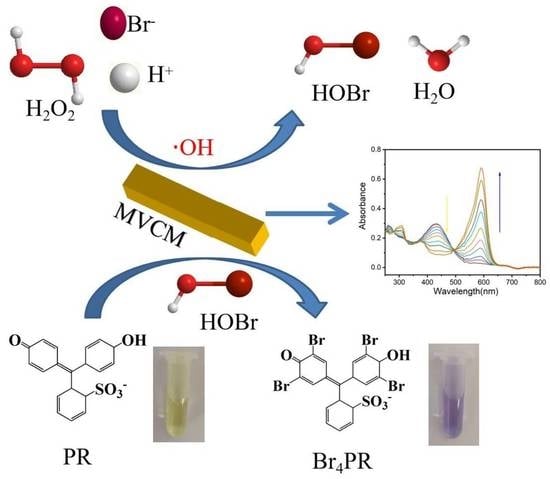
Ce-MOF with Intrinsic Haloperoxidase-Like Activity for Ratiometric Colorimetric Detection of Hydrogen Peroxide
Abstract
:1. Introduction
2. Experiments
2.1. Reagents and Materials
2.2. Apparatus
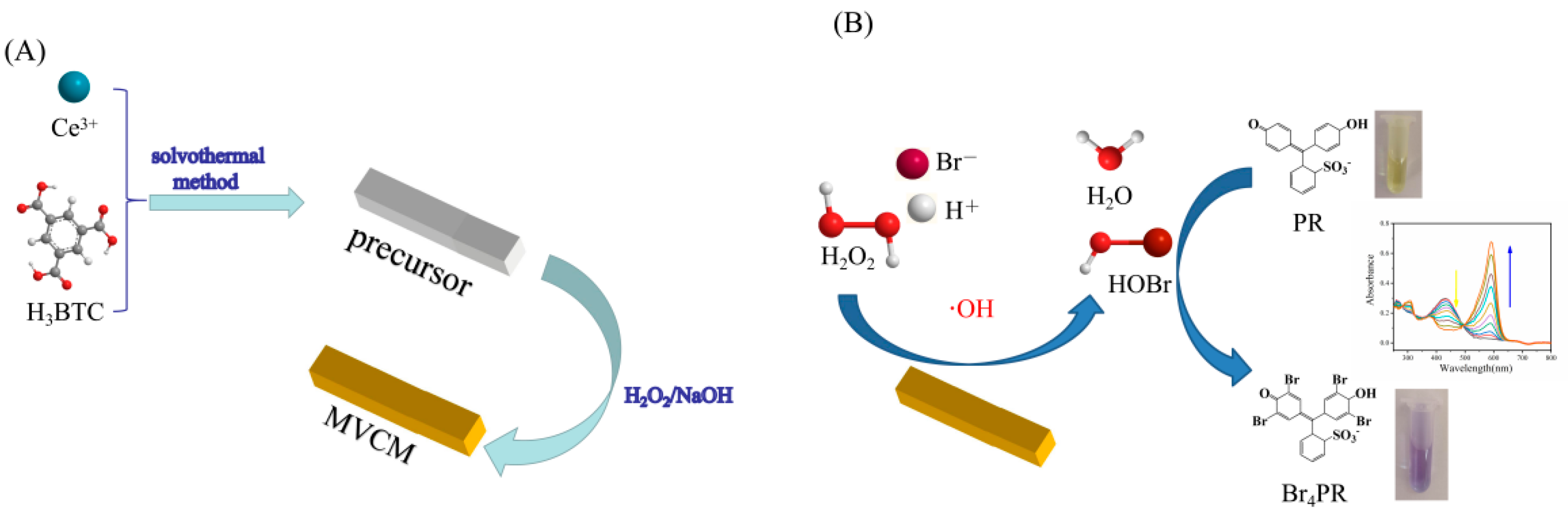
2.3. Synthesis of MVCM
2.4. Catalytic Activity
2.5. Analysis of Active Species
2.6. Kinetic Constant Assay
2.7. H2O2 Detection Using MVCM
2.8. The Analysis of Real Samples
3. Results and Discussion
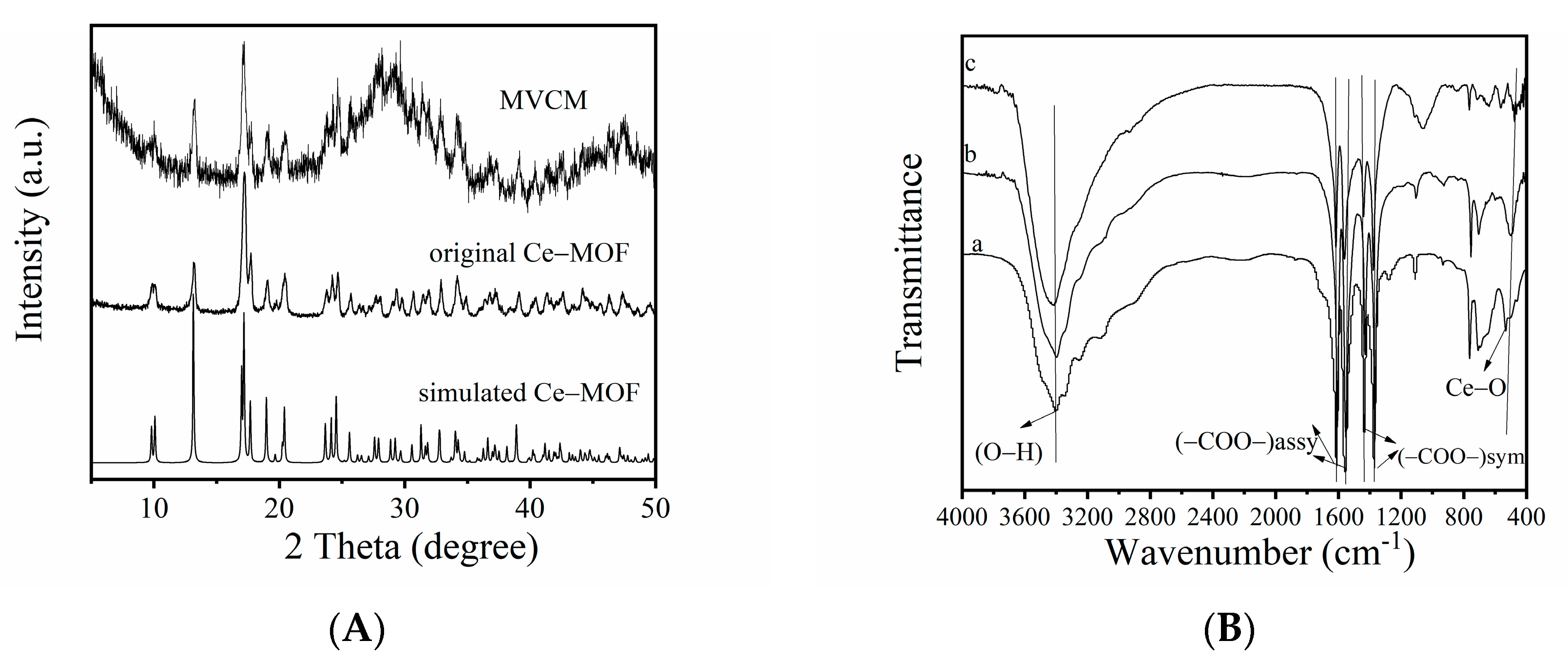
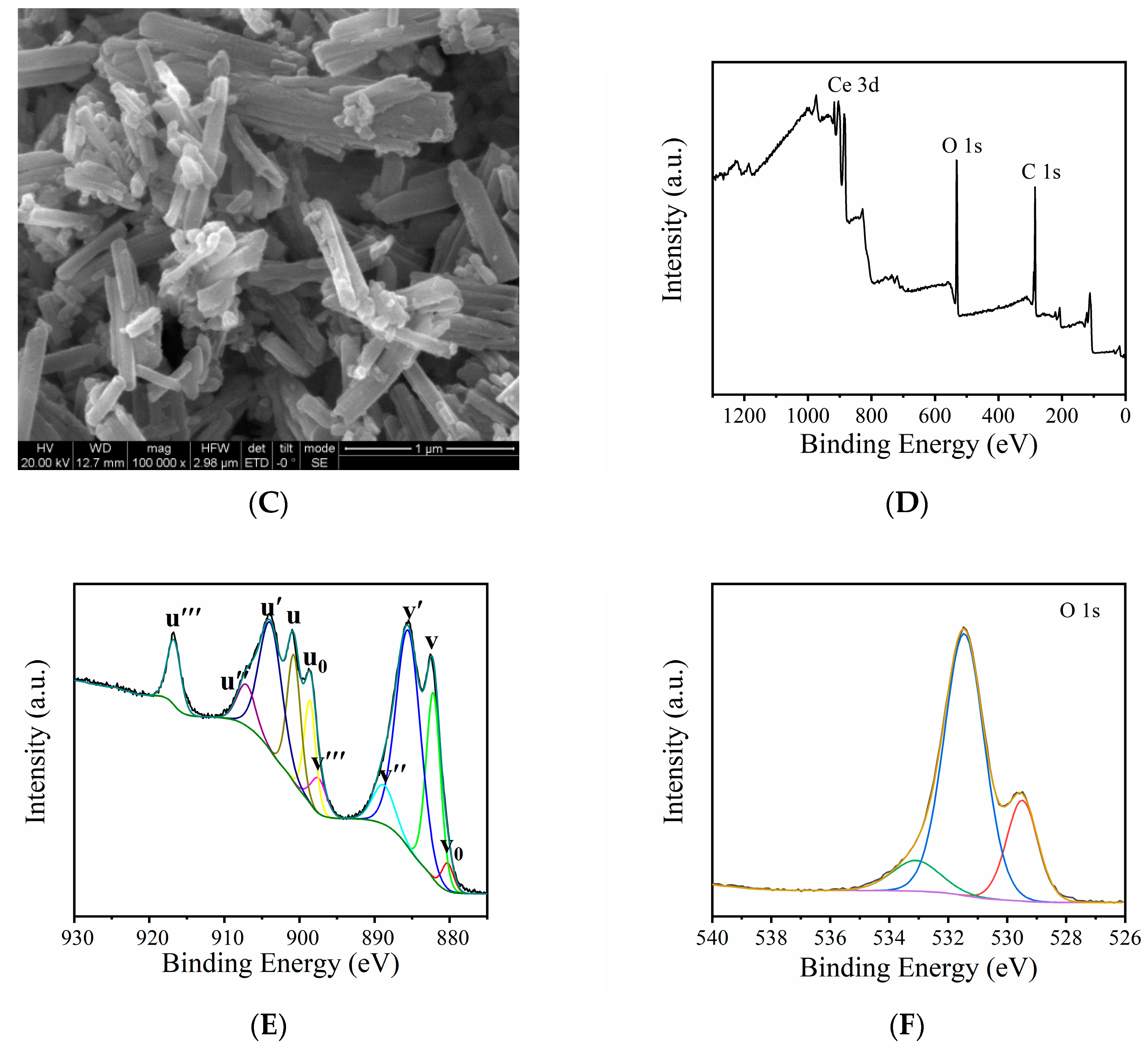
3.1. Characterization of MVCM
3.2. The Intrinsic Haloperoxidase-Like Activity of MVCM
3.3. Active Species Analysis and Catalytic Mechanism
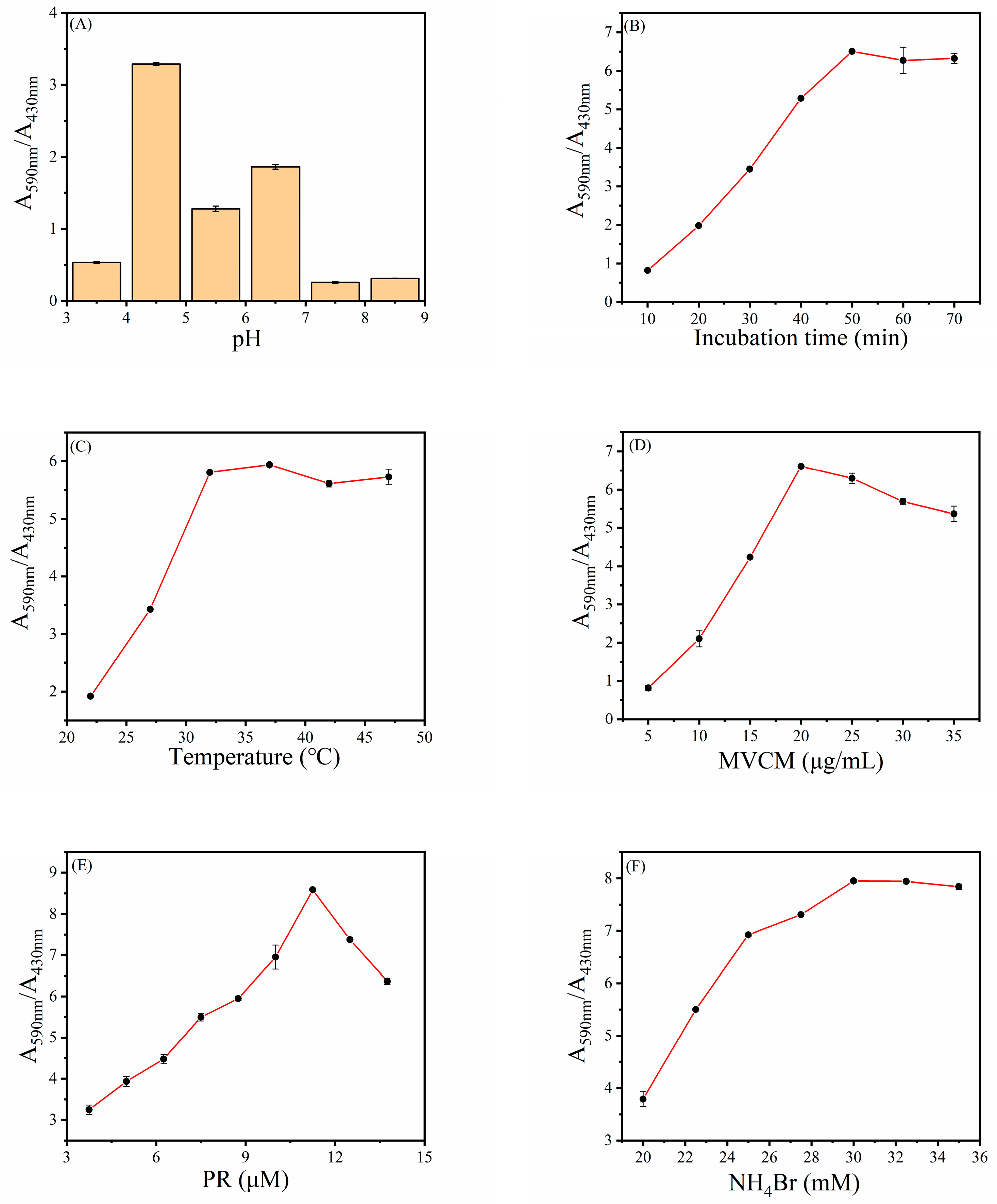
3.4. Optimal Conditions for H2O2 Detection
3.5. Steady-State Kinetics Analysis
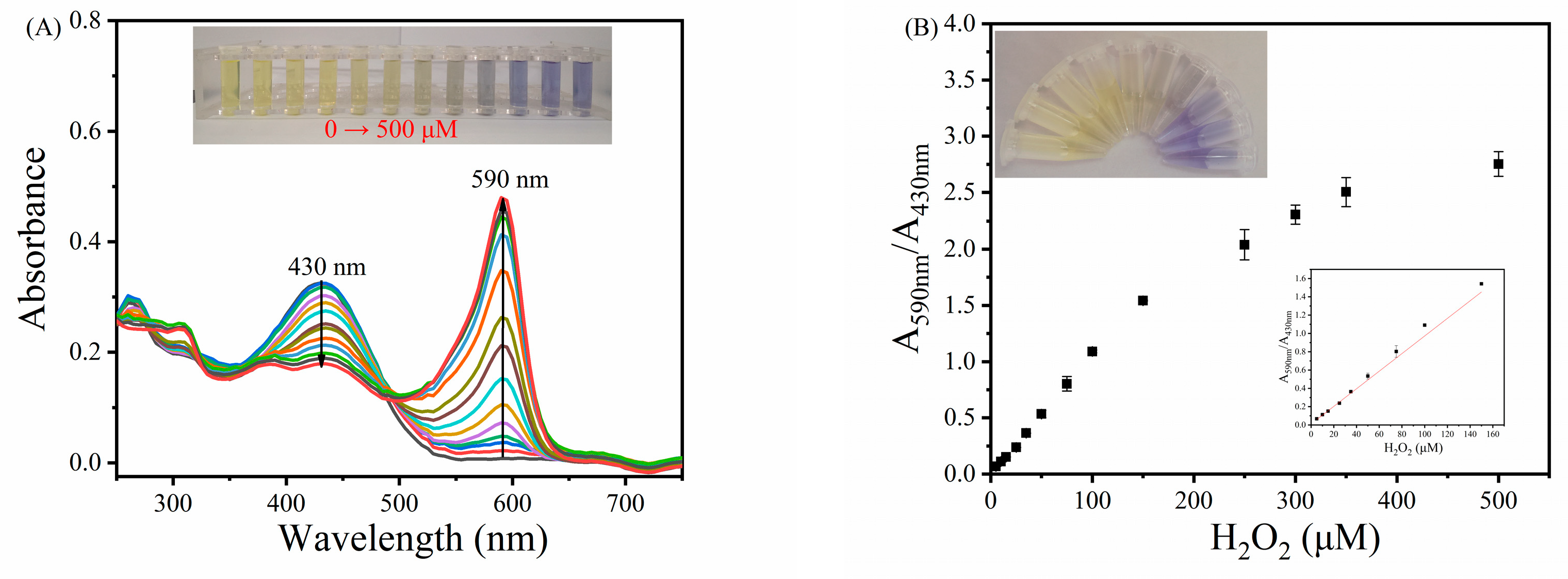
3.6. Ratiometric Colorimetric Sensing of H2O2
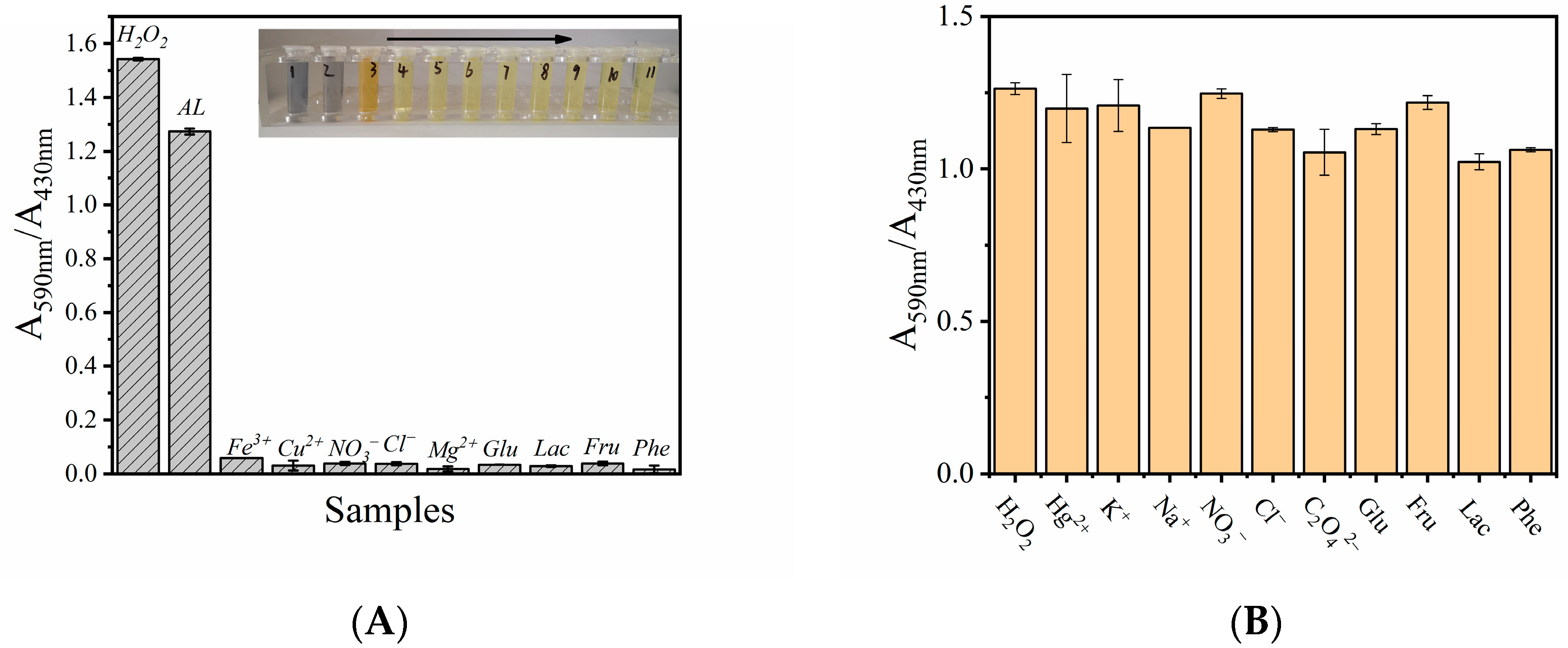
3.7. Selectivity and Applicability of MVCM-Based H2O2 Detection System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Luo, P.; Yang, H.; Pan, S.; Liu, H.; Hu, X. Regulating the enzymatic activities of metal-ATP nanoparticles by metal doping and their application for H2O2 detection. Sens. Actuators B Chem. 2021, 335, 129671. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Luo, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Li, Y.; Yu, C.; Yang, B.; Liu, Z.; Xia, P.; Wang, P. Target-catalyzed hairpin assembly and metal-organic frameworks mediated nonenzymatic co-reaction for multiple signal amplification detection of miR-122 in human serum. Biosens. Bioelectron. 2018, 102, 307–315. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, S.; Cui, X.; Wang, X.; Yang, L. Enzyme-like catalysis of polyoxometalates for chemiluminescence: Application in ultrasensitive detection of H2O2 and blood glucose. Talanta 2019, 205, 120139. [Google Scholar] [CrossRef]
- Ma, X.; Wen, S.; Xue, X.; Guo, Y.; Jin, J.; Song, W.; Zhao, B. Controllable Synthesis of SERS-Active Magnetic Metal-Organic Framework-Based Nanocatalysts and Their Application in Photoinduced Enhanced Catalytic Oxidation. ACS Appl. Mater. Interfaces 2018, 10, 25726–25736. [Google Scholar] [CrossRef]
- Castro, R.; Soares, J.; Ribeiro, D.; Santos, J. Dual-emission ratiometric probe combining carbon dots and CdTe quantum dots for fluorometric and visual determination of H2O2. Sens. Actuators B Chem. 2019, 296, 126665. [Google Scholar] [CrossRef]
- Sun, H.; Liu, X.; Wang, X.; Han, Q.; Qi, C.; Li, Y.; Wang, C.; Chen, Y.; Yang, R. Colorimetric determination of ascorbic acid using a polyallylamine-stabilized IrO2/graphene oxide nanozyme as a peroxidase mimic. Microchim. Acta 2020, 187, 2–9. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.; Yang, B.; Liu, Z.; Zhang, X.; Liu, Q. Iron doped CuSn(OH)6 microspheres as a peroxidase-mimicking artificial enzyme for H2O2 colorimetric detection. ACS Sustain. Chem. Eng. 2018, 6, 14383–14393. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, R.; Zeng, H.; Wang, X.; Luo, S.; Li, W.; Luo, X.; Yang, T. Nucleic acid-based ratiometric electrochemiluminescent, electrochemical and photoelectrochemical biosensors: A review. Microchim. Acta 2019, 186, 405. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Peng, H.; Arabi, M.; Li, J.; Xiong, H.; Choo, J.; Chen, L. Ratiometric fluorescence and colorimetry dual-mode assay based on manganese dioxide nanosheets for visual detection of alkaline phosphatase activity. Sens. Actuators B Chem. 2020, 302, 127176. [Google Scholar] [CrossRef]
- Ye, M.; Lin, B.; Yu, Y.; Li, H.; Wang, Y.; Zhang, L.; Cao, Y.; Guo, M. A ratiometric fluorescence probe based on graphene quantum dots and o-phenylenediamine for highly sensitive detection of acetylcholinesterase activity. Microchim. Acta 2020, 187, 511. [Google Scholar] [CrossRef]
- Su, D.; Wang, M.; Liu, Q.; Chen, J.; Su, X. Dual-emission ratio fluorescence detection of Bleomycin based on nitrogen doped graphene quantum dots@gold nanoclusters assembly. Sens. Actuators B Chem. 2019, 290, 163–169. [Google Scholar] [CrossRef]
- Zhan, T.; Kang, J.; Li, X.; Pan, L.; Li, G.; Hou, W. NiFe layered double hydroxide nanosheets as an efficiently mimic Enzyme for colorimetric determination of glucose and H2O2. Sens. Actuators B Chem. 2018, 255, 2635–2642. [Google Scholar] [CrossRef]
- Zhao, Q.; Zheng, X.; Xing, L.; Tang, Y.; Zhou, X.; Hu, L.; Yao, W.; Yan, Z. 2D Co3O4 stabilizing Rh nano composites developed for visual sensing bioactive urea and toxic p-aminophenol in practice by synergetic-reinforcing oxidase activity. J. Hazard. Mater. 2021, 409, 125019. [Google Scholar] [CrossRef] [PubMed]
- Sandy, M.; Carter-Frankin, J.; Martin, J.; Butler, A. Vanadiumbromoperoxidase from Delisea pulchra: Enzyme-catalyzed formation of bromofuranone and attendant disruption of quorum sensing. Chem. Commun. 2011, 47, 12086–12088. [Google Scholar] [CrossRef]
- Frerichs, H.; Pütz, E.; Pfitzner, F.; Reich, T.; Gazanis, A.; Panthöfer, M.; Jegel, O.; Heermann, R.; Tremel, W. Nanocomposite antimicrobials prevent bacterial growth through the enzyme-like activity of Bi-doped cerium dioxide (Ce1-xBixO2-δ). Nanoscale 2020, 12, 21344–21358. [Google Scholar] [CrossRef]
- Herget, K.; Hubach, P.; Pusch, S.; Deglmann, P.; Götz, H.; Gorelik, T.; Gural’skiy Il’ya, A.; Pfitzner, F.; Link, T.; Schenk, S.; et al. Haloperoxidase mimicry by CeO2−x nanorods combats biofouling. Adv. Mater. 2017, 29, 1603823. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, R.; Yan, X.; Fang, K. Structure and activity of nanozymes: Inspirations for de novo design of nanozymes. Mater. Today 2020, 41, 81–119. [Google Scholar] [CrossRef]
- Wang, D.; Jana, D.; Zhao, Y. Metal-organic framework derived nanozymes in biomedicine. Acc. Chem. Res. 2020, 53, 1389–1400. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.; Roberts, J.; Scheidt, K.; Nguyen, S.; Hupp, J. Metal-Organic Framework Materials as Catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Wu, D.; Li, X.; Hu, N.; Chen, J.; Chen, G.; Wu, Y. Recent progress in the design fabrication of metal-organic frameworks-based nanozymes and their applications to sensing and cancer therapy. Biosens. Bioelectron. 2019, 137, 178–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, L.; Liu, D.; Ma, W.; Dramou, P.; He, H. Nanozymes based on metal-organic frameworks: Construction and prospects. Trends Anal. Chem. 2020, 133, 116080. [Google Scholar] [CrossRef]
- Ai, L.; Li, L.; Zhang, C.; Fu, J.; Jiang, J. MIL-53(Fe): A metal-organic framework with intrinsic peroxidase-like catalytic activity for colorimetric biosensing. Chem. Eur. J. 2013, 19, 15105–15108. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Chen, S.; Ye, F.; Su, L.; Zhang, C.; Shen, S.; Zhao, S. Synthesis of a mixed valence state Ce-MOF as an oxidase mimetic for the colorimetric detection of biothiols. Chem. Commun. 2015, 51, 4635–4638. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Maji, S.; Srivastava, D.; Mondal, A.; Biswas, P.; Paul, P.; Adhikary, B. Synthesis of FeS and FeSe nanoparticles from a single source precursor: A study of their photocatalytic activity, peroxidase-like behavior and electrochemical sensing of H2O2. ACS Appl. Mater. Interfaces 2012, 4, 1919–1927. [Google Scholar] [CrossRef]
- Wang, Z.; Ju, P.; Zhang, Y.; Jiang, F.; Ding, H.; Sun, C. CoMoO4 nanobelts as efficient peroxidase mimics for the colorimetric determination of H2O2. Microchim. Acta 2020, 187, 424. [Google Scholar] [CrossRef]
- Liu, K.; You, H.; Jia, G.; Zheng, Y.; Huang, Y.; Song, Y.; Yang, M.; Zhang, L.; Zhang, H. Hierarchically Nanostructured Coordination Polymer: Facile and Rapid Fabrication and Tunable Morphologies. Cryst. Growth Des. 2010, 10, 790–797. [Google Scholar] [CrossRef]
- Liu, K.; You, H.; Jia, G.; Zheng, Y.; Huang, Y.; Song, Y.; Yang, M.; Zhang, L.; Zhang, H. Hierarchically framework Ce-BTC derivative containing high specific surface area for improving the catalytic activity of CO oxidation reaction. Microporous Mesoporous Mater. 2018, 259, 211–219. [Google Scholar]
- Kouzegaran, V.; Farhadi, K.; Forough, M.; Bahram, M.; Çetinkol, Ö. Highly-sensitive and fast detection of human telomeric G-quadruplex DNA based on a hemin-conjugated fluorescent metal-organic framework platform. Biosens. Bioelectron. 2021, 178, 112999. [Google Scholar] [CrossRef]
- Deshpande, S.; Patil, S.; VNT Kuchibhatla, S.; Seal, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113. [Google Scholar] [CrossRef]
- Wang, L.; Meng, F.; Li, K.; Lu, F. Characterization and optical properties of pole-like nano-CeO2 synthesized by a facile hydrothermal method. Appl. Surf. Sci. 2013, 286, 269–274. [Google Scholar] [CrossRef]


| Material | Substrates | Km (M) | Vmax (M·s−1) |
|---|---|---|---|
| MVCM | H2O2 | 1.0 × 10−4 | 4 × 10−9 |
| Br− | 0.22 | 1.56 × 10−9 |
| Sample | Original (μM) | Added (μM) | Found (μM) | Recovery (%) | RSD (%) (n = 3) |
|---|---|---|---|---|---|
| tap water | N.D. | 9.0 | 8.28 | 92.00 | 2.15 |
| N.D. | 80.0 | 80.94 | 101.17 | 2.93 | |
| N.D. | 120.0 | 115.20 | 96.00 | 1.30 | |
| N.D. | 125.0 | 130.60 | 104.40 | 0.35 | |
| milk | N.D. | 9.0 | 8.76 | 97.33 | 2.53 |
| N.D. | 80.0 | 80.34 | 100.43 | 1.26 | |
| N.D. | 125.0 | 118.86 | 95.09 | 1.15 | |
| contact lens solution | N.D. | 9.0 | 8.75 | 97.22 | 1.95 |
| N.D. | 80.0 | 81.07 | 101.33 | 2.51 | |
| N.D. | 125.0 | 127.90 | 102.32 | 2.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Liang, L.; Ye, F.; Zhao, S. Ce-MOF with Intrinsic Haloperoxidase-Like Activity for Ratiometric Colorimetric Detection of Hydrogen Peroxide. Biosensors 2021, 11, 204. https://doi.org/10.3390/bios11070204
Cheng Y, Liang L, Ye F, Zhao S. Ce-MOF with Intrinsic Haloperoxidase-Like Activity for Ratiometric Colorimetric Detection of Hydrogen Peroxide. Biosensors. 2021; 11(7):204. https://doi.org/10.3390/bios11070204
Chicago/Turabian StyleCheng, Yanyan, Ling Liang, Fanggui Ye, and Shulin Zhao. 2021. "Ce-MOF with Intrinsic Haloperoxidase-Like Activity for Ratiometric Colorimetric Detection of Hydrogen Peroxide" Biosensors 11, no. 7: 204. https://doi.org/10.3390/bios11070204
APA StyleCheng, Y., Liang, L., Ye, F., & Zhao, S. (2021). Ce-MOF with Intrinsic Haloperoxidase-Like Activity for Ratiometric Colorimetric Detection of Hydrogen Peroxide. Biosensors, 11(7), 204. https://doi.org/10.3390/bios11070204