Enhancing Electrochemical Biosensor Performance for 17β-Estradiol Determination with Short Split—Aptamers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus and Instrumentation
2.3. Preparation of Polypyrrole Nanowire
2.4. Synthesis of Polymer Microspheres
2.5. Interaction Study between Split Aptamers and 17β-Estradiol (E2)
2.6. Fabrication of 17β-Estradiol (E2) Aptasensor
2.7. Optimization of 17β-Estradiol (E2) Aptasensor
2.8. Electrochemical Analysis of Aptasensor
2.9. Selectivity, Stability and Reproducibility
2.10. Real Samples
3. Results and Discussion
3.1. Characterization of Polypyrrole Nanowires
3.2. Characterization of the Poly(methacrylic acid-co-n-butyl acrylate) (p(MAA-co-nBA)
3.3. Interaction Study between Sandwich Aptamers and 17β-Estradiol (E2)
3.4. Electrochemical Properties of 17β-Estradiol (E2) Aptasensor
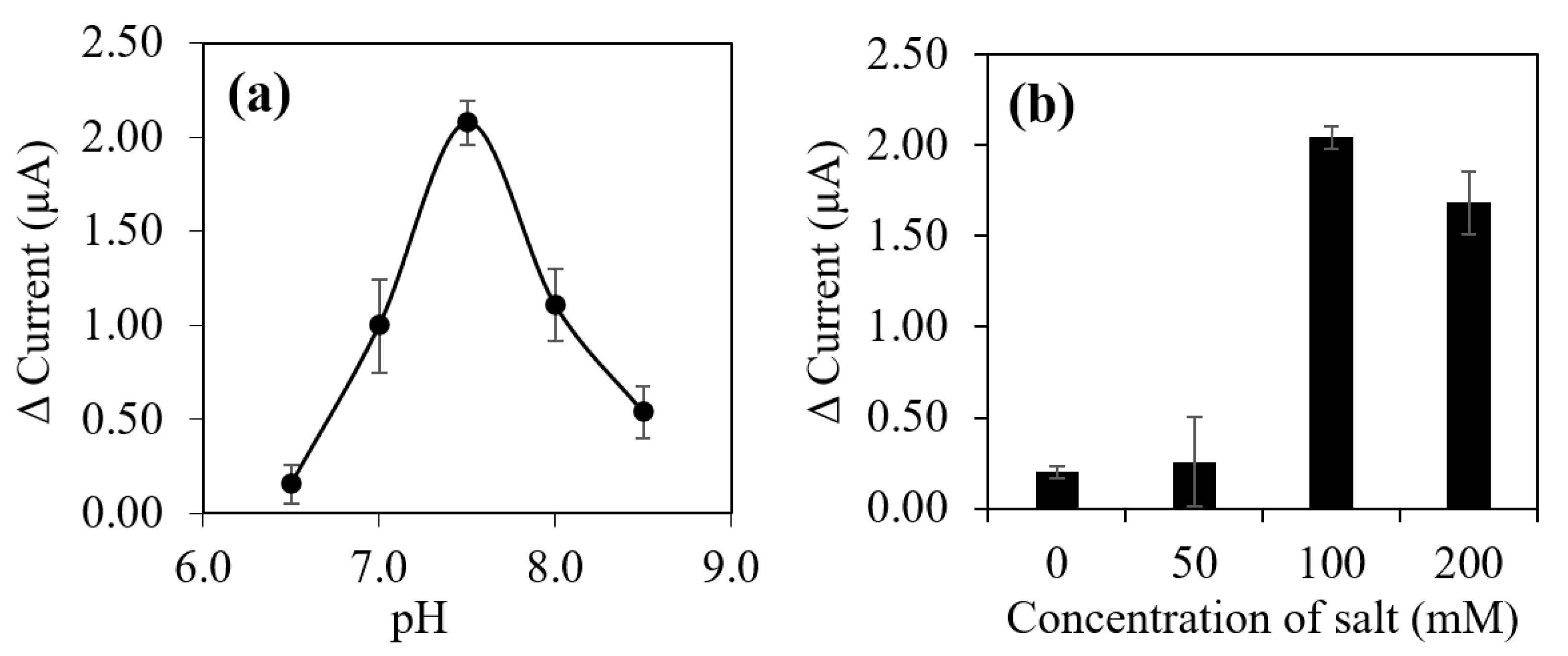
3.5. Optimization and Evaluation of 17β-Estradiol (E2) Aptasensor
3.6. Analytical Performance of 17β-Estradiol (E2) Aptasensor
3.7. Specificity, Stability and Reproducibility
3.8. Real Samples
3.9. A Comparison with Other Similar Aptasensors Reported
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, M.M.; Synmonds, E.M.; Gerber, B.; Hoare, A.; Vleet, E.S.V.; Breitbart, M. Removal of six estrogenic endocrine disrupting compound (EDCs) from municipal wastewater using aluminium electrocoagulation. Water 2016, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Directive, 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy Text with EEA Relevance. Official Journal of European Union 2013, 226/1. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013L0039&from=EN (accessed on 30 October 2022).
- Commission Implementing Decision, 2015/495/EU of 20 March 2015. Official Journal of European Union 2015, 78/40. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015D0495&from=EN (accessed on 30 October 2022).
- Nazari, E.; Suja, F. Effects of 17β-estradiol (E2) on aqueous organisms and its treatment problem: A review. Rev. Environ. Health 2016, 31, 465–491. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Liu, M.; Zhuang, L.; Li, Z.; Fan, L.; Zhao, G. A fetomolar level 17β-estradiol electrochemical aptasensor constructed on hierarchical dendritic gold modified boro-doped diamond electrode. Electrochim. Acta 2014, 137, 146–153. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, G.; Shi, H.; Liu, M. A simple and label-free aptasensor based on nickel hexacyanoferrate nanoparticles as signal probe for highly sensitive detection of 17β-estradiol. Biosens. Bioelectron. 2015, 68, 303–309. [Google Scholar] [CrossRef]
- Du, X.; Dai, L.; Jiang, D.; Li, H.; Hao, N.; You, T.; Mao, H.; Wang, K. Gold nanorods Plasmon-enhanced photoelectrochemical aptasensing based on hematite/N-doped graphene films for ultrasenstitve analysis of 17β-estradiol. Biosens. Bioelectron. 2017, 91, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Hazizan, A.F.; Zulkifli, S.Z.; Mohamat-Yusuff, F.; Omar, H.; Ariozono, K. Determination of 17β-estradiol concentration in aquatic environment of Peninsular Malaysia using the ELISA Technique. Life Sci. J. 2014, 11, 673–679. [Google Scholar]
- Liz, M.; Amaral, B.; Stets, S.; Nagata, N.; Zamora, P.P. Sensitive estrogens determination in wastewater samples by HPLC and fluorescence detection. J. Braz. Chem. Soc. 2016, 28, 1453. [Google Scholar]
- Wooding, M.; Rohwer, E.R.; Naude, Y. Determination of endocrine disrupting chemicals and antiretroviral compounds in surface water: A disposable sorptive sampler with comprehensive gas chromatography-time of flight mass spectrosmetry and large volume injection with ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2017, 1496, 122–132. [Google Scholar] [PubMed] [Green Version]
- Naldi, A.C.; Fayad, P.B.; Prevost, M.; Sauve, S. Analysis of steroid hormones and their conjugated forms in water and urine by on-online solid-phase extraction coupled to liquid chromatography tandem mass spectrosmetry. Chem. Cent. J. 2016, 30. [Google Scholar] [CrossRef] [Green Version]
- Nameghi, M.A.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. An ultrasensitive electrochemical sensor for 17β-estradiol using split aptamers. Anal. Chim. Acta 2019, 1065, 107–112. [Google Scholar] [CrossRef]
- Futra, D.; Lee, Y.H.; Mohamad, Z.; Ulianas, A.; Saeedfar, K.; Tan, L.L. A novel electrochemical sensor for 17β-estradiol from molecularly imprinted polymeric microspheres and multi walled carbon nanotubes grafted with gold nanoparticles. Anal. Methods 2016, 8, 1381–1389. [Google Scholar] [CrossRef]
- Tung, T.T.; Castro, M.; Pillin, I.; Kim, T.Y.; Suh, K.S.; Feller, J.F. Graphene-Fe3O4/PIL-PEDOT for the design of sensitive and stable quantum chemo-resistive VOC sensors. Carbon 2014, l74, 104–122. [Google Scholar] [CrossRef]
- Huang, K.J.; Liu, Y.J.; Zhang, J.Z.; Cao, J.T.; Liu, Y.M. Aptamer/Au nanoparticles/cobalt sulphide nanosheets biosensor for 17β-estradiol detection using a guanine-rich complementary DNA sequences for signal amplification. Biosens. Bioelectron. 2014, 67, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Zaid, M.H.M.; Abdullah, J.; Rozi, N.; Rozlan, A.A.M.; Hanifah, S.A. A sensitive impedimetric aptasensor based on carbon nanodots modified electrode for detection of 17β-estradiol. Nanomaterials 2020, 10, 1346. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lai, G.; Liu, S.; Yu, A. Ultrasensitive electrochemical aptasensing of kanamycin antibiotic by enzymatic signal amplification with a horseradish peroxidase-functionalized gold nanoprobe. Sens. Actuator B Chem. 2018, 273, 1762–1767. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, W. Ultrasensitive aptasensor with DNA tetrahedral nanostructure for Ochratoxin A detection based on hemin/G-quadruplex catalysed polyaniline deposition. Sens. Actuators B Chem. 2018, 276, 1–7. [Google Scholar] [CrossRef]
- Abnous, K.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Taghdisi, S.M. A novel electrochemical sensor for bisphenol A detection based on nontarget-induced extension of aptamer length and formation of a physical barrier. Biosens. Bioelectron. 2018, 119, 204–208. [Google Scholar] [CrossRef]
- Luo, J.; Shen, X.; Li, B.; Li, X.; Zhou, X. Signal amplification by strand displacement in a carbon dot based fluorometric assay for ATP. Microchim. Acta 2018, 185, 392. [Google Scholar] [CrossRef]
- Trinh, K.H.; Kadam, U.S.; Song, J.; Cho, Y.; Kang, C.H.; Lee, K.O.; Lim, C.O.; Chung, W.S.; Hong, J.C. Novel DNA aptameric sensors to detect the toxic insecticide fenitrothion. Int. J. Mol. Sci. 2021, 22, 10846. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, F.; Chen, M.; Zhu, Y.; Xiao, J.; Yang, H.; Chen, X. Rapid and visual detection of aflatoxin B1 in foodstuffs using aptamer/G-quadruplex DNAzyme probe with low background noise. Food Chem. 2019, 271, 581–587. [Google Scholar] [CrossRef]
- Jarczewska, M.; Gorski, L.; Malinowska, E. Electrochemical aptamer-based biosensors as potential tools for clinical diagnostics. Anal. Methods 2016, 8, 3861–3877. [Google Scholar] [CrossRef] [Green Version]
- Sabri, M.Z.; Hamid, A.A.A.; Hitam, S.M.S.; Rahim, M.Z.A. In silico screening of aptamers configuration against hepatitis B surface antigen. Adv. Bioinfom. 2019, 2019, 6912914. [Google Scholar] [CrossRef]
- Seo, H.B.; Gu, M.B. Aptamer-based sandwich-type biosensors. J. Biol. Eng. 2017, 11, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Bai, W.; Niu, S.; Zhu, C.; Yang, S.; Chen, A. Highly sensitive colorimetric detection of 17-β-estradiol using split DNA aptamer immobilized on unmodified gold nanoparticles. Sci. Rep. 2014, 4, 7571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsager, O.A.; Kumar, S.; Zhu, B.; Travas-Sejdic, J.; McNatty, K.P.; Hodgkiss, J.M. Ultrasensitive colorimetric detection of 17β-estradiol: The effect of shortening DNA aptamer sequences. Anal. Chem. 2015, 87, 4201–4209. [Google Scholar] [CrossRef]
- Setka, M.; Drbohlavova, J.; Hubalek, J. Nanostructured polypyrrole based ammonia and volatile organic compound sensors. Sensors 2017, 17, 562. [Google Scholar] [CrossRef]
- Jakhar, P.; Shukla, M.; Singh, V. Electrochemically synthesized polypyrrole nanowiress towards glucose biosensing applications. In Proceedings of the International Workshop on the Physics of Semiconductor and Devices, IWPSD 2017, Delhi, India, 12–15 December 2017. [Google Scholar] [CrossRef]
- Spychalska, K.; Zajac, D.; Cabaj, J. Electrochemical biosensor for detection of 17β-estradiol using semi-conducting polymers and horseradish peroxidase. RSC Adv. 2020, 10, 9079–9087. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.M.; Liu, Q.F.; Qiu, J.H.; Yang, C.; Wei, C.; Liu, C.J.; Lao, L. Design and fabrication of an all-solid-state polymer supercapacitor with highly mechanical flexibility based on polypyrrole hydrogel. ACS Appl. Mater. Interfaces 2017, 9, 33941–33947. [Google Scholar] [CrossRef]
- Xu, L.S.; Jia, M.Y.; Li, Y.; Zhang, S.F.; Jin, X.J. Design and synthesis of graphene/activated carbon/polypyrrole flexible supercapacitor electrodes. RSC Adv. 2017, 7, 31342–31351. [Google Scholar] [CrossRef] [Green Version]
- Britz, B.; Ng, E.; Jiang, H.; Xu, Z.; Kumar, R.; Dong, L. Smart nitrate-selective electrochemical sensors with electrospun nanowiress modified microelectrode. In Proceedings of the IEEE International Conference on Systems, Man and Cybernatics-SMC, San Diego, CA, USA, 5–8 October 2014. [Google Scholar] [CrossRef]
- Esmaeili, C.; Lee, Y.H.; Chew, P.C.; Rashid, Z.A.; Safitri, E.; Marugan, R.S.P.M. A DNA biosensor based on kappa-carragenan-polypyrrole-gold nanoparticles composite for gender determination of arowana fish (scleropages formosus). Sens. Actuators B Chem. 2017, 242, 616–624. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Ahmad, R.; Shrestha, S.; Park, C.H.; Kim, C.S. Globular-shaped polypyrrole doped will-dispersed functionalized multiwall carbon nanotubes/nafion composite for enzymatic glucose biosensor application. Sci. Rep. 2017, 7, 16191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Chen, N.; Mi, H.; Ma, J.; Xie, Y.; Qiu, J. Hierarchical hybrids integrated by dual polypyrrole-based porous carbons foe enhanced capacitive performance. Chem. Eur. J. 2017, 23, 13474–13481. [Google Scholar]
- Ilgu, M.; Nilsen-Hamilton, M. Aptamers in analytics. Analyst 2016, 141, 1551–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novakova, L.; Solich, P.; Matsova, L.; Sicha, J. HPLC determination of estradiol, its degradation product, and preservatives in new topical formulation Estrogen HBF. Anal. Bioanal. Chem. 2004, 379, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, J.; Trchova, T. Conducting polypyrrole nanotubes:a review. Chem. Pap. 2018, 72, 1563–1595. [Google Scholar] [CrossRef]
- Feng, X.M.; Yan, Z.Z.; Li, R.M.; Liu, X.F.; Hou, W.H. The synthesis of shape-controlled polypyrrole/graphene and the study of its capacitance properties. Polym. Bull. 2013, 70, 2291–2304. [Google Scholar] [CrossRef]
- Varga, M.; Kopecka, J.; Moravkova, Z.; Knvka, I.; Trchova, M.; Stejskal, J.; Prokes, J. Effect of oxidants on electronic transport in polypyrrole nanotubes synthesized in the presence of methyl orange. J. Polym. Sci. 2015, 53, 1147–1159. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, J.; Li, B.; Du, W.; Huang, Q.; Zheng, M.; Xue, H.; Pang, H. Facile synthesis of polypyrrole nanowires for high-performance supercapacitor electrode materials. Prog. Nat. Sci. Mater. 2016, 26, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Lampman, G.M.; Pavia, D.L.; Kriz, G.S.; Vyvyan, J.R. Spectroscopy International Edition, 4th ed.; Brook/Cole: Belmont, MA, USA, 2010. [Google Scholar]
- Shen, M.; Han, Y.; Lin, X.; Ding, B.; Zhang, L.; Zhang, X. Preparation and electrochemical performances of porous polypyrrole film by interfacial polymerization. J. Appl. Polym. Sci. 2013, 12, 2938–2944. [Google Scholar] [CrossRef]
- Valtera, S.; Prokes, J.; Kopecka, J.; Vrnata, M.; Trchova, M.; Varga, M.; Stejskal, J.; Kopecky, D. Dye-stimulated control of conducting polypyrrole morphology. RSC Adv. 2017, 7, 51495–51505. [Google Scholar] [CrossRef] [Green Version]
- Harun, N.A.; Tan, Y.T.; Liew, P.C.; Zainuddin, A.A.; Kassim, S. Copolymerization of methacrylic acid (MAA) and butyl acrylate (BuA) via emulsion polymerization technique. MJChem 2019, 21, 20–28. [Google Scholar]
- Belkadi, S.; Bendaikha, H.; Lebsir, F.; Ould-Kada, S. Synthesis, characterization and swelling study of poly(methacrylic acid-co-maleic acid) hydrogels. Orient. J. Chem. 2018, 34, 948. [Google Scholar] [CrossRef]
- Bajpai, S.; Rai, J.S.P.; Nigam, I. Swelling behaviour of poly(MMA-co-BA-PPGDA) polymers. J. Appl. Polym. Sci. 2009, 112, 2374–2382. [Google Scholar] [CrossRef]
- El-Said, W.A.; Abdelshakour, M.; Choi, J.H.; Choi, J.W. Application of conducting polymer nanostrucutres to electrochemical biosensors. Molecules 2020, 25, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alagappan, M.; Immanuel, S.; Sivasubramanian, R.; Kandaswamy, A. Development of cholesterol biosensor using Au nanoparticles decorated f-MWCNT covered with polypyrrole network. Arab. J. Chem. 2020, 13, 2001–2010. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Khoddami, E.; Rezaei, B. Aptamer@Au-o-phenylenediamine modified pencil graphite electrode: A new selective electrochemical impedance biosensor for the determination of insulin. Colloids Surf. B Biointerfaces 2017, 159, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.P.; Yang, T.H. Sensing performance of EGFET pH sensors with CuO nanowires fabricated on glass substrate. Int. J. Electrochem. Sci. 2012, 7, 5020–5027. [Google Scholar]
- Taib, M.; Tan, L.L.; Karim, N.H.A.; Goh, C.T.; Lee, Y.H.; Khalid, B. Reflectance aptasensor based on metal salphen label for rapid and facile determination of insulin. Talanta 2020, 207, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Perrin, D.; Boyd, D. Buffers for pH and Metal Ion Control (Chapman and Hall Laboratory Manuals in Physical Chemistry and Biochemistry); Chapman and Hall: Boca Raton, FL, USA, 1974. [Google Scholar]
- Zhang, G.; Li, T.; Zhang, J.; Chen, A. A simple FRET-based turn-on fluorescent aptasensor for 17β-estradiol determination in environmental water, urine and milk samples. Sens. Actuators B Chem. 2018, 273, 1648–1653. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Feng, J.; Ren, X.; Zhang, Y.; Yan, T.; Liu, X.; Wei, Q. A self-powered photoelectrochemical cathodic aptasensor for the detection of 17β-estradiol based on FeOOH/In2S3. Biosens. Bioelectron. 2020, 154, 112089. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.T.N.; Chikae, M.; Ukita, Y.; Takamura, Y. Labelless impedance immunosensor based on polypyrrole-pyrolecarboxylic acid copolymer for hCG detection. Talanta 2011, 85, 2576–2580. [Google Scholar] [CrossRef] [PubMed]
- Minopoli, A.; Sakac, N.; Lenyk, B.; Campanile, R.; Mayer, D.; Offenhausser, A.; Velotta, R.; Ventura, B.D. LSPR-based colorimetric immunosensor for rapid and sensitive 17β-estradiol detection in tap water. Sens. Actuator B Chem. 2020, 308, 127699. [Google Scholar] [CrossRef]
- Zhu, B.; Alsager, O.A.; Kumar, S.; Hodgkiss, J.M. Label-free electrochemical aptasensor for femtomolar detection 17β-estradiol. Biosens. Bioelectron. 2015, 70, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Na, W.; Park, J.W.; An, J.H.; Jang, J. Size-controllable ultrathin carboxylated polypyrrole nanotube transducer for extremely sensitive 17β-estradiol FET-type biosensors. J. Mater. Chem. B 2016, 4, 5025–5034. [Google Scholar] [CrossRef] [PubMed]











| E2 Concentration Added (nM) to Sample | E2 Concentration Found (nM) | Recovery (%) | RSD (% n = 3) |
|---|---|---|---|
| 0.0 | non-detected | - | - |
| 1.0 | 0.99 | 99.0 | 0.65 |
| 5.0 | 4.89 | 97.7 | 0.62 |
| 10.0 | 10.13 | 101.3 | 0.45 |
| 25.0 | 24.63 | 98.5 | 0.59 |
| 50.0 | 48.78 | 97.5 | 0.43 |
| 75.0 | 73.51 | 98.0 | 0.42 |
| 100.0 | 101.00 | 101.0 | 0.83 |
| Sample | Detection Method | Linear Range (M) | LOD (M) | Ref. |
|---|---|---|---|---|
| AuNP/35-mer aptamer | Colorimetry | 5.00 10−8 to 8.00 10−7 | 2.00 10−9 | Alsager et al. 2015 [27] |
| AuNP/33-mer & 43-mer aptamer | Colorimetry | 3.67 10−10 to 3.67 10−4 | 3.67 10−10 | Liu et al. 2014 [26] |
| Polyclonal antibodies/Colloidal AuNP | Colorimetry | 1.10 10−11 to 1.00 10−7 | 11 10−12 | Minopoli et al. 2020 [58] |
| Poly 4,7-bis-(3,4-ethylenedioxylthiophene) thiophen-2-yl)benzothiadiazole/H2O2/Horseradish peroxidase enzyme | Electrochemistry (CV, DPV) | 1.00 10−7 to 1.20 10−4 | 1 10−6 | Spychalska et al. 2020 [30] |
| Poly(pyrrole-co-3-pyrrolacrylic acid)copolymer/75 aptamer | Electrochemistry (EIS) | 1.00 10−15 to 1.00 10−6 | 1.00 10−15 | Zhu et al. 2015 [59] |
| Ultrathin carboxylated polypyrrole nanotube/76 aptamer | Field effect transistor (FET) method | 1.00 10−15 to 1.00 10−6 | 1.00 10−15 | Na et al. 2016 [60] |
| Gold SPE/self-assembled 33-mer & 43-mer aptamer | Electrochemistry (DPV) | 1.20 10−12 to 1.00 10−10 7.00 10−9 to 1.00 10−10 | 5.00 10−13 | Nameghi et al. 2019 [12] |
| PPY/PMAA-NBA/12-mer & 14mer aptamer | Electrochemistry (DPV) | 1.00 10−4 to 1.00 10−12 | 4.80 10−13 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozi, N.; Hanifah, S.A.; Abd Karim, N.H.; Heng, L.Y.; Higashi, S.L.; Ikeda, M. Enhancing Electrochemical Biosensor Performance for 17β-Estradiol Determination with Short Split—Aptamers. Biosensors 2022, 12, 1077. https://doi.org/10.3390/bios12121077
Rozi N, Hanifah SA, Abd Karim NH, Heng LY, Higashi SL, Ikeda M. Enhancing Electrochemical Biosensor Performance for 17β-Estradiol Determination with Short Split—Aptamers. Biosensors. 2022; 12(12):1077. https://doi.org/10.3390/bios12121077
Chicago/Turabian StyleRozi, Normazida, Sharina Abu Hanifah, Nurul Huda Abd Karim, Lee Yook Heng, Sayuri L. Higashi, and Masato Ikeda. 2022. "Enhancing Electrochemical Biosensor Performance for 17β-Estradiol Determination with Short Split—Aptamers" Biosensors 12, no. 12: 1077. https://doi.org/10.3390/bios12121077
APA StyleRozi, N., Hanifah, S. A., Abd Karim, N. H., Heng, L. Y., Higashi, S. L., & Ikeda, M. (2022). Enhancing Electrochemical Biosensor Performance for 17β-Estradiol Determination with Short Split—Aptamers. Biosensors, 12(12), 1077. https://doi.org/10.3390/bios12121077





