Enabling Continuous Wearable Reflectance Pulse Oximetry at the Sternum
Abstract
:1. Introduction
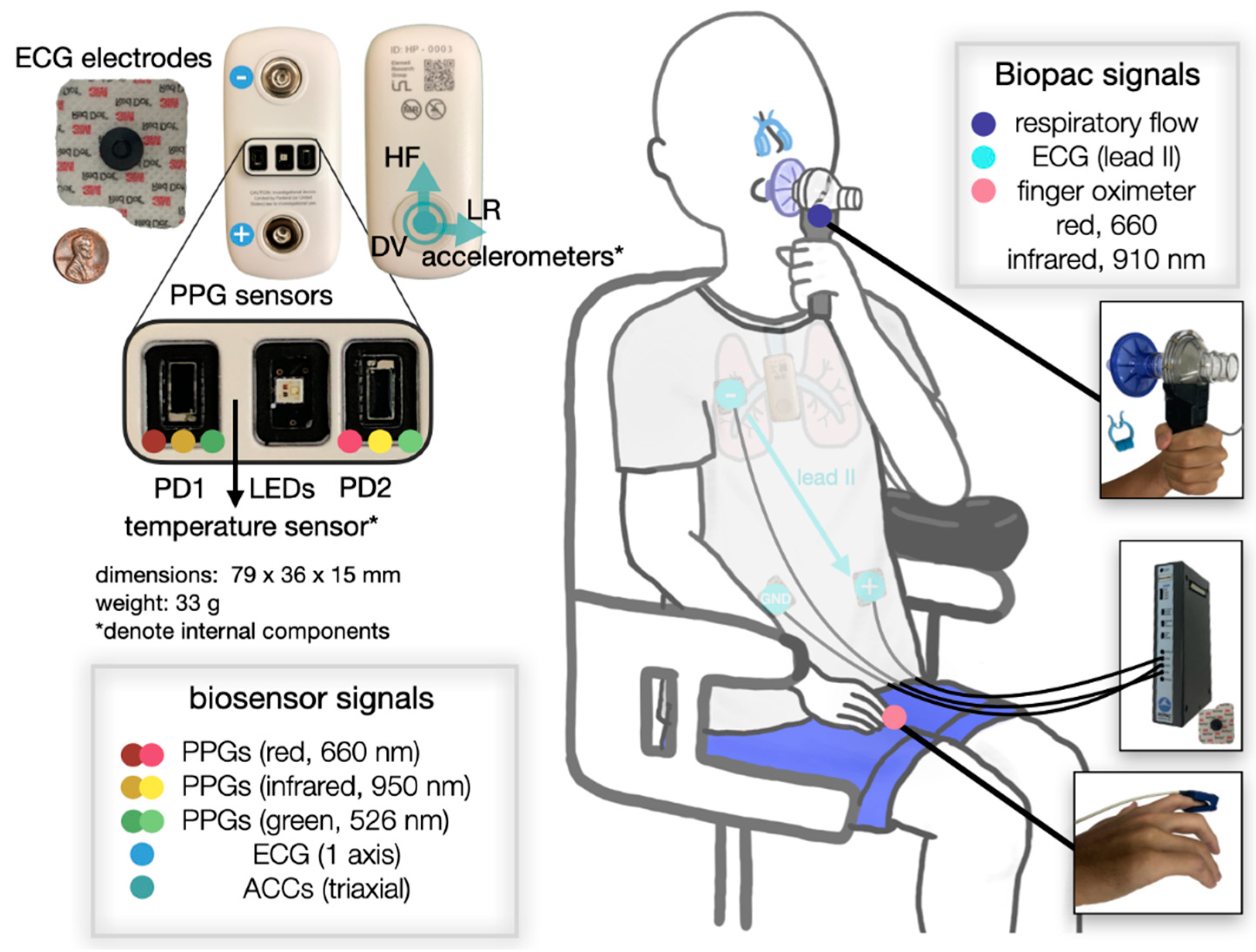
2. Materials and Methods
2.1. Principle of PPG
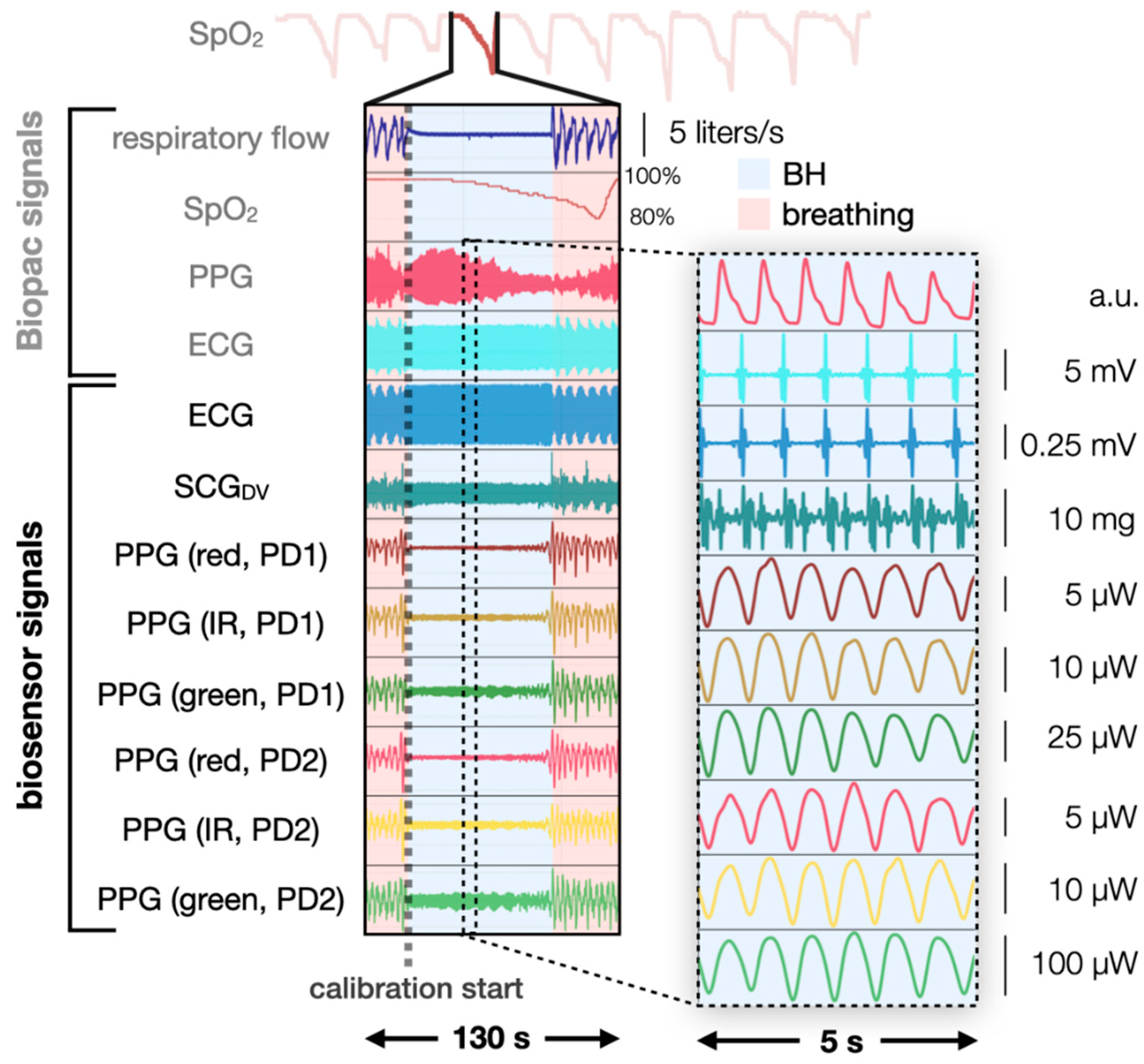
2.2. Breath-Hold Study Design
2.3. Manual Labeling
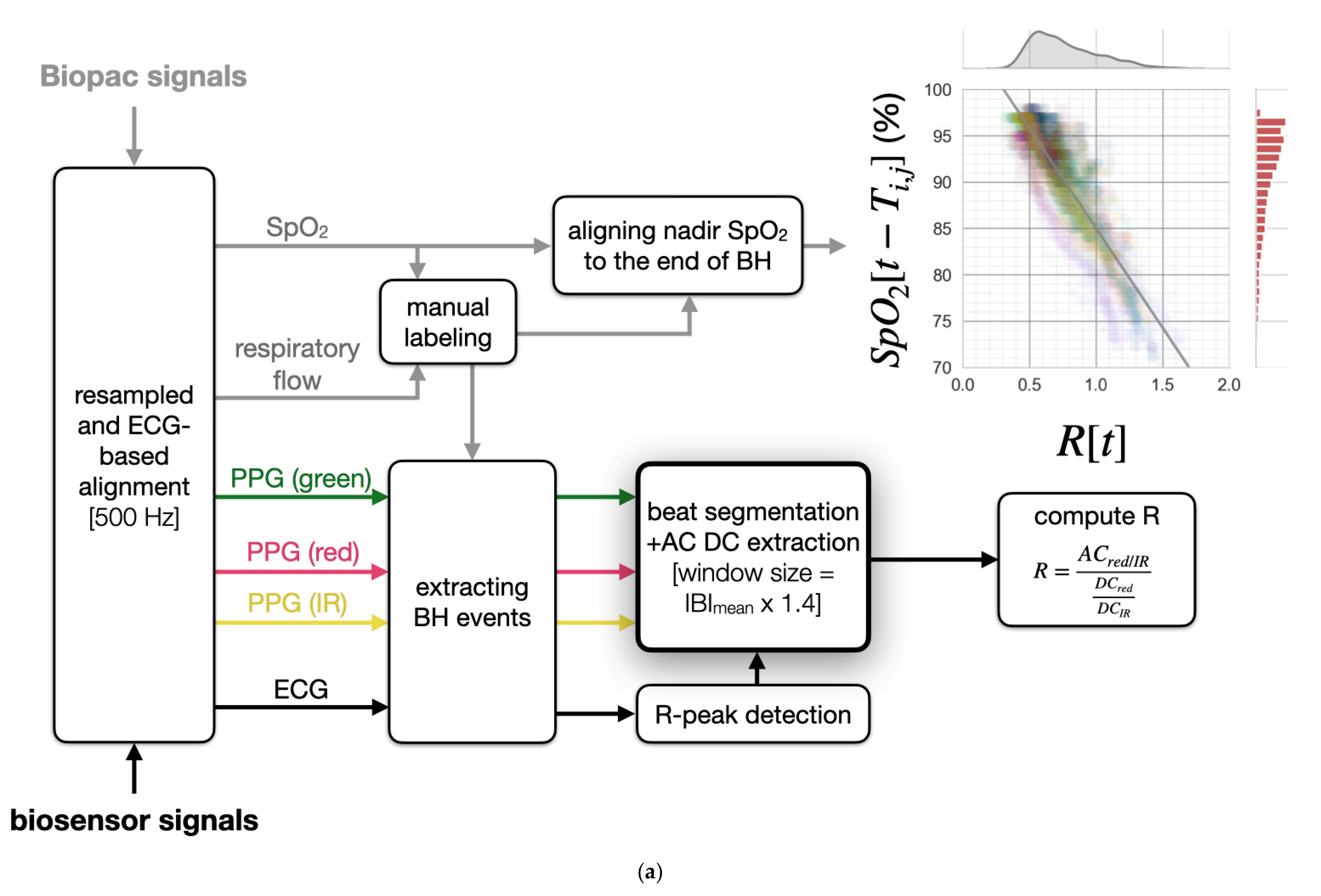
2.4. Signal Processing Pipeline
2.4.1. Principle of Pulse Oximetry
2.4.2. Preprocessing Overview
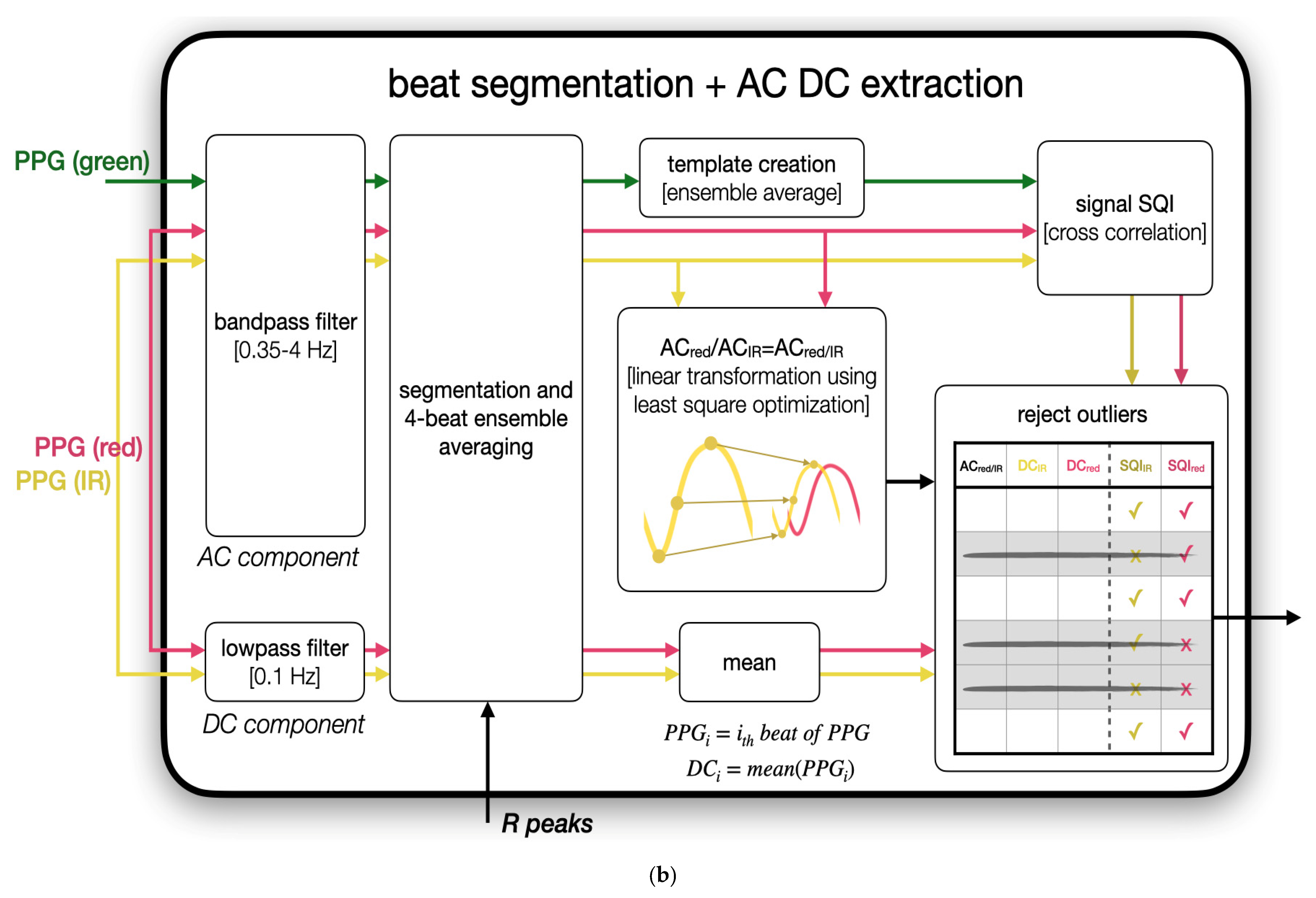
2.4.3. Robust Feature Extraction via Linear Transformation
2.4.4. PPGgreen-Based Outlier Rejection
2.4.5. Computation of R
2.5. SpO2 Estimation
2.5.1. Linear Regression
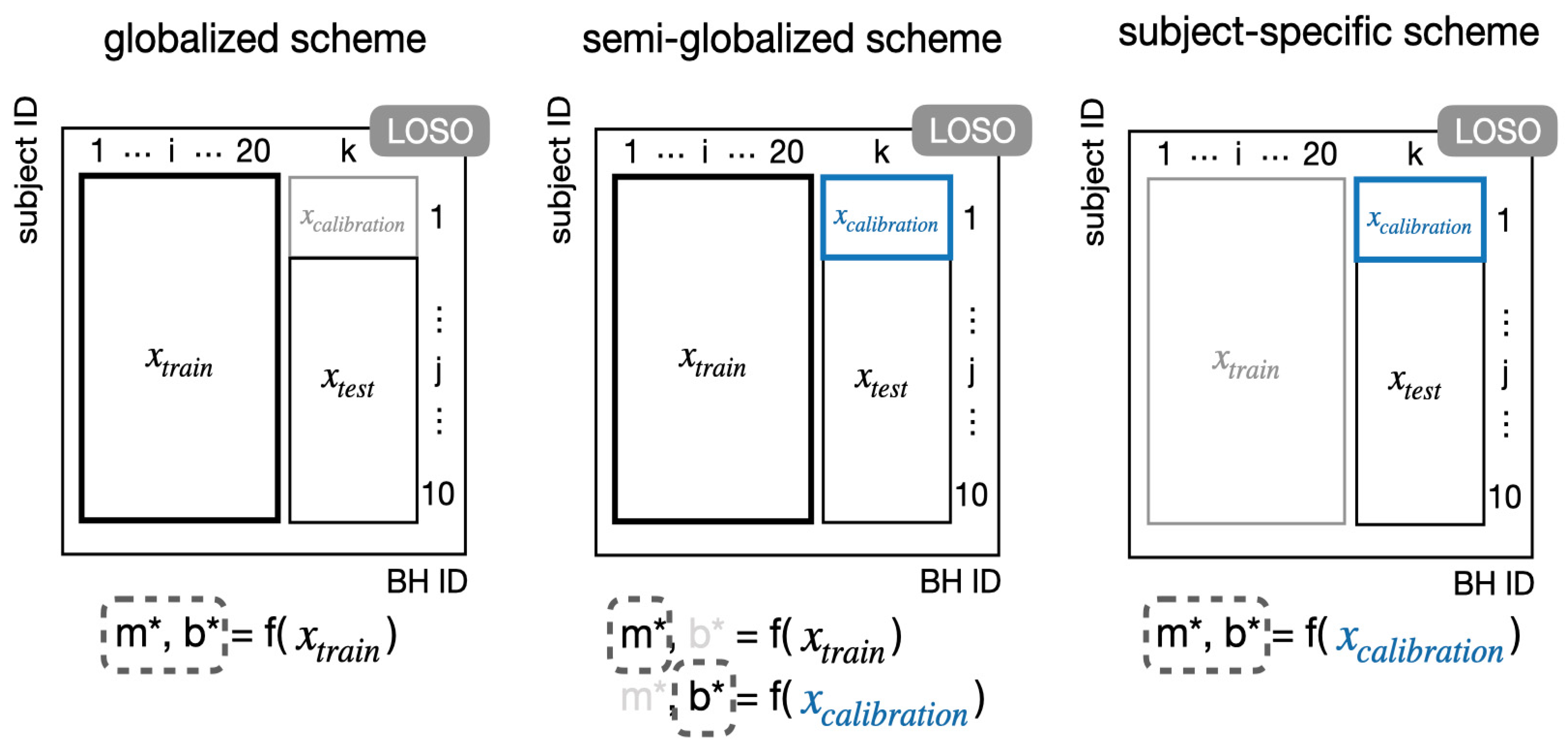
2.5.2. Training and Calibration Schemes
2.5.3. Evaluating Model Performance
3. Results
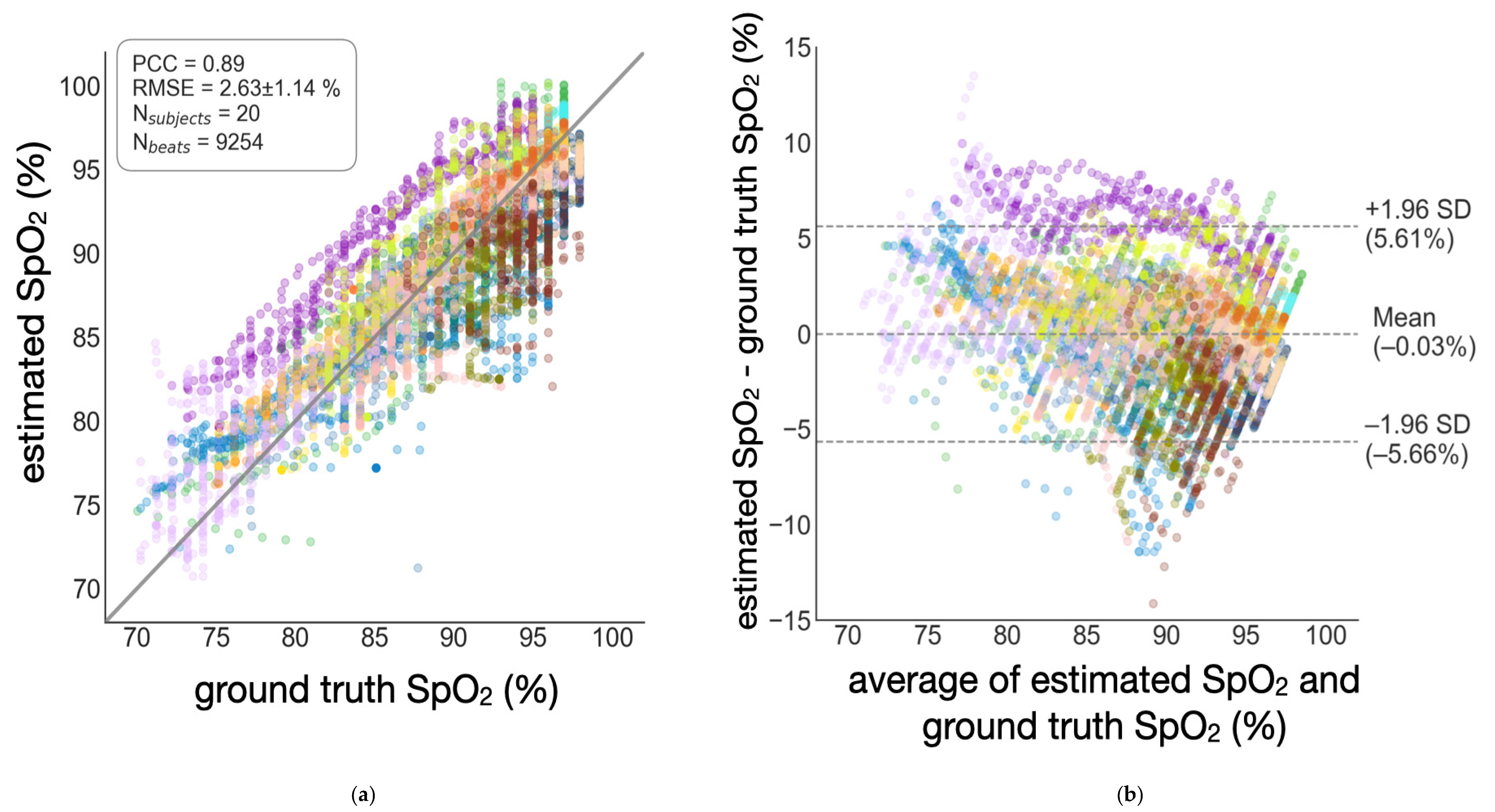
3.1. Accuracy of SpO2 Estimation
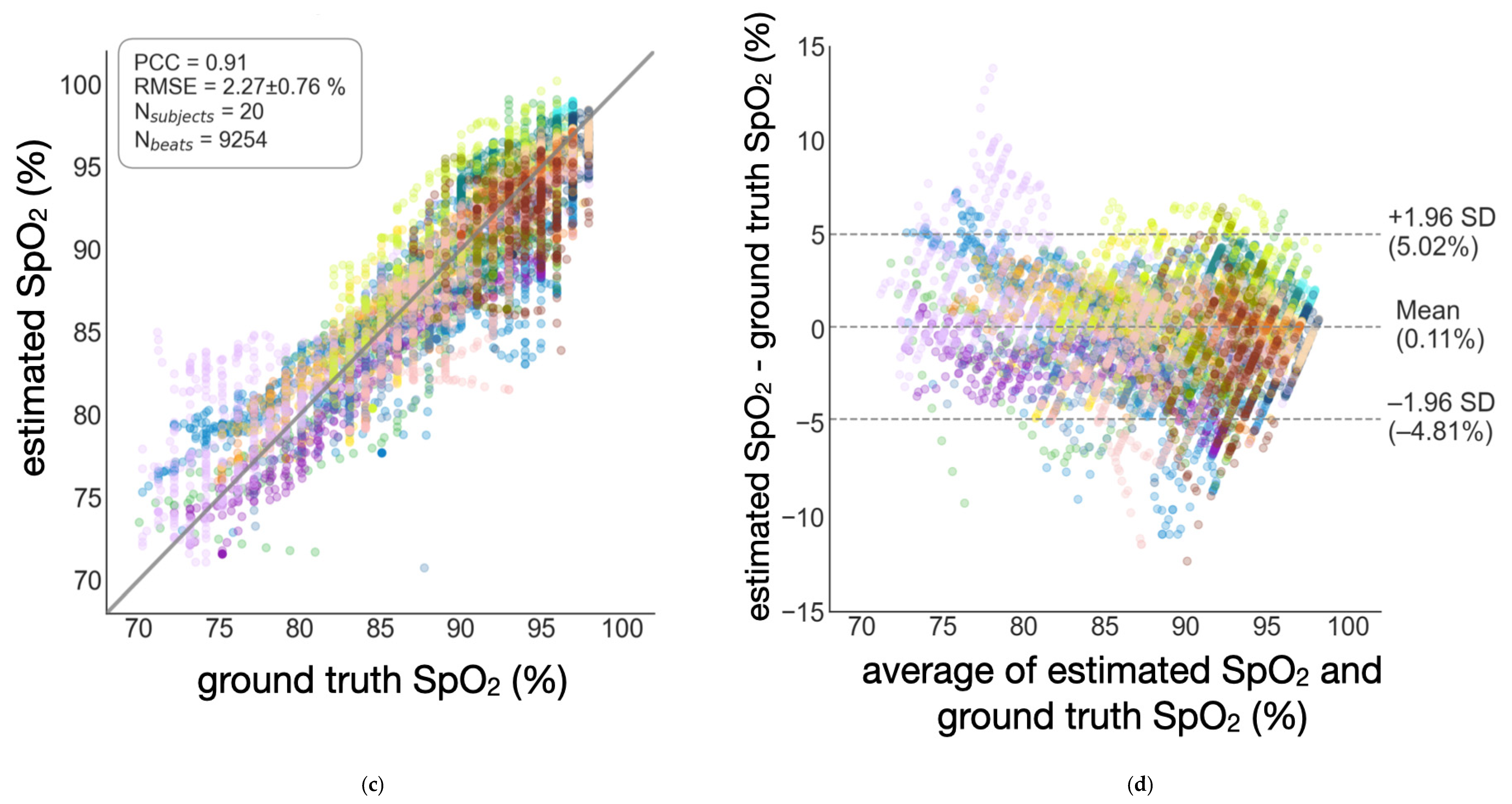
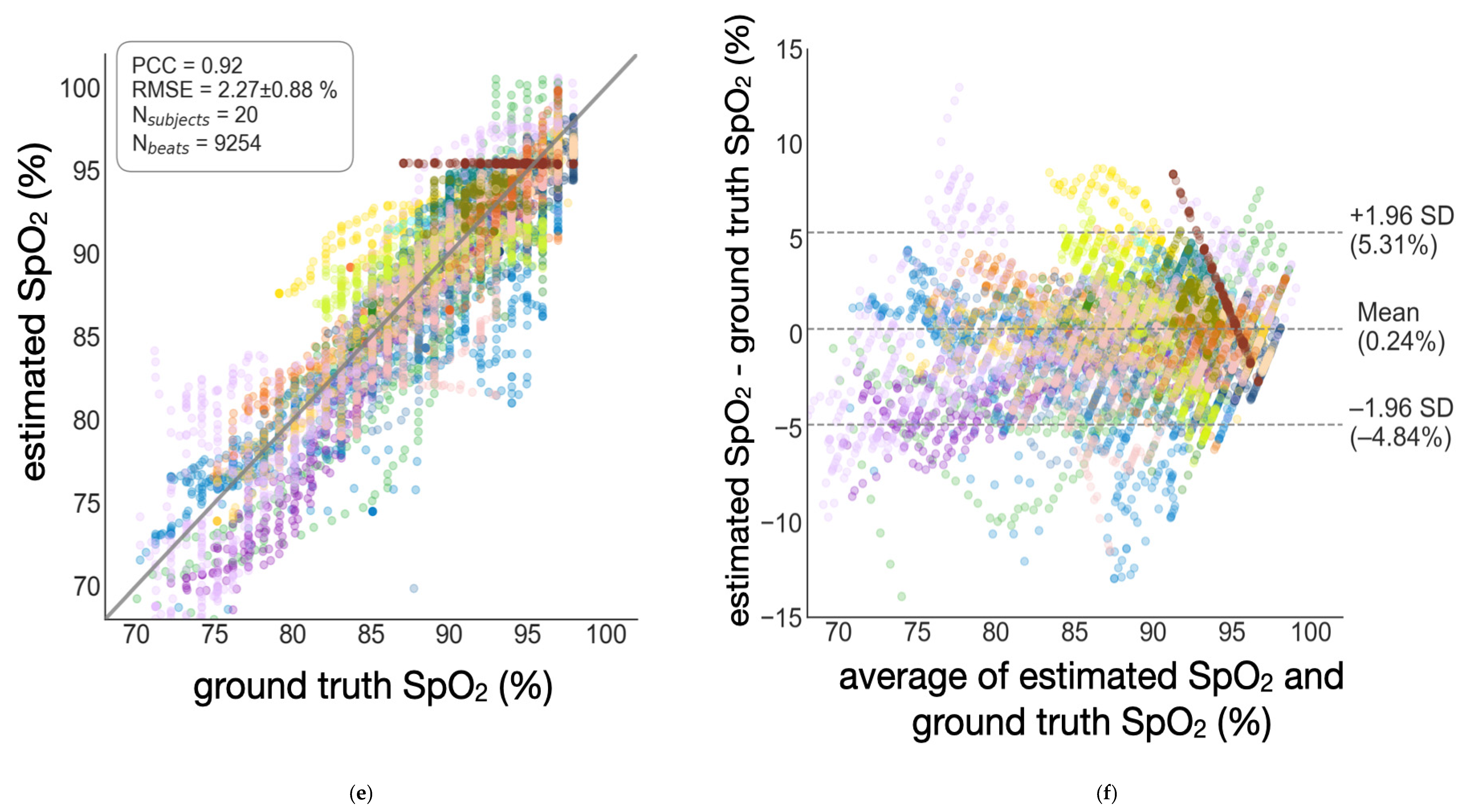
3.2. Semi-Globalized Scheme vs. Subject-Specific Scheme
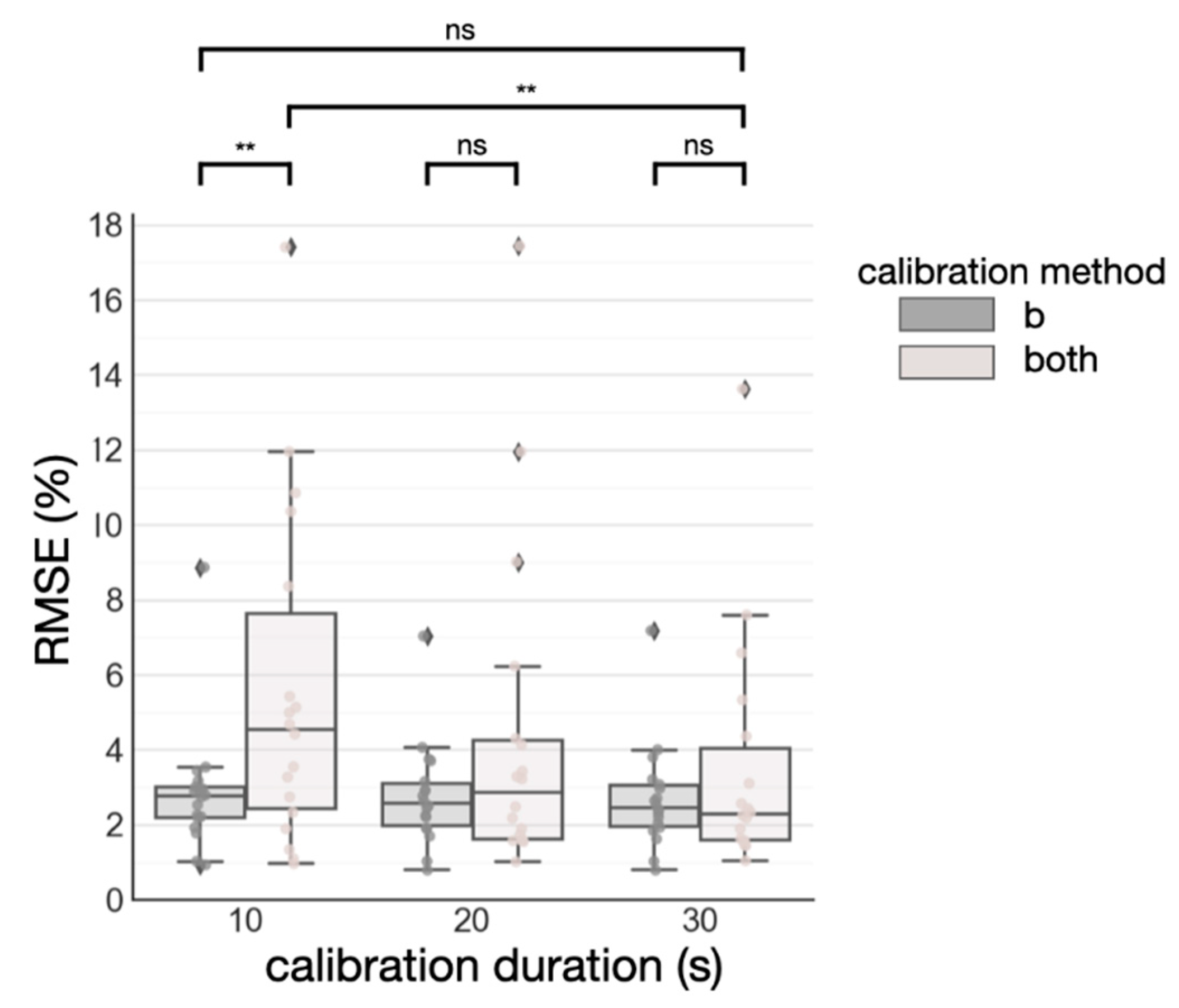
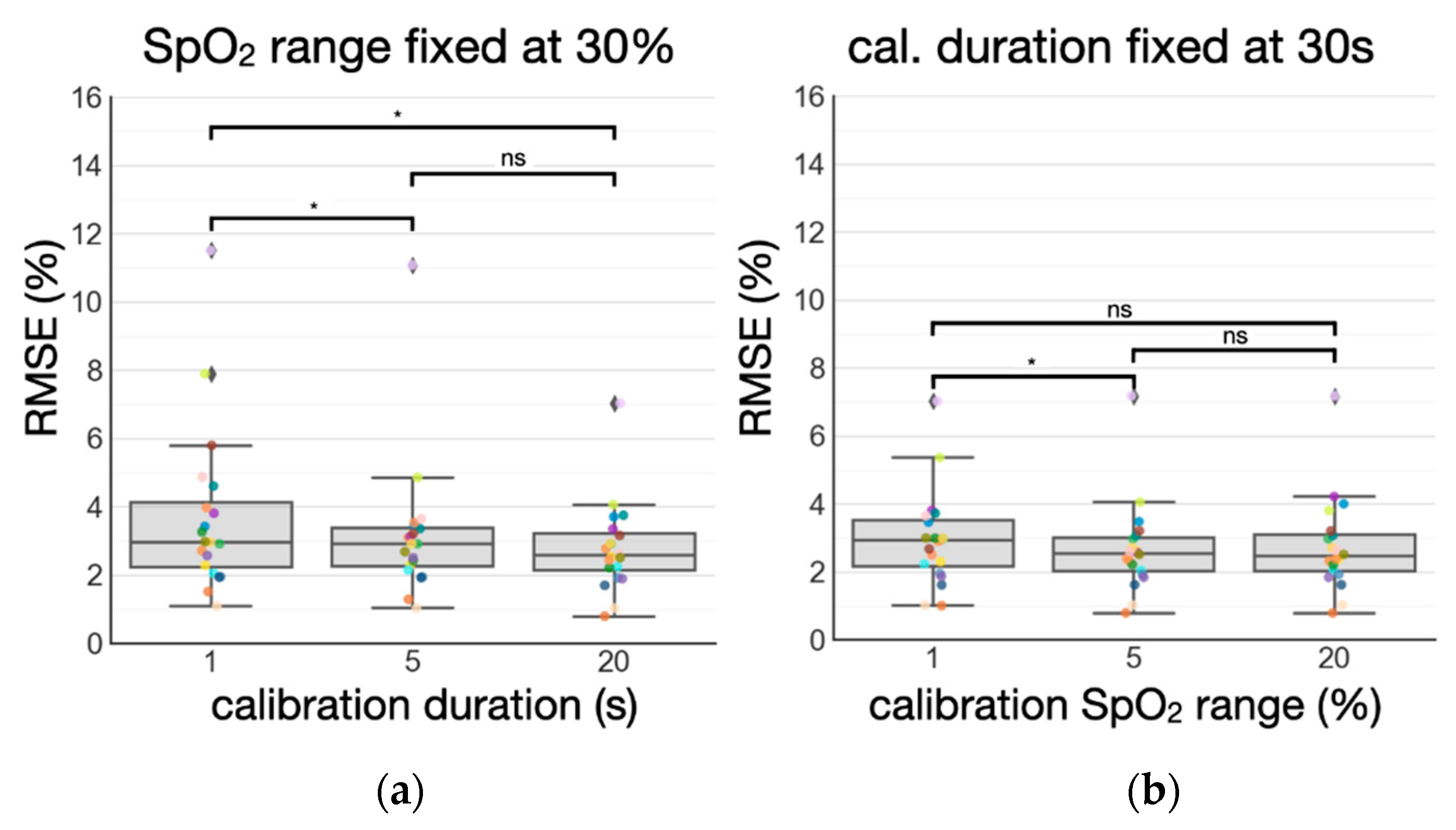
3.3. Standardizing Subject-Specific Calibration: Duration vs. SpO2 Range
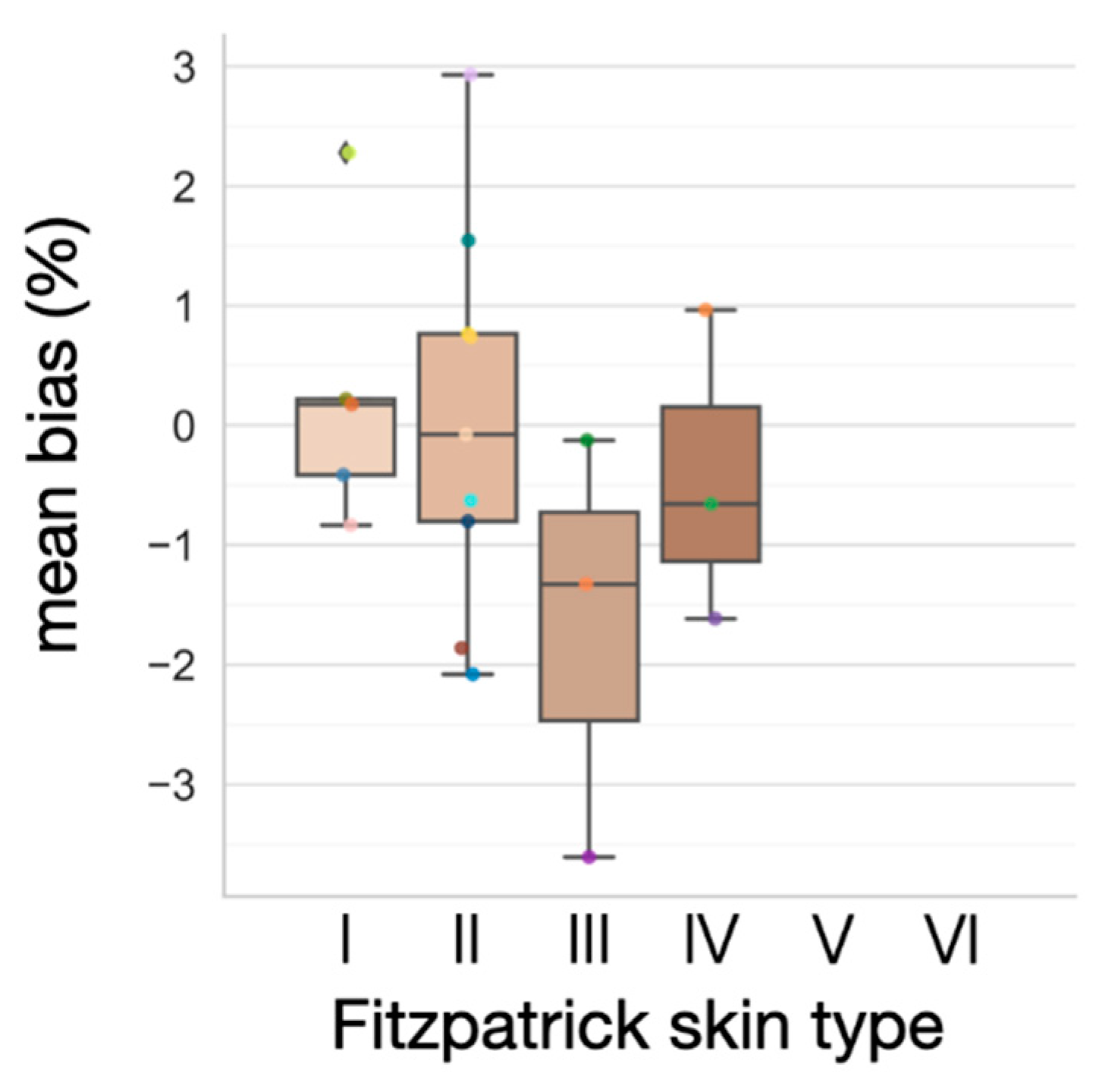
3.4. Effect of Varying Melanin Content
4. Discussion
4.1. Accurate SpO2 Estimation
4.2. Standardization of Subject-Specific Calibration
4.3. Practical Use Case
4.4. Limitations
4.5. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Pimentel, M.A.F.; Redfern, O.C.; Hatch, R.; Young, J.D.; Tarassenko, L.; Watkinson, P.J. Trajectories of Vital Signs in Patients with COVID-19. Resuscitation 2020, 156, 99–106. [Google Scholar] [CrossRef]
- Shah, S.; Majmudar, K.; Stein, A.; Gupta, N.; Suppes, S.; Karamanis, M.; Capannari, J.; Sethi, S.; Patte, C. Novel Use of Home Pulse Oximetry Monitoring in COVID-19 Patients Discharged from the Emergency Department Identifies Need for Hospitalization. Acad. Emerg. Med. 2020, 27, 681–692. [Google Scholar] [CrossRef]
- Longmore, S.K.; Lui, G.Y.; Naik, G.; Breen, P.P.; Jalaludin, B.; Gargiulo, G.D. A Comparison of Reflective Photoplethysmography for Detection of Heart Rate, Blood Oxygen Saturation, and Respiration Rate at Various Anatomical Locations. Sensors 2019, 19, 1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, H.J.; Williams, I.; Peters, N.S.; Mandic, D.P. In-Ear SpO2: A Tool for Wearable, Unobtrusive Monitoring of Core Blood Oxygen Saturation. Sensors 2020, 20, 4879. [Google Scholar] [CrossRef] [PubMed]
- Sugino, S.; Kanaya, N.; Mizuuchi, M.; Nakayama, M.; Namiki, A. Forehead Is as Sensitive as Finger Pulse Oximetry during General Anesthesia. Can. J. Anesth. 2004, 51, 432–436. [Google Scholar] [CrossRef] [Green Version]
- Bouten, J.; Bourgois, J.G.; Boone, J. Hold Your Breath: Peripheral and Cerebral Oxygenation during Dry Static Apnea. Eur. J. Appl. Physiol. 2020, 120, 2213–2222. [Google Scholar] [CrossRef]
- Inan, O.T.; Pouyan, M.B.; Javaid, A.Q.; Dowling, S.; Etemadi, M.; Dorier, A.; Heller, J.A.; Bicen, A.O.; Roy, S.; De Marco, T.; et al. Novel Wearable Seismocardiography and Machine Learning Algorithms Can Assess Clinical Status of Heart Failure Patients. Circ.-Heart. Fail. 2018, 11, e004313. [Google Scholar] [CrossRef]
- Semiz, B.; Carek, A.M.; Johnson, J.C.; Ahmad, S.; Heller, J.A.; Garcia-Vicente, F.G.; Caron, S.; Hogue, C.W.; Etemadi, M.; Inan, O. Non-Invasive Wearable Patch Utilizing Seismocardiography for Peri-Operative Use in Surgical Patients. IEEE J. Biomed. Health Inform. 2021, 25, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, V.B.; Nagesh, S.; Shandhi, M.M.H.; Fan, J.; Klein, L.; Etemadi, M.; Heller, J.A.; Inan, O.T.; Rehg, J.M. Classification of Decompensated Heart Failure From Clinical and Home Ballistocardiography. IEEE Trans. Bio-Med. Eng. 2020, 67, 1303–1313. [Google Scholar] [CrossRef]
- Shandhi, M.M.H.; Bartlett, W.H.; Heller, J.A.; Etemadi, M.; Young, A.; Plötz, T.; Inan, O.T. Estimation of Instantaneous Oxygen Uptake During Exercise and Daily Activities Using a Wearable Cardio-Electromechanical and Environmental Sensor. IEEE J. Biomed. Health Inform. 2021, 25, 634–646. [Google Scholar] [CrossRef]
- Schreiner, C.; Catherwood, P.; Anderson, J.; McLaughlin, J. Blood Oxygen Level Measurement with a Chest-Based Pulse Oximetry Prototype System. In Proceedings of the Computing in Cardiology, Belfast, UK, 26–29 September 2010; pp. 537–540. [Google Scholar]
- AKiruthiga, A.; Alex, A.; Balamugesh, T.; Prabhu, D.; Christopher, D.J.; Preejith, S.P.; Joseph, J.; Sivaprakasam, M. Reflectance Pulse Oximetry for Blood Oxygen Saturation Measurement from Diverse Locations-A Preliminary Analysis. In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018; pp. 1–6. [Google Scholar]
- Kramer, M.; Lobbestael, A.; Barten, E.; Eian, J.; Rausch, G. Wearable Pulse Oximetry Measurements on the Torso, Arms, and Legs: A Proof of Concept. Mil. Med. 2017, 182, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Vetter, R.; Rossini, L.; Ridolfi, A.; Sola, J.; Chetelat, O.; Correvon, M.; Krauss, J. Frequency Domain SpO2 Estimation Based on Multichannel Photoplethysmographic Measurements at the Sternum. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Munich, Germany, 7–12 September 2009; pp. 326–329, ISBN 978-3-642-03881-5. [Google Scholar]
- Näslund, E.; Lindberg, L.-G.; Lund, I.; Näslund-Koch, L.; Larsson, A.; Frithiof, R. Measuring Arterial Oxygen Saturation from an Intraosseous Photoplethysmographic Signal Derived from the Sternum. J. Clin. Monit. Comput. 2020, 34, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Pulse Oximeters—Premarket Notification Submissions [510(k)s] Guidance for Industry and Food and Drug Administration Staff. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pulse-oximeters-premarket-notification-submissions-510ks-guidance-industry-and-food-and-drug (accessed on 12 December 2021).
- King, P.H. Design Of Pulse Oximeters. IEEE Eng. Med. Biol. 1998, 17, 117. [Google Scholar] [CrossRef]
- Arnold, M. The Surgical Anatomy of Sternal Blood Supply. J. Thorac. Cardiovasc. Surg. 1972, 64, 596–610. [Google Scholar] [CrossRef]
- Andersson, J.; Schagatay, E. Effects of Lung Volume and Involuntary Breathing Movements on the Human Diving Response. Eur. J. Appl. Physiol. 1997, 77, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Reisner, A.; Shaltis, P.A.; McCombie, D.; Asada, H.H.; Warner, D.S.; Warner, M.A. Utility of the Photoplethysmogram in Circulatory Monitoring. Anesthesiology 2008, 108, 950–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayarangan, S.; Suresh, P.; SP, P.; Joseph, J.; Sivaprakasam, M. Proceedings of Robust Modelling of Reflectance Pulse Oximetry for SpO2 Estimation. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 374–377. [Google Scholar]
- Priem, G.; Martinez, C.; Bodinier, Q.; Carrault, G. Clinical Grade SpO2 Prediction through Semi-Supervised Learning. In Proceedings of the 2020 IEEE 20th International Conference on Bioinformatics and Bioengineering (BIBE), Cincinnati, OH, USA, 26–28 October 2020; pp. 914–921. [Google Scholar]
- Ganti, V.; Carek, A.M.; Jung, H.; Srivatsa, A.V.; Cherry, D.; Johnson, L.N.; Inan, O.T. Enabling Wearable Pulse Transit Time-Based Blood Pressure Estimation for Medically Underserved Areas and Health Equity: Comprehensive Evaluation Study. JMIR mHealth uHealth 2021, 9, e27466. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.M.; Nicolson, S.C.; Steven, J.M.; Escobar, A.; McGonigle, M.E.; Jobes, D.R. Influence of Sensor Site Location on Pulse Oximetry Kinetics in Children. Anesth. Analg. 1993, 76, 751–754. [Google Scholar] [CrossRef]
- Pan, J.; Tompkins, W.J. A Real-Time QRS Detection Algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar] [CrossRef]
- Tanveejul, A.P.M.; Alex, A.; Preejith, S.P.; Christopher, D.J.; Chandy, S.; Joseph, J.; Sivaprakasam, M. A Study on the Subject and Location Specificity in Reflectance Based SpO2 Estimation Using R-Value Based Calibration Curve. In Proceedings of the 2020 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Bari, Italy, 1 June–1 July 2020; pp. 1–6. [Google Scholar]
- Leys, C.; Ley, C.; Klein, O.; Bernard, P.; Licata, L. Detecting Outliers: Do Not Use Standard Deviation around the Mean, Use Absolute Deviation around the Median. J. Exp. Soc. Psychol. 2013, 49, 764–766. [Google Scholar] [CrossRef] [Green Version]
- Andersson, J.P.A.; Linér, M.H.; Rünow, E.; Schagatay, E.K.A. Diving Response and Arterial Oxygen Saturation during Apnea and Exercise in Breath-Hold Divers. J. Appl. Physiol. 2002, 93, 882–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, J.; Schagatay, E. Arterial Oxygen Desaturation during Apnea in Humans. Undersea Hyperb. Med. 1998, 25, 21–25. [Google Scholar] [PubMed]
- Stewart, I.B.; Bulmer, A.C.; Sharman, J.E.; Ridgway, L. Arterial Oxygen Desaturation Kinetics during Apnea. Med. Sci. Sports Exerc. 2005, 37, 1871–1876. [Google Scholar] [CrossRef]
- Matsumura, K.; Toda, S.; Kato, Y. RGB and Near-Infrared Light Reflectance/Transmittance Photoplethysmography for Measuring Heart Rate during Motion. IEEE Access 2020, 8, 80233–80242. [Google Scholar] [CrossRef]
- Krasteva, V.; Jekova, I. QRS Template Matching for Recognition of Ventricular Ectopic Beats. Ann. Biomed. Eng. 2007, 35, 2065–2076. [Google Scholar] [CrossRef]
- Ganti, V.G.; Carek, A.; Nevius, B.N.; Heller, J.; Etemadi, M.; Inan, O. Wearable Cuff-Less Blood Pressure Estimation at Home via Pulse Transit Time. IEEE J. Biomed. Health Inform. 2021, 25, 1926–1937. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B. The Validity and Practicality of Sun-Reactive Skin Types I Through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Feiner, J.R.; Severinghaus, J.W.; Bickler, P.E. Dark Skin Decreases the Accuracy of Pulse Oximeters at Low Oxygen Saturation: The Effects of Oximeter Probe Type and Gender. Anesth. Analg. 2007, 105, S18–S23. [Google Scholar] [CrossRef] [Green Version]
- Bickler, P.E. Effects of Skin Pigmentation on Pulse Oximeter Accuracy at Low Saturation. J. Am. Soc. Anesthesiol. 2005, 102, 715–719. [Google Scholar] [CrossRef]
- Parkes, M.J. Breath-Holding and Its Breakpoint. Exp. Physiol. 2006, 91, 1–15. [Google Scholar] [CrossRef]
- Glass, K.L.; Dillard, T.A.; Phillips, Y.Y.; Torrington, K.G.; Thompson, J.C. Pulse Oximetry Correction for Smoking Exposure. Mil. Med. 1996, 161, 273–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, E.D.; Chan, M.M.; Chan, M.M. Pulse Oximetry: Understanding Its Basic Principles Facilitates Appreciation of Its Limitations. Respir. Med. 2013, 107, 789–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | Female (N = 6) | Male (N = 14) | |
|---|---|---|---|
| Demographics | Age (years) | 28.00 (2.00) | 26.15 (2.19) |
| Fitzpatrick skin type (I–VI) | 1.83 (1.17) | 2.36 (0.93) | |
| Weight (kg) | 56.35 (11.61) | 76.44 (11.97) | |
| Height (cm) | 163.03 (7.46) | 178.03 (7.09) | |
| BMI (kg/m2) | 21.02 (2.86) | 24.05 (2.84) | |
| Breath-hold response | Baseline SpO2 (%) | 96.50 (0.84) | 96.29 (1.27) |
| Nadir SpO2 (%) | 88.80 (4.81) | 80.52 (8.70) | |
| Breath-hold duration (s) | 44.07 (25.64) | 55.99 (15.52) | |
| Approximate finger SpO2 delay (s) | 24.91 (8.69) | 27.42 (12.49) | |
| Perfusion index (PI) | Red PPG (%) | 0.05 (0.03) | 0.07 (0.04) |
| Infrared PPG (%) | 0.08 (0.04) | 0.10 (0.06) | |
| Green PPG (%) | 0.56 (0.23) | 0.60 (0.40) | |
| RMSE | PCC | bias | 95% LOR | |
|---|---|---|---|---|
| Globalized | 2.63 ± 1.14% | 0.89 | −0.03% | [−5.66%, 5.61%] |
| Semi-globalized | 2.27 ± 0.76% | 0.91 | 0.11% | [−4.81%, 5.02%] |
| Subject-specific | 2.27 ± 0.88% | 0.92 | 0.24% | [−4.84%, 5.31%] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, M.; Ganti, V.G.; Heller, J.A.; Abdallah, C.A.; Etemadi, M.; Inan, O.T. Enabling Continuous Wearable Reflectance Pulse Oximetry at the Sternum. Biosensors 2021, 11, 521. https://doi.org/10.3390/bios11120521
Chan M, Ganti VG, Heller JA, Abdallah CA, Etemadi M, Inan OT. Enabling Continuous Wearable Reflectance Pulse Oximetry at the Sternum. Biosensors. 2021; 11(12):521. https://doi.org/10.3390/bios11120521
Chicago/Turabian StyleChan, Michael, Venu G. Ganti, J. Alex Heller, Calvin A. Abdallah, Mozziyar Etemadi, and Omer T. Inan. 2021. "Enabling Continuous Wearable Reflectance Pulse Oximetry at the Sternum" Biosensors 11, no. 12: 521. https://doi.org/10.3390/bios11120521
APA StyleChan, M., Ganti, V. G., Heller, J. A., Abdallah, C. A., Etemadi, M., & Inan, O. T. (2021). Enabling Continuous Wearable Reflectance Pulse Oximetry at the Sternum. Biosensors, 11(12), 521. https://doi.org/10.3390/bios11120521





