.; Bergaud, C.; Uzun, L. Progress in conducting polymers for biointerfacing and biorecognition applications. Sens. Actuators Rep. 2021, 3, 100035. [Google Scholar] [CrossRef]
- Kim, D.H.; Wiler, J.A.; Anderson, D.J.; Kipke, D.R.; Martin, D.C. Conducting polymers on hydrogel-coated neural electrode provide sensitive neural recordings in auditory cortex. Acta Biomater. 2010, 6, 57–62. [Google Scholar] [CrossRef]
- Luo, X.; Weaver, C.L.; Zhou, D.D.; Greenberg, R.; Cui, X.T. Highly stable carbon nanotube doped poly(3,4-ethylenedioxythiophene) for chronic neural stimulation. Biomaterials 2011, 32, 5551–5557. [Google Scholar] [CrossRef] [Green Version]
- Vajrala, V.S.; Saunier, V.; Nowak, L.G.; Flahaut, E.; Bergaud, C.; Maziz, A. Nanofibrous PEDOT-Carbon Composite on Flexible Probes for Soft Neural Interfacing. Front. Bioeng. Biotechnol. 2021, 9, 780197. [Google Scholar] [CrossRef]
- Sohal, H.S.; Clowry, G.J.; Jackson, A.; O’Neill, A.; Baker, S.N. Mechanical Flexibility Reduces the Foreign Body Response to Long-Term Implanted Microelectrodes in Rabbit Cortex. PLoS ONE 2016, 11, e0165606. [Google Scholar] [CrossRef] [Green Version]
- Kee-Keun, L.; Jiping, H.; Amarjit, S.; Stephen, M.; Gholamreza, E.; Bruce, K.; Gregory, R. Polyimide-based intracortical neural implant with improved structural stiffness. J. Micromech. Microeng. 2004, 14, 32. [Google Scholar] [CrossRef]
- Felix, S.H.; Shah, K.G.; Tolosa, V.M.; Sheth, H.J.; Tooker, A.C.; Delima, T.L.; Jadhav, S.P.; Frank, L.M.; Pannu, S.S. Insertion of flexible neural probes using rigid stiffeners attached with biodissolvable adhesive. J. Vis. Exp. 2013, 79, e50609. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Kuo, J.T.; Hara, S.A.; Lee, C.D.; Yu, L.; Gutierrez, C.A.; Hoang, T.Q.; Pikov, V.; Meng, E. 3D Parylene sheath neural probe for chronic recordings. J. Neural Eng. 2013, 10, 045002. [Google Scholar] [CrossRef] [Green Version]
- Kozai, T.D.; Kipke, D.R. Insertion shuttle with carboxyl terminated self-assembled monolayer coatings for implanting flexible polymer neural probes in the brain. J. Neurosci. Methods 2009, 184, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, S.; Ziegler, D.; Yoshida, Y.; Mabuchi, K.; Suzuki, T. Parylene flexible neural probes integrated with microfluidic channels. Lab Chip 2005, 5, 519–523. [Google Scholar] [CrossRef]
- Foley, C.P.; Nishimura, N.; Neeves, K.B.; Schaffer, C.B.; Olbricht, W.L. Flexible microfluidic devices supported by biodegradable insertion scaffolds for convection-enhanced neural drug delivery. Biomed. Microdev. 2009, 11, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Tien, L.W.; Wu, F.; Tang-Schomer, M.D.; Yoon, E.; Omenetto, F.G.; Kaplan, D.L. Silk as a Multifunctional Biomaterial Substrate for Reduced Glial Scarring around Brain-Penetrating Electrodes. Adv. Funct. Mater. 2013, 23, 3185–3193. [Google Scholar] [CrossRef] [Green Version]
- Jeon, M.; Cho, J.; Kim, Y.K.; Jung, D.; Yoon, E.-S.; Shin, S.; Cho, I.-J. Partially flexible MEMS neural probe composed of polyimide and sucrose gel for reducing brain damage during and after implantation. J. Micromech. Microeng. 2014, 24, 025010. [Google Scholar] [CrossRef]
- Xiang, Z.; Yen, S.-C.; Xue, N.; Sun, T.; Tsang, W.M.; Zhang, S.; Liao, L.-D.; Thakor, N.V.; Lee, C. Ultra-thin flexible polyimide neural probe embedded in a dissolvable maltose-coated microneedle. J. Micromech. Microeng. 2014, 24, 065015. [Google Scholar] [CrossRef] [Green Version]
- Kil, D.; Bovet Carmona, M.; Ceyssens, F.; Deprez, M.; Brancato, L.; Nuttin, B.; Balschun, D.; Puers, R. Dextran as a Resorbable Coating Material for Flexible Neural Probes. Micromachines 2019, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Pas, J.; Rutz, A.L.; Quilichini, P.P.; Slézia, A.; Ghestem, A.; Kaszas, A.; Donahue, M.J.; Curto, V.F.; O’Connor, R.P.; Bernard, C.; et al. A bilayered PVA/PLGA-bioresorbable shuttle to improve the implantation of flexible neural probes. J. Neural Eng. 2018, 15, 065001. [Google Scholar] [CrossRef]
- Lecomte, A.; Castagnola, V.; Descamps, E.; Dahan, L.; Blatché, M.C.; Dinis, T.M.; Leclerc, E.; Egles, C.; Bergaud, C. Silk and PEG as means to stiffen a parylene probe for insertion in the brain: Toward a double time-scale tool for local drug delivery. J. Micromech. Microeng. 2015, 25, 125003. [Google Scholar] [CrossRef]
- Lecomte, A.; Descamps, E.; Bergaud, C. A review on mechanical considerations for chronically-implanted neural probes. J. Neural Eng. 2018, 15, 031001. [Google Scholar] [CrossRef]
- Deisseroth, K.; Feng, G.P.; Majewska, A.K.; Miesenbock, G.; Ting, A.; Schnitzer, M.J. Next-generation optical technologies for illuminating genetically targeted brain circuits. J. Neurosci. 2006, 26, 10380–10386. [Google Scholar] [CrossRef] [Green Version]
- Tye, K.M.; Deisseroth, K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat. Rev. Neurosci. 2012, 13, 251–266. [Google Scholar] [CrossRef]
- Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P.; Bamberg, E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 2003, 100, 13940–13945. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.-P.; Boyden, E.S.; Deisseroth, K. Channelrhodopsin-2 and optical control of excitable cells. Nat. Methods 2006, 3, 785–792. [Google Scholar] [CrossRef]
- Gradinaru, V.; Zhang, F.; Ramakrishnan, C.; Mattis, J.; Prakash, R.; Diester, I.; Goshen, I.; Thompson, K.R.; Deisseroth, K. Molecular and Cellular Approaches for Diversifying and Extending Optogenetics. Cell 2010, 141, 154–165. [Google Scholar] [CrossRef] [Green Version]
- Gradinaru, V.; Thompson, K.R.; Zhang, F.; Mogri, M.; Kay, K.; Schneider, M.B.; Deisseroth, K. Targeting and Readout Strategies for Fast Optical Neural Control In Vitro and In Vivo. J. Neurosci. 2007, 27, 14231–14238. [Google Scholar] [CrossRef] [Green Version]
- Aravanis, A.M.; Wang, L.P.; Zhang, F.; Meltzer, L.A.; Mogri, M.Z.; Schneider, M.B.; Deisseroth, K. An optical neural interface: In vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 2007, 4, S143–S156. [Google Scholar] [CrossRef]
- Emara, M.S.; Pisanello, M.; Sileo, L.; de Vittorio, M.; Pisanello, F. A Wireless Head-Mountable Device With Tapered Optical Fiber-Coupled Laser Diode for Light Delivery in Deep Brain Regions. IEEE Trans. Biomed. Eng. 2019, 66, 1996–2009. [Google Scholar] [CrossRef] [Green Version]
- Sridharan, A.; Rajan, S.D.; Muthuswamy, J. Long-term changes in the material properties of brain tissue at the implant–tissue interface. J. Neural Eng. 2013, 10, 066001. [Google Scholar] [CrossRef] [Green Version]
- Kampasi, K.; Stark, E.; Seymour, J.; Na, K.; Winful, H.G.; Buzsáki, G.; Wise, K.D.; Yoon, E. Fiberless multicolor neural optoelectrode for in vivo circuit analysis. Sci. Rep. 2016, 6, 30961. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.W.; Jun, Y.S.; Park, Y.-G.; Jang, J.; Park, J.-U. Recent advances in electronic devices for monitoring and modulation of brain. Nano Res. 2021, 14, 3070–3095. [Google Scholar] [CrossRef]
- Sharma, K.; Jäckel, Z.; Schneider, A.; Paul, O.; Diester, I.; Ruther, P. Multifunctional optrode for opsin delivery, optical stimulation, and electrophysiological recordings in freely moving rats. J. Neural Eng. 2021, 18, 066013. [Google Scholar] [CrossRef]
- Park, S.; Guo, Y.; Jia, X.; Choe, H.K.; Grena, B.; Kang, J.; Park, J.; Lu, C.; Canales, A.; Chen, R.; et al. One-step optogenetics with multifunctional flexible polymer fibers. Nat. Neurosci. 2017, 20, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Canales, A.; Jia, X.; Froriep, U.P.; Koppes, R.A.; Tringides, C.M.; Selvidge, J.; Lu, C.; Hou, C.; Wei, L.; Fink, Y.; et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 2015, 33, 277–284. [Google Scholar] [CrossRef]
- Rubehn, B.; Wolff, S.B.E.; Tovote, P.; Lüthi, A.; Stieglitz, T. A polymer-based neural microimplant for optogenetic applications: Design and first in vivo study. Lab A Chip 2013, 13, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Son, Y.; Chae, U.; Kim, J.; Choi, N.; Lee, H.J.; Woo, J.; Cho, Y.; Yang, S.H.; Lee, C.J.; et al. Multifunctional multi-shank neural probe for investigating and modulating long-range neural circuits in vivo. Nat. Commun. 2019, 10, 3777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.; Jeong, S.; Lee, J.-H.; Sun, W.; Choi, N.; Cho, I.-J. 3D high-density microelectrode array with optical stimulation and drug delivery for investigating neural circuit dynamics. Nat. Commun. 2021, 12, 492. [Google Scholar] [CrossRef]
- Sanja, M.; Stefano, P.; Helton Maia, P.; George, C.D.N.; Klas, K.; Adriano, B.L.T.; Richardson, N.L. On the photovoltaic effect in local field potential recordings. Neurophotonics 2016, 3, 015002. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Fan, Y.; Li, L.; Wen, F.; Guo, B.; Jin, M.; Xu, J.; Zhou, Y.; Kang, X.; Ji, B.; et al. Flexible Neural Probes with Optical Artifact-Suppressing Modification and Biofriendly Polypeptide Coating. Micromachines 2022, 13, 199. [Google Scholar] [CrossRef]
- Kim, K.; Vöröslakos, M.; Seymour, J.P.; Wise, K.D.; Buzsáki, G.; Yoon, E. Artifact-free and high-temporal-resolution in vivo opto-electrophysiology with microLED optoelectrodes. Nat. Commun. 2020, 11, 2063. [Google Scholar] [CrossRef]
- Packer, A.M.; Roska, B.; Häusser, M. Targeting neurons and photons for optogenetics. Nat. Neurosci. 2013, 16, 805–815. [Google Scholar] [CrossRef] [Green Version]
- Ayling, O.G.S.; Harrison, T.C.; Boyd, J.D.; Goroshkov, A.; Murphy, T.H. Automated light-based mapping of motor cortex by photoactivation of channelrhodopsin-2 transgenic mice. Nat. Methods 2009, 6, 219–224. [Google Scholar] [CrossRef]
- Han, X.; Qian, X.; Bernstein, J.G.; Zhou, H.-H.; Franzesi, G.T.; Stern, P.; Bronson, R.T.; Graybiel, A.M.; Desimone, R.; Boyden, E.S. Millisecond-Timescale Optical Control of Neural Dynamics in the Nonhuman Primate Brain. Neuron 2009, 62, 191–198. [Google Scholar] [CrossRef]
- Cardin, J.A.; Carlén, M.; Meletis, K.; Knoblich, U.; Zhang, F.; Deisseroth, K.; Tsai, L.-H.; Moore, C.I. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat. Protoc. 2010, 5, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Zatonyi, A.; Borhegyi, Z.; Srivastava, M.; Cserpan, D.; Somogyvari, Z.; Kisvarday, Z.; Fekete, Z. Functional brain mapping using optical imaging of intrinsic signals and simultaneous high-resolution cortical electrophysiology with a flexible, transparent microelectrode array. Sens. Actuators B Chem. 2018, 273, 519–526. [Google Scholar] [CrossRef]
- Guo, B.; Fan, Y.; Wang, M.; Cheng, Y.; Ji, B.; Chen, Y.; Wang, G. Flexible Neural Probes with Electrochemical Modified Microelectrodes for Artifact-Free Optogenetic Applications. Int. J. Mol. Sci. 2021, 22, 11528. [Google Scholar] [CrossRef]
- Ji, B.; Ge, C.; Guo, Z.; Wang, L.; Wang, M.; Xie, Z.; Xu, Y.; Li, H.; Yang, B.; Wang, X.; et al. Flexible and stretchable opto-electric neural interface for low-noise electrocorticogram recordings and neuromodulation in vivo. Biosens. Bioelectron. 2020, 153, 112009. [Google Scholar] [CrossRef]
- Guo, Z.J.; Ji, B.W.; Wang, M.H.; Ge, C.F.; Wang, L.C.; Gu, X.W.; Yang, B.; Wang, X.L.; Li, C.Y.; Liu, J.Q. A Polyimide-Based Flexible Optoelectrodes for Low-Noise Neural Recording. IEEE Electron Device Lett. 2019, 40, 1190–1193. [Google Scholar] [CrossRef]
- Kampasi, K.; English, D.F.; Seymour, J.; Stark, E.; McKenzie, S.; Vöröslakos, M.; Buzsáki, G.; Wise, K.D.; Yoon, E. Dual color optogenetic control of neural populations using low-noise, multishank optoelectrodes. Microsyst. Nanoeng. 2018, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.C.; Ge, C.F.; Wang, M.H.; Ji, B.W.; Guo, Z.J.; Wang, X.L.; Yang, B.; Li, C.Y.; Liu, J.Q. An artefact-resist optrode with internal shielding structure for low-noise neural modulation. J. Neural Eng. 2020, 17, 046024. [Google Scholar] [CrossRef]
- Kim, K.; English, D.; McKenzie, S.; Wu, F.; Stark, E.; Seymour, J.; Ku, P.C.; Wise, K.; Buzsaki, G.; Yoon, E. GaN-on-Si μLED optoelectrodes for high-spatiotemporal-accuracy optogenetics in freely behaving animals. In Proceedings of the 2016 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 3–7 December 2016; pp. 26.25.21–26.25.24. [Google Scholar]
- Laxpati, N.G.; Mahmoudi, B.; Gutekunst, C.-A.; Newman, J.P.; Zeller-Townson, R.; Gross, R.E. Real-time in vivo optogenetic neuromodulation and multielectrode electrophysiologic recording with NeuroRighter. Front. Neuroeng. 2014, 7, 40. [Google Scholar] [CrossRef]
- Budai, D.; Vizvári, A.D.; Bali, Z.K.; Márki, B.; Nagy, L.V.; Kónya, Z.; Madarász, D.; Henn-Mike, N.; Varga, C.; Hernádi, I. A novel carbon tipped single micro-optrode for combined optogenetics and electrophysiology. PLoS ONE 2018, 13, e0193836. [Google Scholar] [CrossRef] [Green Version]
- Khurram, A.; Seymour, J.P. Investigation of the photoelectrochemical effect in optoelectrodes and potential uses for implantable electrode characterization. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2013, 2013, 3032–3035. [Google Scholar] [CrossRef]
- Park, J.; Sun, F.; Xie, Y.; Xiong, Z.; Xu, G. Low-Impedance Low-Artifact PEDOT: PSS-Coated Graphene Electrodes Towards High Density Optogenetic Electrophysiology. IEEE Electron Device Lett. 2020, 41, 1261–1264. [Google Scholar] [CrossRef]
- Wang, L.; Huang, K.; Zhong, C.; Wang, L.; Lu, Y. Fabrication and modification of implantable optrode arrays for in vivo optogenetic applications. Biophys. Rep. 2018, 4, 82–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chen, G.; Xie, K.; Zaia, K.A.; Zhang, S.; Tsien, J.Z. Large-scale neural ensemble recording in the brains of freely behaving mice. J. Neurosci. Methods 2006, 155, 28–38. [Google Scholar] [CrossRef]
- Wang, M.-H.; Ji, B.-W.; Gu, X.-W.; Guo, Z.-J.; Wang, X.-L.; Yang, B.; Li, C.-Y.; Liu, J.-Q. A novel assembly method for 3-dimensional microelectrode array with micro-drive. Sens. Actuators B Chem. 2018, 264, 100–109. [Google Scholar] [CrossRef]
- Wang, M.H.; Gu, X.W.; Ji, B.W.; Wang, L.C.; Guo, Z.J.; Yang, B.; Wang, X.L.; Li, C.Y.; Liu, J.Q. Three-dimensional drivable optrode array for high-resolution neural stimulations and recordings in multiple brain regions. Biosens. Bioelectron. 2019, 131, 9–16. [Google Scholar] [CrossRef]
- Wang, L.; Ge, C.; Wang, F.; Guo, Z.; Hong, W.; Jiang, C.; Ji, B.; Wang, M.; Li, C.; Sun, B.; et al. Dense Packed Drivable Optrode Array for Precise Optical Stimulation and Neural Recording in Multiple-Brain Regions. ACS Sens. 2021, 6, 4126–4135. [Google Scholar] [CrossRef]
- Stocke, S.K.; Samuelsen, C.L. A drivable optrode for use in chronic electrophysiology and optogenetic experiments. J. Neurosci. Methods 2021, 348, 108979. [Google Scholar] [CrossRef]
- Zorzos, A.N.; Scholvin, J.; Boyden, E.S.; Fonstad, C.G. Three-dimensional multiwaveguide probe array for light delivery to distributed brain circuits. Opt. Lett. 2012, 37, 4841–4843. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; He, F.; Wei, X.; Lin, S.; Xie, C. Parallel, minimally-invasive implantation of ultra-flexible neural electrode arrays. J. Neural Eng. 2019, 16, 035001. [Google Scholar] [CrossRef]
- Hassler, C.; Boretius, T.; Stieglitz, T. Polymers for neural implants. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 18–33. [Google Scholar] [CrossRef]
- Eickenscheidt, M.; Herrmann, T.; Weisshap, M.; Mittnacht, A.; Rudmann, L.; Zeck, G.; Stieglitz, T. An optoelectronic neural interface approach for precise superposition of optical and electrical stimulation in flexible array structures. Biosens. Bioelectron. 2022, 205, 114090. [Google Scholar] [CrossRef]
- Reddy, J.W.; Kimukin, I.; Stewart, L.T.; Ahmed, Z.; Barth, A.L.; Towe, E.; Chamanzar, M. High Density, Double-sided, Flexible Optoelectronic Neural Probes With Embedded mu LEDs. Front. Neurosci. 2019, 13, 745. [Google Scholar] [CrossRef] [Green Version]
- Kohler, F.; Gkogkidis, C.A.; Bentler, C.; Wang, X.; Gierthmuehlen, M.; Fischer, J.; Stolle, C.; Reindl, L.M.; Rickert, J.; Stieglitz, T.; et al. Closed-loop interaction with the cerebral cortex: A review of wireless implant technology. Brain-Comput. Interfaces 2017, 4, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Gwon, T.M.; Kim, C.; Shin, S.; Park, J.H.; Kim, J.H.; Kim, S.J. Liquid Crystal Polymer (LCP)-based Neural Prosthetic Devices. Biomed. Eng. Lett. 2016, 6, 148–163. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Cai, X.; Xie, Y.; Wang, T.; Cheng, D.; Li, L.; Li, R.; Deng, Y.; Ding, H.; et al. A wireless, implantable optoelectrochemical probe for optogenetic stimulation and dopamine detection. Microsyst. Nanoeng. 2020, 6, 64. [Google Scholar] [CrossRef]
- Reddy, J.W.; Chamanzar, M. Low-loss flexible Parylene photonic waveguides for optical implants. Opt. Lett. 2018, 43, 4112–4115. [Google Scholar] [CrossRef]
- Schwaerzle, M.; Pothof, F.; Paul, O.; Ruther, P. High-resolution neural depth probe with integrated 460 NM light emitting diode for optogenetic applications. In Proceedings of the 2015 Transducers-2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015; pp. 1774–1777. [Google Scholar]
- Ji, B.; Wang, M.; Kang, X.; Gu, X.; Li, C.; Yang, B.; Wang, X.; Liu, J. Flexible Optoelectric Neural Interface Integrated Wire-Bonding μ LEDs and Microelectrocorticography for Optogenetics. IEEE Trans. Electron. Dev. 2017, 64, 2008–2015. [Google Scholar] [CrossRef]
- Kim, T.-I.; McCall, J.G.; Jung, Y.H.; Huang, X.; Siuda, E.R.; Li, Y.; Song, J.; Song, Y.M.; Pao, H.A.; Kim, R.-H.; et al. Injectable, Cellular-Scale Optoelectronics with Applications for Wireless Optogenetics. Science 2013, 340, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.-M.; Hu, L.; Fu, X. Integrated Giant Magnetoresistance Technology for Approachable Weak Biomagnetic Signal Detections. Sensors 2018, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Peng, L.; Ye, C. Measurement of Triaxial Magnetocardiography Using High Sensitivity Tunnel Magnetoresistance Sensor. IEEE Sens. J. 2019, 19, 9610–9615. [Google Scholar] [CrossRef]
- Gaster, R.S.; Hall, D.A.; Nielsen, C.H.; Osterfeld, S.J.; Yu, H.; Mach, K.E.; Wilson, R.J.; Murmann, B.; Liao, J.C.; Gambhir, S.S.; et al. Matrix-insensitive protein assays push the limits of biosensors in medicine. Nat. Med. 2009, 15, 1327–1332. [Google Scholar] [CrossRef]
- Cardoso, S.; Leitao, D.C.; Dias, T.M.; Valadeiro, J.; Silva, M.D.; Chicharo, A.; Silverio, V.; Gaspar, J.; Freitas, P.P. Challenges and trends in magnetic sensor integration with microfluidics for biomedical applications. J. Phys. D Appl. Phys. 2017, 50, 213001. [Google Scholar] [CrossRef]
- Graham, D.L.; Ferreira, H.A.; Freitas, P.P. Magnetoresistive-based biosensors and biochips. Trends Biotechnol. 2004, 22, 455–462. [Google Scholar] [CrossRef]
- Gaster, R.S.; Xu, L.; Han, S.-J.; Wilson, R.J.; Hall, D.A.; Osterfeld, S.J.; Yu, H.; Wang, S.X. Quantification of protein interactions and solution transport using high-density GMR sensor arrays. Nat. Nanotechnol. 2011, 6, 314–320. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Sun, S.; Wilson, R.J.; White, R.L.; Pourmand, N.; Wang, S.X. Spin valve sensors for ultrasensitive detection of superparamagnetic nanoparticles for biological applications. Sens. Actuators A Phys. 2006, 126, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Germano, J.; Martins, V.C.; Cardoso, F.A.; Almeida, T.M.; Sousa, L.; Freitas, P.P.; Piedade, M.S. A portable and autonomous magnetic detection platform for biosensing. Sensors 2009, 9, 4119–4137. [Google Scholar] [CrossRef] [Green Version]
- Martins, V.C.; Cardoso, F.A.; Germano, J.; Cardoso, S.; Sousa, L.; Piedade, M.; Freitas, P.P.; Fonseca, L.P. Femtomolar limit of detection with a magnetoresistive biochip. Biosens. Bioelectron. 2009, 24, 2690–2695. [Google Scholar] [CrossRef]
- Schulz, L.; Heinisch, P.; Richter, I. Calibration of Off-the-Shelf Anisotropic Magnetoresistance Magnetometers. Sensors 2019, 19, 1850. [Google Scholar] [CrossRef] [Green Version]
- Binasch, G.; Grünberg, P.; Saurenbach, F.; Zinn, W. Enhanced magnetoresistance in layered magnetic structures with antiferromagnetic interlayer exchange. Phys. Rev. B 1989, 39, 4828–4830. [Google Scholar] [CrossRef]
- Baibich, M.N.; Broto, J.M.; Fert, A.; Van Dau, F.N.; Petroff, F.; Etienne, P.; Creuzet, G.; Friederich, A.; Chazelas, J. Giant Magnetoresistance of (001)Fe/(001)Cr Magnetic Superlattices. Phys. Rev. Lett. 1988, 61, 2472–2475. [Google Scholar] [CrossRef] [Green Version]
- Dieny, B.; Speriosu, V.S.; Parkin, S.S.P.; Gurney, B.A.; Wilhoit, D.R.; Mauri, D. Giant magnetoresistive in soft ferromagnetic multilayers. Phys. Rev. B 1991, 43, 1297–1300. [Google Scholar] [CrossRef]
- Heim, D.E.; Fontana, R.E.; Tsang, C.; Speriosu, V.S.; Gurney, B.A.; Williams, M.L. Design and operation of spin valve sensors. IEEE Trans. Magn. 1994, 30, 316–321. [Google Scholar] [CrossRef]
- Freitas, P.P.; Leal, J.L.; Melo, L.V.; Oliveira, N.J.; Rodrigues, L.; Sousa, A.T. Spin-valve sensors exchange-biased by ultrathin TbCo films. Appl. Phys. Lett. 1994, 65, 493–495. [Google Scholar] [CrossRef]
- Dieny, B. Chapter 2—Spin valves. In Magnetoelectronics; Johnson, M., Ed.; Academic Press: San Diego, CA, USA, 2004; pp. 67–377. [Google Scholar] [CrossRef]
- Wilson, A.H.; Fowler, R.H. The electrical conductivity of the transition metals. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1938, 167, 580–593. [Google Scholar] [CrossRef] [Green Version]
- Yuasa, S.; Djayaprawira, D. Giant tunnel magnetoresistance in magnetic tunnel junctions with a crystalline MgO (0 0 1) barrier. J. Phys. D Appl. Phys. 2007, 40, R337. [Google Scholar] [CrossRef] [Green Version]
- Julliere, M. Tunneling between ferromagnetic films. Phys. Lett. A 1975, 54, 225–226. [Google Scholar] [CrossRef]
- Fermon, C.; Van de Voorde, M. Nanomagnetism: Applications and Perspectives; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Amaral, J.; Cardoso, S.; Freitas, P.P.; Sebastião, A.M. Toward a system to measure action potential on mice brain slices with local magnetoresistive probes. J. Appl. Phys. 2011, 109, 07B308. [Google Scholar] [CrossRef]
- Freitas, P.; Cardoso, F.; Martins, V.; Martins, S.; Loureiro, J.; Amaral, J.; Chaves, R.; Cardoso, S.; Fonseca, L.; Sebastião, A. Spintronic platforms for biomedical applications. Lab A Chip 2012, 12, 546–557. [Google Scholar] [CrossRef]
- Amaral, J.; Gaspar, J.; Pinto, V.; Costa, T.; Sousa, N.; Cardoso, S.; Freitas, P. Measuring brain activity with magnetoresistive sensors integrated in micromachined probe needles. Appl. Phys. A 2013, 111, 407–412. [Google Scholar] [CrossRef]
- Amaral, J.; Pinto, V.; Costa, T.; Gaspar, J.; Ferreira, R.; Paz, E.; Cardoso, S.; Freitas, P.P. Integration of TMR Sensors in Silicon Microneedles for Magnetic Measurements of Neurons. IEEE Trans. Magn. 2013, 49, 3512–3515. [Google Scholar] [CrossRef]
- Valadeiro, J.; Silva, M.; Cardoso, S.; Martins, M.; Gaspar, J.; Freitas, P.P.; Sebastião, A.M. Microneedles with integrated magnetoresistive sensors: A precision tool in biomedical instrumentation. In Proceedings of the 2017 IEEE Sensors Applications Symposium (SAS), Glassboro, NJ, USA, 13–15 March 2017; pp. 1–6. [Google Scholar]
- Valadeiro, J.; Amaral, J.; Leitao, D.C.; Silva, A.V.; Gaspar, J.; Silva, M.; Costa, M.; Martins, M.; Franco, F.; Fonseca, H.; et al. Bending Effect on Magnetoresistive Silicon Probes. IEEE Trans. Magn. 2015, 51, 4401104. [Google Scholar] [CrossRef]
- Chopin, C.; Torrejon, J.; Solignac, A.; Fermon, C.; Pannetier-Lecoeur, M. Magnetoresistive Sensor in Two-Dimension on a 25 μm Thick Silicon Substrate for In Vivo Neuronal Measurements. ACS Sens. 2020, 5, 3493–3500. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.P.; Gervasoni, G.; Albisetti, E.; D’Ercoli, F.; Monticelli, M.; Moretti, D.; Forte, N.; Rocchi, A.; Ferrari, G.; Baldelli, P.; et al. Towards a magnetoresistive platform for neural signal recording. AIP Adv. 2017, 7, 056706. [Google Scholar] [CrossRef] [Green Version]
- Moretti, D.; DiFrancesco, M.L.; Sharma, P.P.; Dante, S.; Albisetti, E.; Monticelli, M.; Bertacco, R.; Petti, D.; Baldelli, P.; Benfenati, F. Biocompatibility of a Magnetic Tunnel Junction Sensor Array for the Detection of Neuronal Signals in Culture. Front. Neurosci. 2018, 12, 909. [Google Scholar] [CrossRef]
- Vanhove, E.; Tsopéla, A.; Bouscayrol, L.; Desmoulin, A.; Launay, J.; Temple-Boyer, P. Final capping passivation layers for long-life microsensors in real fluids. Sens. Actuators B Chem. 2013, 178, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Monticelli, M.; Conca, D.V.; Albisetti, E.; Torti, A.; Sharma, P.P.; Kidiyoor, G.; Barozzi, S.; Parazzoli, D.; Ciarletta, P.; Lupi, M.; et al. Magnetic domain wall tweezers: A new tool for mechanobiology studies on individual target cells. Lab A Chip 2016, 16, 2882–2890. [Google Scholar] [CrossRef]
- Okada, Y.C.; Wu, J.; Kyuhou, S. Genesis of MEG signals in a mammalian CNS structure. Electroencephalogr. Clin. Neurophysiol. 1997, 103, 474–485. [Google Scholar] [CrossRef]
- Xie, Y.F.; Jackson, M.F.; Macdonald, J.F. Optogenetics and synaptic plasticity. Acta Pharm. Sin. 2013, 34, 1381–1385. [Google Scholar] [CrossRef]
- Huff, M.L.; Miller, R.L.; Deisseroth, K.; Moorman, D.E.; LaLumiere, R.T. Posttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in rats. Proc. Natl. Acad. Sci. USA 2013, 110, 3597–3602. [Google Scholar] [CrossRef] [Green Version]
- Rolls, A.; Colas, D.; Adamantidis, A.; Carter, M.; Lanre-Amos, T.; Heller, H.C.; de Lecea, L. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc. Natl. Acad. Sci. USA 2011, 108, 13305–13310. [Google Scholar] [CrossRef] [Green Version]
- Xiong, W.; Jin, X. Optogenetic field potential recording in cortical slices. J. Neurosci. Methods 2012, 210, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Kohl, M.M.; Shipton, O.A.; Deacon, R.M.; Rawlins, J.N.; Deisseroth, K.; Paulsen, O. Hemisphere-specific optogenetic stimulation reveals left-right asymmetry of hippocampal plasticity. Nat. Neurosci. 2011, 14, 1413–1415. [Google Scholar] [CrossRef]
- Chun, S.; Bayazitov, I.T.; Blundon, J.A.; Zakharenko, S.S. Thalamocortical long-term potentiation becomes gated after the early critical period in the auditory cortex. J. Neurosci. 2013, 33, 7345–7357. [Google Scholar] [CrossRef] [Green Version]
- Shibata, A.C.E.; Ueda, H.H.; Eto, K.; Onda, M.; Sato, A.; Ohba, T.; Nabekura, J.; Murakoshi, H. Photoactivatable CaMKII induces synaptic plasticity in single synapses. Nat. Commun. 2021, 12, 751. [Google Scholar] [CrossRef]
- Liewald, J.F.; Brauner, M.; Stephens, G.J.; Bouhours, M.; Schultheis, C.; Zhen, M.; Gottschalk, A. Optogenetic analysis of synaptic function. Nat. Methods 2008, 5, 895–902. [Google Scholar] [CrossRef]
- Adamantidis, A.R.; Tsai, H.C.; Boutrel, B.; Zhang, F.; Stuber, G.D.; Budygin, E.A.; Touriño, C.; Bonci, A.; Deisseroth, K.; de Lecea, L. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J. Neurosci. 2011, 31, 10829–10835. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Beppu, K.; Tanaka, K.F.; Fukazawa, Y.; Shigemoto, R.; Matsui, K. Application of an optogenetic byway for perturbing neuronal activity via glial photostimulation. Proc. Natl. Acad. Sci. USA 2012, 109, 20720–20725. [Google Scholar] [CrossRef] [Green Version]
- Su, D.; Wu, K.; Saha, R.; Peng, C.; Wang, J.-P. Advances in Magnetoresistive Biosensors. Micromachines 2019, 11, 34. [Google Scholar] [CrossRef]
- Drew, L. The brain-reading devices helping paralysed people to move, talk and touch. Nature 2022, 604, 416–419. [Google Scholar] [CrossRef] [PubMed]



| Type | Source | Magnitude | Suppression Methods |
|---|---|---|---|
| PV | Metal electrodes exposed to optical radiation | Tens to hundreds μVs | Heavily boron-doped silicon substrates [100] |
| Transparent electrode materials [52,105] | |||
| Flexible polymer-based substrates [106,107,108] | |||
| EMI | Active μ-LEDs and their interconnects | Several mVs | Metal shielding layer [100,108,110] |
| Dual-metal-layer shielding [111] | |||
| Transient pulse shaping [110] | |||
| PEC | Metal–electrolyte interface | Tens to hundreds μVs | Electrochemical modification [52,106,115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Xue, N.; Chen, J. A Review: Research Progress of Neural Probes for Brain Research and Brain–Computer Interface. Biosensors 2022, 12, 1167. https://doi.org/10.3390/bios12121167
Luo J, Xue N, Chen J. A Review: Research Progress of Neural Probes for Brain Research and Brain–Computer Interface. Biosensors. 2022; 12(12):1167. https://doi.org/10.3390/bios12121167
Chicago/Turabian StyleLuo, Jiahui, Ning Xue, and Jiamin Chen. 2022. "A Review: Research Progress of Neural Probes for Brain Research and Brain–Computer Interface" Biosensors 12, no. 12: 1167. https://doi.org/10.3390/bios12121167
APA StyleLuo, J., Xue, N., & Chen, J. (2022). A Review: Research Progress of Neural Probes for Brain Research and Brain–Computer Interface. Biosensors, 12(12), 1167. https://doi.org/10.3390/bios12121167







