An Electrochemical Immunosensor Based on Carboxylated Graphene/SPCE for IgG-SARS-CoV-2 Nucleocapsid Determination
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Samples
2.2. Apparatus and Procedures
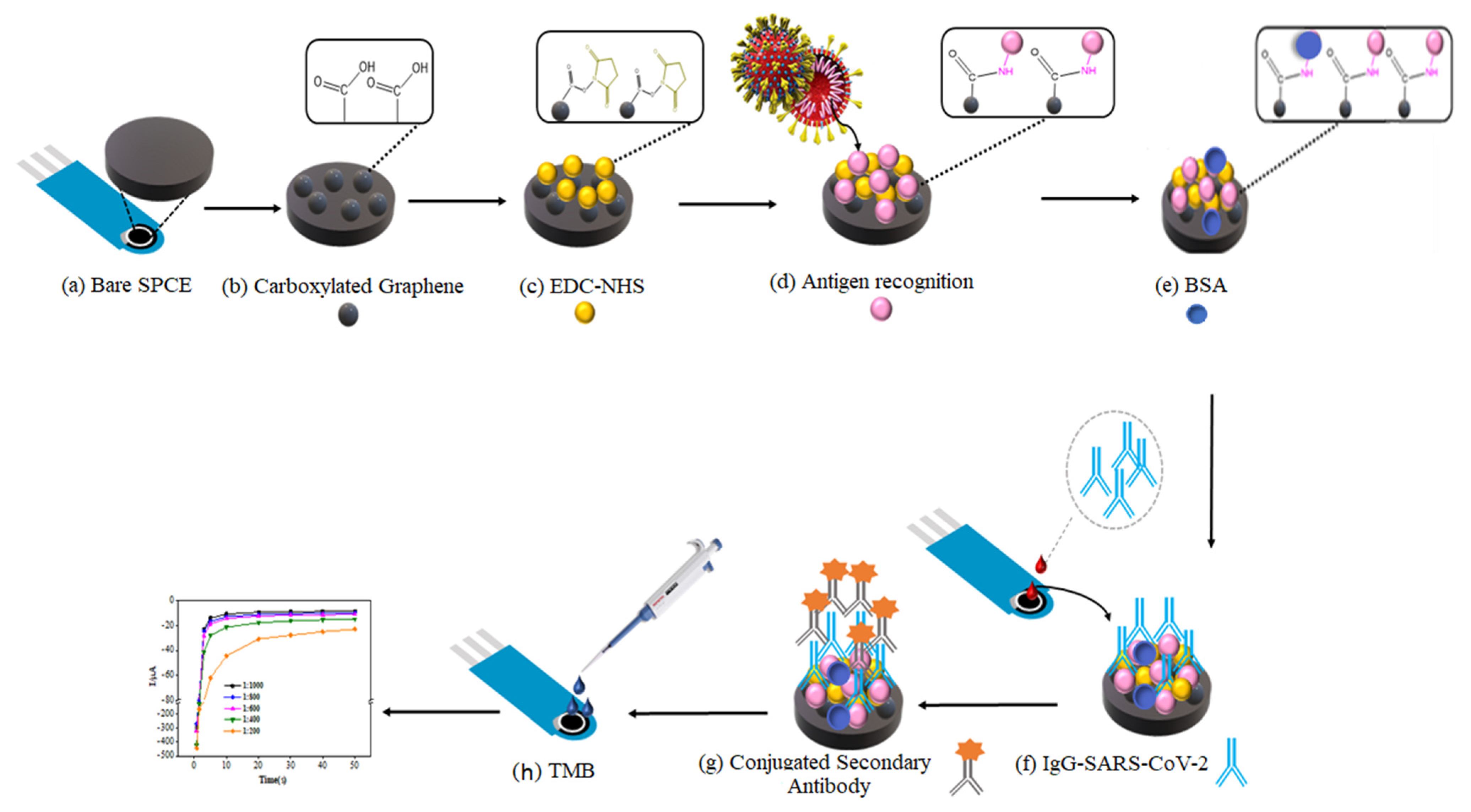
2.3. Assembly of the Biosensor
2.4. Synthesis of Carboxylate Graphene
2.5. Electrochemical Detection
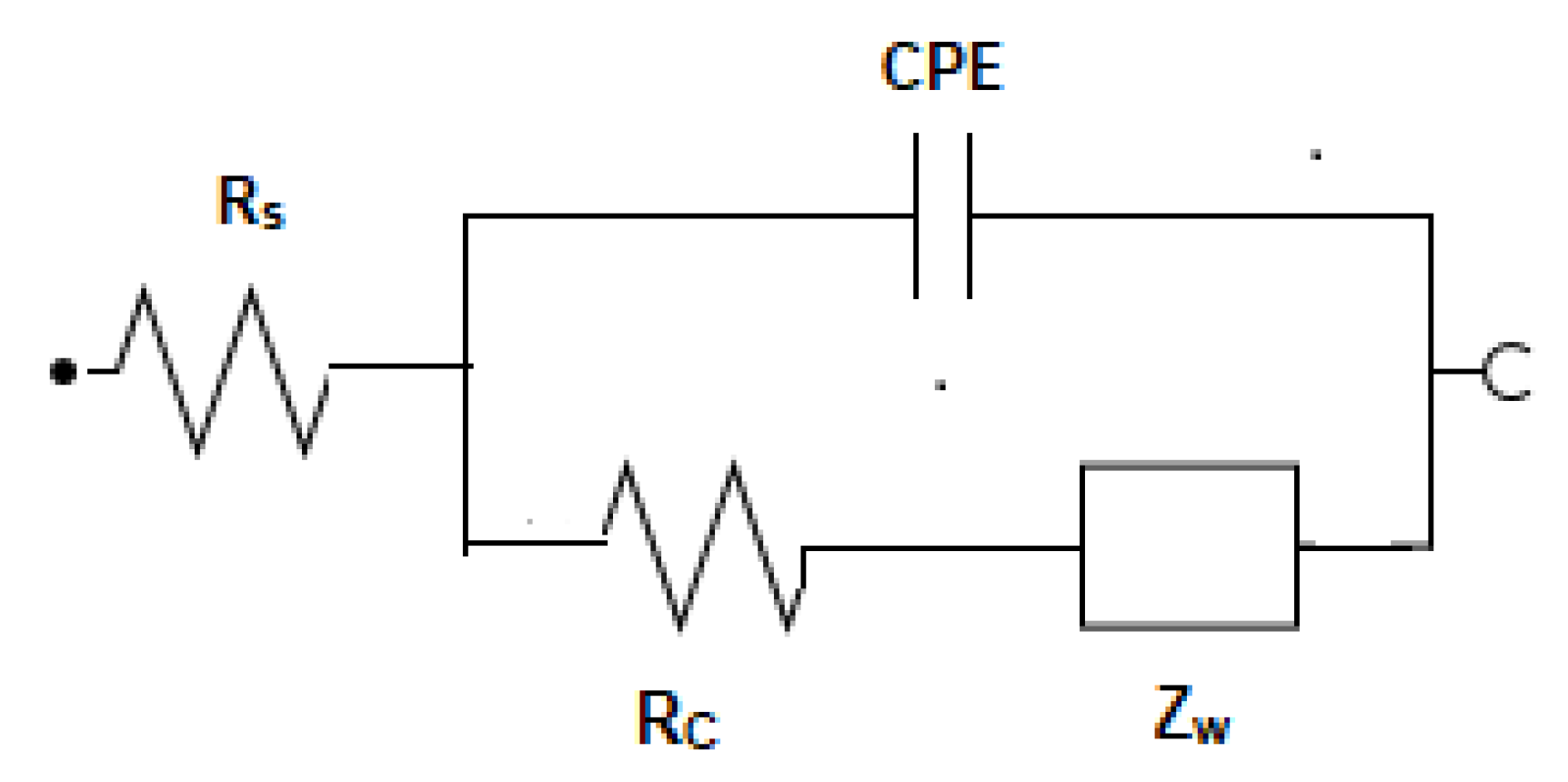
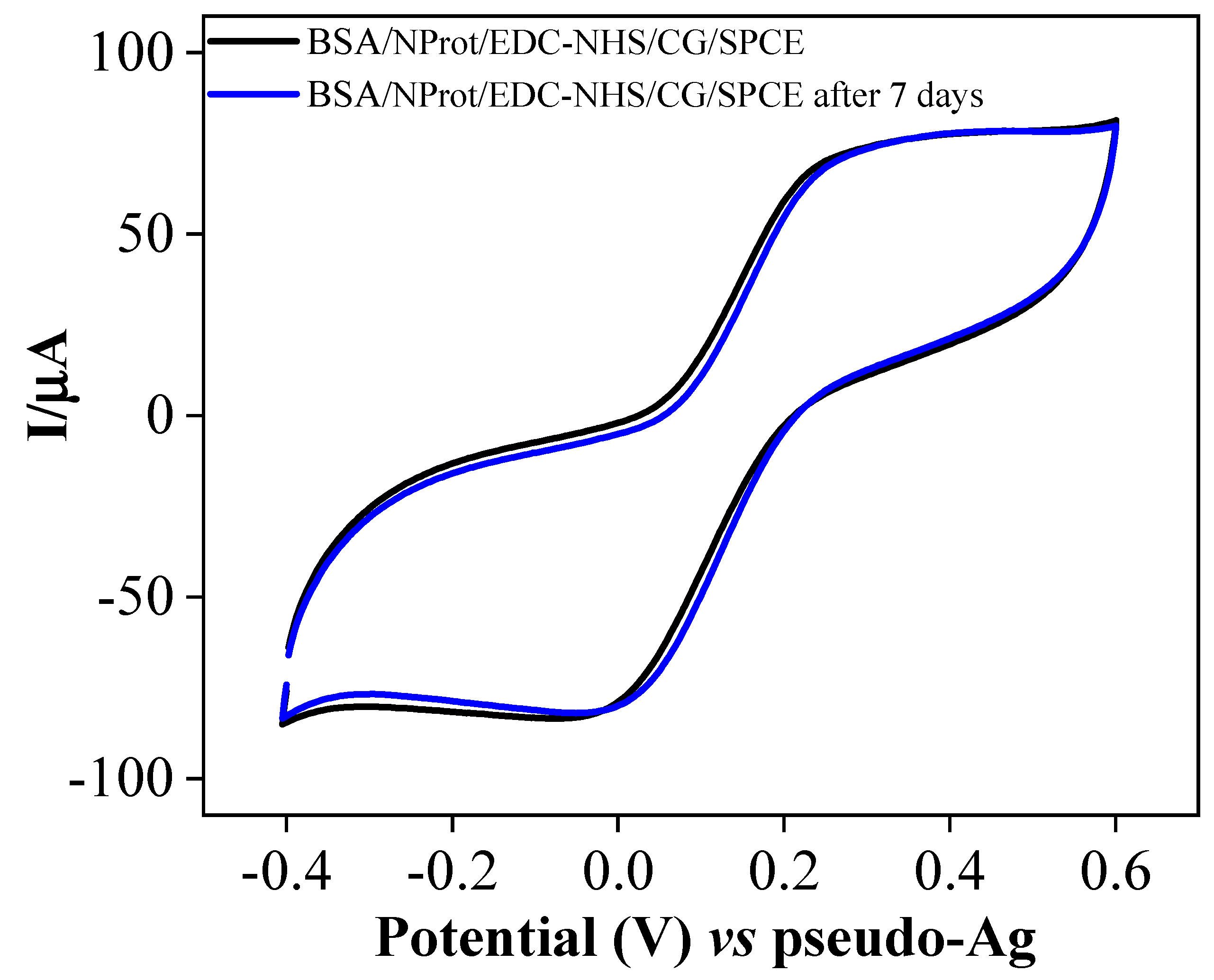
3. Results and Discussion
3.1. Optimization of Antigen Immobilization
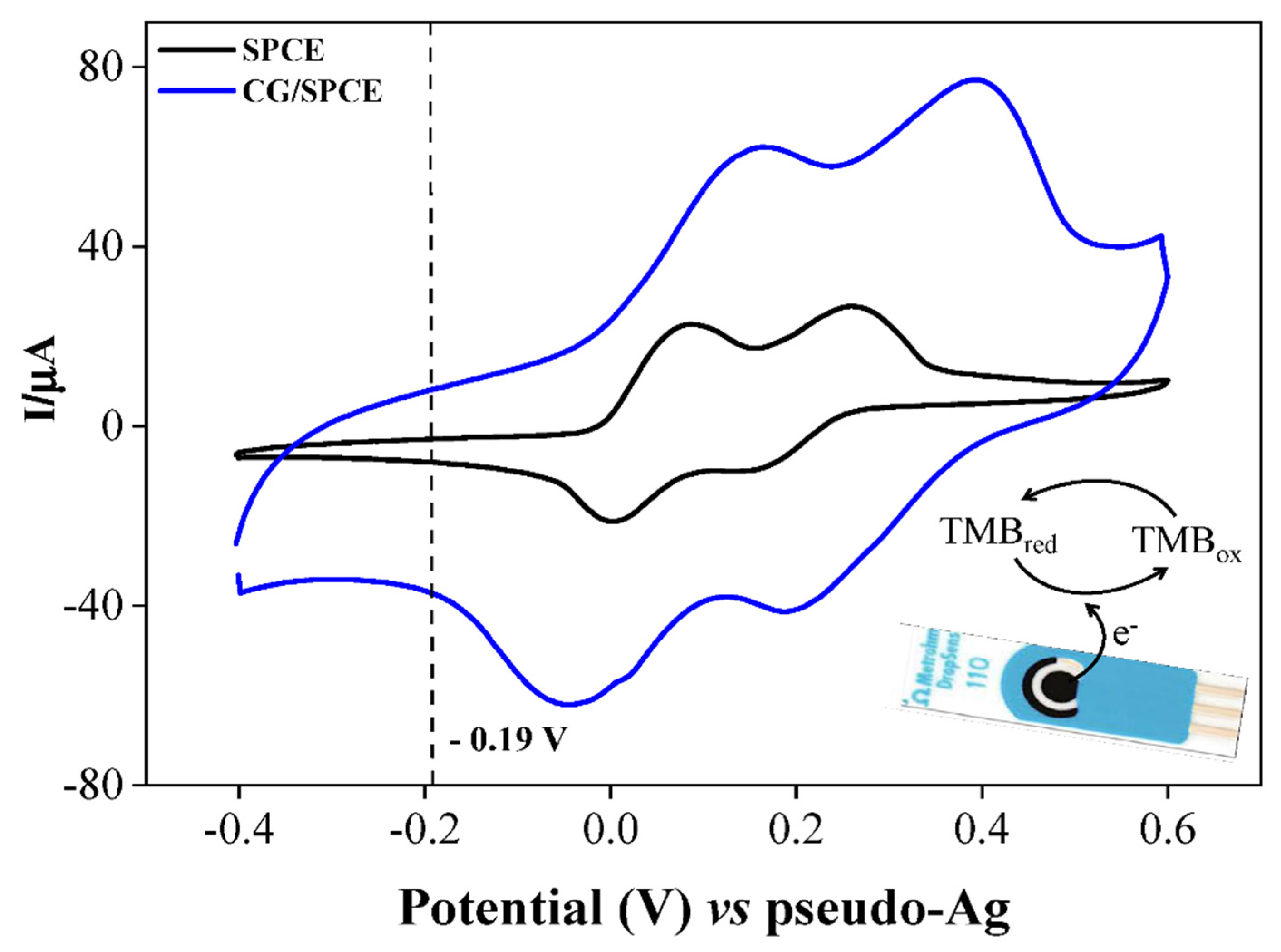
3.2. Cyclic Voltammetric and Chronoamperometric Studies of TMB at SPCEs
3.3. Immunosensor Calibration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OMS. Archived: WHO Timeline—COVID-19. 2021. Available online: https://www.who.int/news/item/27-04-2020-who-timeline---covid-19 (accessed on 9 September 2022).
- Dhama, C.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus Disease 2019-COVID-19. Clin. Microbiol. Rev. 2020, 33, e00028-20. [Google Scholar] [CrossRef] [PubMed]
- Surjit, M.; Lal, S.K. The Nucleocapsid Protein of the SARS Coronavirus: Structure, Function and Therapeutic Potential. In Molecular Biology of the SARS-Coronavirus, 1st ed.; Lal, S.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–328. [Google Scholar]
- Ward, S.; Lindsley, A.; Courter, J.; Assa, A. Clinical testing for COVID-19. J. Allergy Clin. Immunol. 2020, 146, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Döhla, M.; Boesecke, C.; Schulte, B.; Diegmann, C.; Sib, E.; Richter, E.; Eschbach-Bludau, M.; Aldabbagh, S.; Marx, B.; Eis-Hübinger, A.M.; et al. Rapid point-of-care testing for SARS-CoV-2 in a community screening setting shows low sensitivity. Public Health 2020, 182, 170–172. [Google Scholar] [CrossRef]
- Testes Para a COVID-19: Como São e Quando Devem ser Feitos. 2022. Available online: https://portal.fiocruz.br/noticia/testes-para-covid-19-como-sao-e-quando-devem-ser-feitos (accessed on 9 September 2022).
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical biosensors—Sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Mehrvar, M.; Abdi, M. Recent developments, characteristics, and potential applications of electrochemical biosensors. Anal. Sci. 2004, 20, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, Z.; Roushani, M.; Hosseini, H.; Choobin, H. An electrochemical immunosensor using SARS-CoV-2 spike protein-nickel hydroxide nanoparticles bio-conjugate modified SPCE for ultrasensitive detection of SARS-CoV-2 antibodies. Microchem. J. 2021, 170, 106718. [Google Scholar] [CrossRef]
- Zhang, W.; Du, R.H.; Li, B.; Zheng, X.S.; Yang, X.L.; Hu, B.; Wang, Y.Y.; Xiao, G.F.; Yan, B.; Shi, Z.L.; et al. Molecular and serological investigation of 2019-nCoV infected patients: Implication of multiple shedding routes. Emerg. Microbes Infect. 2020, 9, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Antuña-Jiménez, D.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Screen-printed electrodes modified with metal nanoparticles for small molecule sensing. Biosensors 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Amine, A.; Majorani, C.; Di Giorgio, F.; De Felicis, D.; Cataldo, F.; Moscone, D.; Palleschi, G. High performance electrochemical sensor based on modified screen-printed electrodes with cost-effective dispersion of nanostructured carbon black. Electrochem. Commun. 2010, 12, 346–350. [Google Scholar] [CrossRef]
- Hatala, M.; Gemeiner, P.; Lorencová, L.; Mikula, M.; Hvojnik, M.; Pavličková, M.; Ház, A.; Kosnáč, D.; Bertók, T.; Tkáč, J. Screen-printed conductive carbon layers for dye-sensitized solar cells and electrochemical detection of dopamine. Chem. Pap. 2021, 75, 3817–3829. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.; Shin, D.; Ryoo, S.; Hong, B.H.; Min, D. Biomedical applications of graphene and graphene oxide. Accounts of Chemical Research. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-Based Nanomaterials: Multifunctional Materials for Biomedical Engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xu, C.; Lin, J.; Wang, C.; Wang, X.; Zhang, C.; Zhou, X.; Guo, S.; Cui, D. Folic Acid-conjugated Graphene Oxide loaded with Photosensitizers for Targeting Photodynamic Therapy. Theranostics 2012, 1, 240–250. [Google Scholar] [CrossRef]
- Jin, L.; Yang, K.; Yao, K.; Zhang, S.; Tao, H.; Lee, S.T.; Liu, Z.; Peng, R. Functionalized graphene oxide in enzyme engineering: A selective modulator for enzyme activity and thermostability. ACS Nano 2012, 6, 4864–4875. [Google Scholar] [CrossRef]
- Li, Q.; Fan, F.; Wang, Y.; Feng, W.; Ji, P. Enzyme immobilization on carboxyl-functionalized graphene oxide for catalysis in organic solvent. Ind. Eng. Chem. Res. 2013, 52, 6343–6348. [Google Scholar] [CrossRef]
- Shen, J.; Shi, M.; Yan, B.; Ma, H.; Li, N.; Hu, Y.; Ye, M. Covalent attaching protein to graphene oxide via diimide-activated amidation. Colloids Surf. B Biointerfaces 2010, 81, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Y.; Wu, Q.; Gao, Y.; Zhang, H.; Bai, Y.; Song, D. Preparation of graphene oxide-based surface plasmon resonance biosensor with Au bipyramid nanoparticles as sensitivity enhancer. Colloids Surf. B Biointerfaces 2014, 116, 211–218. [Google Scholar] [CrossRef]
- Ezenarro, J.J.; Párraga-Niño, N.; Sabrià, M.; Del Campo, F.J.; Muñoz-Pascual, F.X.; Mas, J.; Uria, N. Rapid detection of legionella pneumophila in drinking water, based on filter immunoassay and chronoamperometric measurement. Biosensors 2020, 10, 102. [Google Scholar] [CrossRef]
- Baldrich, E.; del Campo, F.J.; Muñoz, F.X. Biosensing at disk microelectrode arrays. Inter-electrode functionalisation allows formatting into miniaturised sensing platforms of enhanced sensitivity. Biosens. Bioelectron. 2009, 25, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Cawthraw, S.A.; Feldman, R.A.; Sayers, A.R.; Newell, D.G. Long-term antibody responses following human infection with Campylobacter jejuni. Clin. Exp. Immunol. 2002, 130, 101–106. [Google Scholar] [CrossRef]
- Fanjul-Bolado, P.; González-García, M.B.; Costa-García, A. Amperometric detection in TMB/HRP-based assays. Anal. Bioanal. Chem. 2005, 382, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xu, S.; Ma, X.; Qiu, B.; Lin, Z.; Chen, G. Dual-color plasmonic enzyme-linked immunosorbent assay based on enzyme-mediated etching of Au nanoparticles. Sci. Rep. 2016, 6, 32755. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhang, L.; Wen, W.; Zhang, X.; Wang, S. Enzyme catalytic amplification of miRNA-155 detection with graphene quantum dot-based electrochemical biosensor. Biosens. Bioelectron. 2016, 77, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Josephy, P.D.; Eling, T.; Mason, R.P. The horseradish peroxidase-catalyzed oxidation of 3,5,3’,5’-tetramethylbenzidine. Free radical and charge-transfer complex intermediates. J. Biol. Chem. 1982, 257, 3669–3675. [Google Scholar] [CrossRef] [PubMed]
- Kadir, M.A.; Tothill, I.E. Immunosensor for Aflatoxin B1 Using Gold Working Electrode. In Proceedings of the Cranfield Multi-Strand Conference: Creating Wealth Through Research and Innovation (CMC 2008), Cranfield, UK, 5–6 May 2008; pp. 440–446. [Google Scholar]
- Li, J.; Lillehoj, P.B. Microfluidic Magneto Immunosensor for Rapid, High Sensitivity Measurements of SARS-CoV-2 Nucleocapsid Protein in Serum. ACS Sensors 2021, 6, 1270–1278. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Liu, G.; Wai, C.M.; Lin, Y. Magnetic beads-based bioelectrochemical immunoassay of polycyclic aromatic hydrocarbons. Electrochem. Commun. 2007, 9, 1547–1552. [Google Scholar] [CrossRef]
- Berka, A.N.; Tocksteinov, Z.; Zima, J.I.G. Voltammetric determination of benzidine and its derivatives, at a glassy-carbon electrode. Talanta 1986, 33, 811–815. [Google Scholar]
- Lalwani, P.; Salgado, B.B.; Filho, I.V.P.; da Silva, D.S.S.; de Morais, T.B.d.N.; Jordão, M.F.; Barbosa, A.R.C.; Cordeiro, I.B.; Neto, J.N.d.S.; de Assunção, E.N.; et al. SARS-CoV-2 seroprevalence and associated factors in Manaus, Brazil: Baseline results from the DETECTCoV-19 cohort study. Int. J. Infect. Dis. 2021, 110, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Barthelmebs, L.; Sassolas, A.; Marty, J.L. An electrochemical immunosensor based on covalent immobilization of okadaic acid onto screen printed carbon electrode via diazotization-coupling reaction. Talanta 2011, 85, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Stanković, V.; Đurđić, S.; Ognjanović, M.; Antić, B.; Kalcher, K.; Mutić, J.; Stanković, D.M. Anti-human albumin monoclonal antibody immobilized on EDC-NHS functionalized carboxylic graphene/AuNPs composite as promising electrochemical HSA immunosensor. J. Electroanal. Chem. 2020, 860, 113928. [Google Scholar] [CrossRef]
- Cui, G.; Yoo, Y.H.; Lee, J.S.; Yoo, J.; Uhm, J.H.; Cha, G.S.; Nam, H. Effect of pre-treatment on the surface and electrochemical properties of screen-printed carbon paste electrodes. Analyst 2001, 126, 1399–1403. [Google Scholar] [CrossRef]
- Verdoodt, N.; Basso, C.R.; Rossi, B.F.; Pedrosa, V.A. Development of a rapid and sensitive immunosensor for the detection of bacteria. Food Chem. 2017, 221, 1792–1796. [Google Scholar] [CrossRef]
- Jaiswal, N.; Pandey, C.M.; Soni, A.; Tiwari, I.; Rosillo-Lopez, M.; Salzmann, C.G.; Malhotra, B.D.; Sumana, G. Electrochemical genosensor based on carboxylated graphene for detection of water-borne pathogen. Sens. Actuators B Chem. 2018, 275, 312–321. [Google Scholar] [CrossRef]
- Ngunjiri, J.N.; Stark, D.J.; Tian, T.; Briggman, K.A.; Garno, J.C. Immobilization of proteins on carboxylic acid functionalized nanopatterns. Anal. Bioanal. Chem. 2013, 405, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, N.; Ikada, Y. Mechanism of Amide Formation by Carbodiimide for Bioconjugation in Aqueous Media. Bioconjug. Chem. 1995, 6, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Mavrikou, S.; Moschopoulou, G.; Zafeirakis, A.; Kalogeropoulou, K.; Giannakos, G.; Skevis, A.; Kintzios, S. An ultra-rapid biosensory point-of-care (POC) assay for prostate-specific antigen (PSA) detection in human serum. Sensors 2018, 18, 3834. [Google Scholar] [CrossRef] [PubMed]
- Biscay, J.; García, M.B.G.; García, A.C. Electrochemical biotin determination based on a screen printed carbon electrode array and magnetic beads. Sens. Actuators B Chem. 2014, 205, 426–432. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; Mercader, J.V.; Abad-Fuentes, A.; Checa-Orrego, B.I.; Costa-García, A.; de la Escosura-Muñiz, A. Direct competitive immunosensor for Imidacloprid pesticide detection on gold nanoparticle-modified electrodes. Talanta 2020, 209, 120465. [Google Scholar] [CrossRef] [PubMed]
- Pasakon, P.; Mensing, J.P.; Phokaratkul, D.; Karuwan, C.; Lomas, T.; Wisitsoraat, A.; Tuantranont, A. A high-performance, disposable screen-printed carbon electrode modified with multi-walled carbon nanotubes/graphene for ultratrace level electrochemical sensors. J. Appl. Electrochem. 2019, 49, 217–227. [Google Scholar] [CrossRef]
- Kanso, H.; Pankratova, G.; Bollella, P.; Leech, D.; Hernandez, D.; Gorton, L. Sunlight photocurrent generation from thylakoid membranes on gold nanoparticle modified screen-printed electrodes. J. Electroanal. Chem. 2018, 816, 259–264. [Google Scholar] [CrossRef]
- Fanjul-Bolado, P.; Queipo, P.; Lamas-Ardisana, P.J.; Costa-García, A. Manufacture and evaluation of carbon nanotube modified screen-printed electrodes as electrochemical tools. Talanta 2007, 74, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, R.S.; de Faria, R.A.D.; Zanoni, M.V.B.; Nalin, M.; De Lima Gomes, P.C.F.; Messaddeq, Y. Simple, fast and environmentally friendly method to determine ciprofloxacin in wastewater samples based on an impedimetric immunosensor. RSC Adv. 2020, 10, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.Z.; Chen, C.J.; Settu, K.; Lin, Y.F.; Chen, C.L.; Liu, J.T. Screen-printed carbon electrode-based electrochemical immunosensor for rapid detection of microalbuminuria. Biosens. Bioelectron. 2016, 77, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Ahammad, A.J.S. Hydrogen Peroxide Biosensors Based on Horseradish Peroxidase and Hemoglobin. J. Biosens. Bioelectron. 2012, S9, 1–11. [Google Scholar] [CrossRef]
- Chhabra, V.A.; Deep, A.; Kaur, R.; Kumar, R. Functionalization of Graphene using Carboxylation Process. Int. J. Sci. Emerg. Technol. with Latest Trends 2012, 4, 13–19. [Google Scholar]
- Eigler, S.; Dotzer, C.; Hirsch, A.; Enzelberger, M.; Müller, P. Formation and decomposition of CO2 intercalated graphene oxide. Chem. Mater. 2012, 24, 1276–1282. [Google Scholar] [CrossRef]
- Chhabra, V.; Singh, M.; Deep, A. Carboxylated Graphene-Indium Tin Oxide Hetrojunction for Bio-Sensing. Nanotechnol. Nov. Perspect. Prospect. 2015, 1, 414–420. [Google Scholar]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Naebe, M.; Wang, J.; Amini, A.; Khayyam, H.; Hameed, N.; Li, L.H.; Chen, Y.; Fox, B. Mechanical Property and Structure of Covalent Functionalised Graphene/Epoxy Nanocomposites. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vasile, E.; Pandele, A.M.; Andronescu, C.; Selaru, A.; Dinescu, S.; Costache, M.; Hanganu, A.; Raicopol, M.D.; Teodorescu, M. Hema-Functionalized Graphene Oxide: A Versatile Nanofiller for Poly(Propylene Fumarate)-Based Hybrid Materials. Sci. Rep. 2019, 9, 18685. [Google Scholar] [CrossRef]
- Shao, G.; Liu, P.; Zhang, K.; Li, W.; Chen, X.; Ma, F. Mechanical properties of graphene nanoplates reinforced copper matrix composites prepared by electrostatic self-assembly and spark plasma sintering. Mater. Sci. Eng. A 2019, 739, 329–334. [Google Scholar] [CrossRef]
- Park, K.W.; Jung, J.H. Spectroscopic and electrochemical characteristics of a carboxylated graphene-ZnO composites. J. Power Sources 2012, 199, 379–385. [Google Scholar] [CrossRef]
- Gu, Y.; Ju, C.; Li, Y.; Shang, Z.; Wu, Y.; Jia, Y.; Niu, Y. Detection of circulating tumor cells in prostate cancer based on carboxylated graphene oxide modified light addressable potentiometric sensor. Biosens. Bioelectron. 2015, 66, 24–31. [Google Scholar] [CrossRef]




| Patients | Immunosensor | ELISA |
|---|---|---|
| 1 | + | + |
| 2 | + | + |
| 3 | + | + |
| 4 | + | + |
| 5 | + | + |
| 6 | − | − |
| 7 | − | − |
| 8 | − | − |
| 9 | − | − |
| 10 | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza Freire, L.; Ruzo, C.M.; Salgado, B.B.; Gandarilla, A.M.D.; Romaguera-Barcelay, Y.; Tavares, A.P.M.; Sales, M.G.F.; Cordeiro, I.; Lalwani, J.D.B.; Matos, R.; et al. An Electrochemical Immunosensor Based on Carboxylated Graphene/SPCE for IgG-SARS-CoV-2 Nucleocapsid Determination. Biosensors 2022, 12, 1161. https://doi.org/10.3390/bios12121161
de Souza Freire L, Ruzo CM, Salgado BB, Gandarilla AMD, Romaguera-Barcelay Y, Tavares APM, Sales MGF, Cordeiro I, Lalwani JDB, Matos R, et al. An Electrochemical Immunosensor Based on Carboxylated Graphene/SPCE for IgG-SARS-CoV-2 Nucleocapsid Determination. Biosensors. 2022; 12(12):1161. https://doi.org/10.3390/bios12121161
Chicago/Turabian Stylede Souza Freire, Luciana, Camila Macena Ruzo, Bárbara Batista Salgado, Ariamna María Dip Gandarilla, Yonny Romaguera-Barcelay, Ana P. M. Tavares, Maria Goreti Ferreira Sales, Isabelle Cordeiro, Jaila Dias Borges Lalwani, Robert Matos, and et al. 2022. "An Electrochemical Immunosensor Based on Carboxylated Graphene/SPCE for IgG-SARS-CoV-2 Nucleocapsid Determination" Biosensors 12, no. 12: 1161. https://doi.org/10.3390/bios12121161
APA Stylede Souza Freire, L., Ruzo, C. M., Salgado, B. B., Gandarilla, A. M. D., Romaguera-Barcelay, Y., Tavares, A. P. M., Sales, M. G. F., Cordeiro, I., Lalwani, J. D. B., Matos, R., Fonseca Filho, H., Astolfi-Filho, S., Ţălu, Ş., Lalwani, P., & Brito, W. R. (2022). An Electrochemical Immunosensor Based on Carboxylated Graphene/SPCE for IgG-SARS-CoV-2 Nucleocapsid Determination. Biosensors, 12(12), 1161. https://doi.org/10.3390/bios12121161












