Liquid Biopsy-Based Biosensors for MRD Detection and Treatment Monitoring in Non-Small Cell Lung Cancer (NSCLC)
Abstract
:1. Introduction
2. MRD in Lung Cancer
3. Liquid Biopsy as an Alternative Technique for Tissue Biopsy
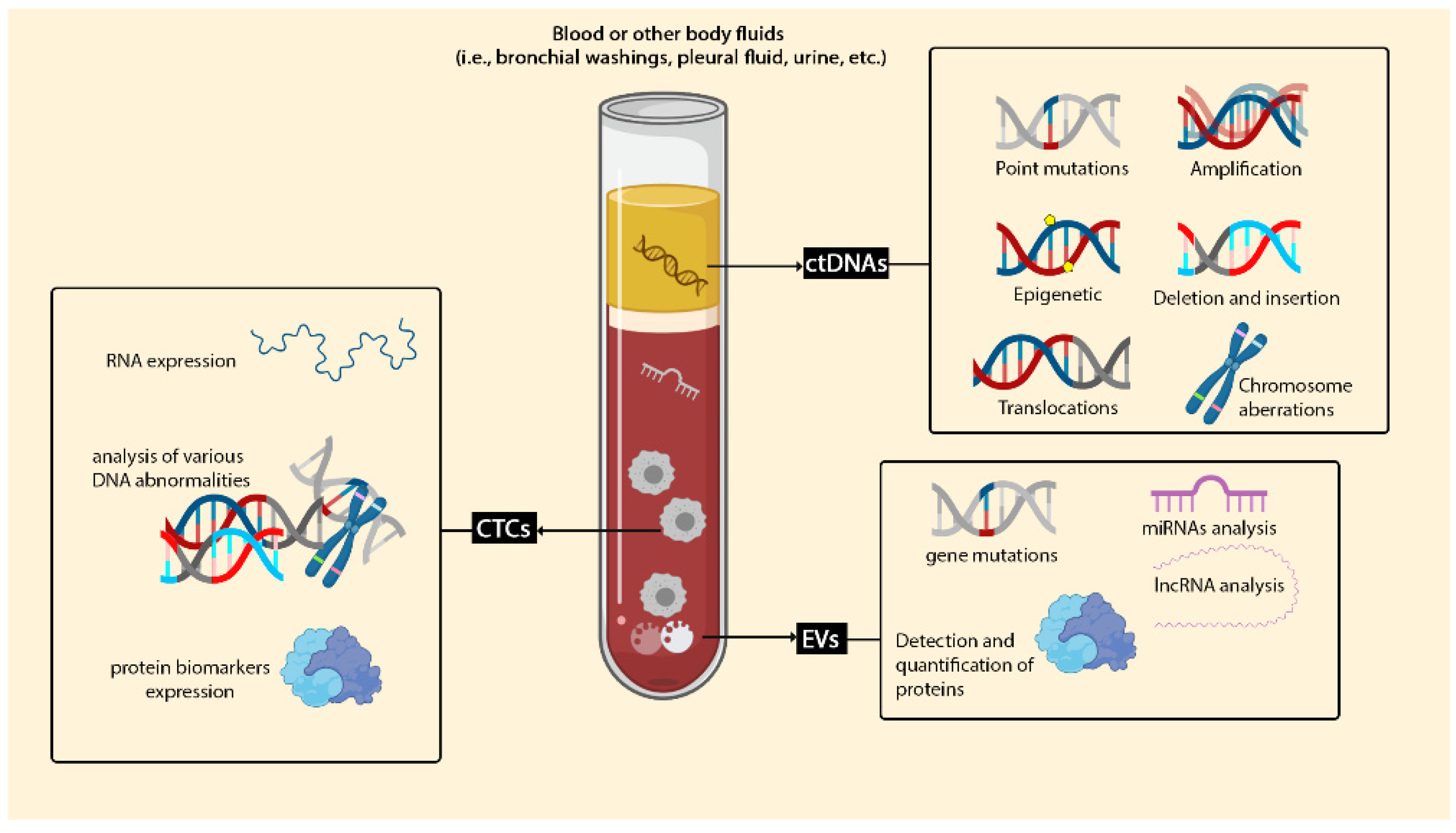
3.1. Circulating Cell-Free DNA (cfDNA) and Circulating Tumour DNA (ctDNA)
3.2. Circulating Tumor Cells (CTCs)
3.3. Extracellular Vesicles (EVs)
4. Type of Biosensors for Detection of Lung Cancer Biomarkers in Liquid Biopsy
4.1. Electrochemical Sensor
4.2. Electro-Kinetic Biosensors
4.3. Magnetic Biosensor
4.4. Optical Biosensors
4.4.1. Surface Enhanced Raman Spectroscopy (SERS)
4.4.2. Surface Plasmon Resonance Biosensors (SPR)
4.4.3. Fluorescence-Based Biosensing
4.4.4. Lateral Flow Immunoassay (LFIA)
4.4.5. Chemiluminescence
4.5. Other Biosensors
5. Challenges and Future Prospective of Liquid MRD
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ettinger, D.S.; Wood, D.E.; Aggarwal, C.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J. Natl. Compr. Canc. Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef]
- Wu, C.-F.; Fu, J.-Y.; Yeh, C.-J.; Liu, Y.-H.; Hsieh, M.-J.; Wu, Y.-C.; Tsai, Y.-H.; Chou, W.-C. Recurrence Risk Factors Analysis for Stage I Non-Small Cell Lung Cancer. Medicine 2015, 94, e1337. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Crowley, J.; Chansky, K.; Giroux, D.J.; Groome, P.A.; Rami-Porta, R.; Postmus, P.; Rusch, V.; Sobin, L. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the TNM Stage Groupings in the Forthcoming (Seventh) Edition of the TNM Classification of Malignant Tumours. J. Thorac. Oncol. 2007, 2, 706–714. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Wakelee, H.A. Adjuvant Chemotherapy of Completely Resected Early Stage Non-Small Cell Lung Cancer (NSCLC). Transl. Lung Cancer Res. 2013, 2, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Blandin Knight, S.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussel, T.; Dive, C. Progress and Prospects of Early Detection in Lung Cancer. Open Biol. 2017, 7, 170070. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.-H.; Luo, L.; Wampfler, A.J.; Wang, Y.; Liu, D.; Chen, Y.-M.; Adjei, A.A.; Midthun, E.D.; Yang, P. 5-Year Overall Survival in Patients with Lung Cancer Eligible or Ineligible for Screening According to US Preventive Services Task Force Criteria: A Prospective, Observational Cohort Study. Lancet Oncol. 2019, 20, 1098–1108. [Google Scholar] [CrossRef] [Green Version]
- Uramoto, H.; Tanaka, F. Recurrence after Surgery in Patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242–249. [Google Scholar] [CrossRef]
- Anandappa, G.; Starling, N.; Begum, R.; Bryant, A.; Sharma, S.; Renner, D.; Aresu, M.; Peckitt, C.; Sethi, H.; Feber, A.; et al. Minimal Residual Disease (MRD) Detection with Circulating Tumor DNA (Ctdna) from Personalized Assays in Stage II-III Colorectal Cancer Patients in a U.K. Multicenter Prospective Study (TRACC). J. Clin. Oncol. 2021, 39, 102. [Google Scholar] [CrossRef]
- Ofiara, L.; Navasakulpong, A.; Ezer, N.; Gonzalez, A. The Importance of a Satisfactory Biopsy for the Diagnosis of Lung Cancer in the Era of Personalized Treatment. Curr. Oncol. 2012, 19, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghamir, S.M.K.; Heshmat, R.; Ebrahimi, M.; Khatami, F. Liquid Biopsy: The Unique Test for Chasing the Genetics of Solid Tumors. Epigenetics Insights 2020, 13. [Google Scholar] [CrossRef]
- Pesapane, F.; Suter, M.B.; Rotili, A.; Penco, S.; Nigro, O.; Cremonesi, M.; Bellomi, M.; Jereczek-Fossa, B.A.; Pinotti, G.; Cassano, E. Will Traditional Biopsy be Substituted by Radiomics and Liquid Biopsy for Breast Cancer Diagnosis and Characterisation? Med. Oncol. 2020, 37, 29. [Google Scholar] [CrossRef]
- Pisapia, P.; Malapelle, U.; Troncone, G. Liquid Biopsy and Lung Cancer. Acta Cytol. 2019, 63, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.; Ayala, R.; Martínez-López, J. Minimal Residual Disease Monitoring with Next-Generation Sequencing Methodologies in Hematological Malignancies. Int. J. Mol. Sci. 2019, 20, 2832. [Google Scholar] [CrossRef] [Green Version]
- Coccaro, N.; Tota, G.; Anelli, L.; Zagaria, A.; Specchia, G.; Albano, F. Digital PCR: A Reliable Tool for Analyzing and Monitoring Hematologic Malignancies. Int. J. Mol. Sci. 2020, 21, 3141. [Google Scholar] [CrossRef] [PubMed]
- Chin, R.-I.; Chen, K.; Usmani, A.; Chua, C.; Harris, P.K.; Binkley, M.S.; Azad, T.D.; Dudley, J.C.; Chaudhuri, A.A. Detection of Solid Tumor Molecular Residual Disease (MRD) Using Circulating Tumor DNA (ctDNA). Mol. Diagn. 2019, 23, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Robert, C. A Decade of Immune-Checkpoint Inhibitors in Cancer Therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Gubens, A.M.; Davies, M. NCCN Guidelines Updates: New Immunotherapy Strategies for Improving Outcomes in Non-Small Cell Lung Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 574–578. [Google Scholar]
- Augustus, E.; Zwaenepoel, K.; Siozopoulou, V.; Raskin, J.; Jordaens, S.; Baggerman, G.; Sorber, L.; Roeyen, G.; Peeters, M.; Pauwels, P. Prognostic and Predictive Biomarkers in Non-Small Cell Lung Cancer Patients on Immunotherapy—The Role of Liquid Biopsy in Unraveling the Puzzle. Cancers 2021, 13, 1675. [Google Scholar] [CrossRef] [PubMed]
- El Rassy, E.; Assi, T.; Kattan, J. Is minimal Residual Disease a Convincing Tool to Determine the Treatment Duration of Immune Checkpoint Inhibitors? Futur. Oncol. 2016, 13, 381–383. [Google Scholar] [CrossRef] [Green Version]
- Nagasaka, M.; Uddin, M.H.; Al-Hallak, M.N.; Rahman, S.; Balasubramanian, S.; Sukari, A.; Azmi, A.S. Liquid Biopsy for Therapy Monitoring in Early-Stage Non-Small Cell Lung Cancer. Mol. Cancer 2021, 20, 82. [Google Scholar] [CrossRef]
- Bailey, C.; Black, J.R.; Reading, J.L.; Litchfield, K.; Turajlic, S.; McGranahan, N.; Jamal-Hanjani, M.; Swanton, C. Tracking Cancer Evolution through the Disease Course. Cancer Discov. 2021, 11, 916–932. [Google Scholar] [CrossRef] [PubMed]
- Jamal-Hanjani, M.; Wilson, G.A.; McGranahan, N.; Birkbak, N.; Watkins, T.B.; Veeriah, S.; Shafi, S.; Johnson, D.H.; Mitter, R.; Rosenthal, R.; et al. Tracking the Evolution of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2109–2121. [Google Scholar] [CrossRef] [Green Version]
- Arya, S.K.; Bhansali, S. Lung Cancer and Its Early Detection Using Biomarker-Based Biosensors. Chem. Rev. 2011, 111, 6783–6809. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Tang, C.; Deng, Y.; Huang, H.; He, Z. Recent Advances in Biosensor for Detection of Lung Cancer Biomarkers. Biosens. Bioelectron. 2019, 141, 111416. [Google Scholar] [CrossRef]
- Lin, X.-H.; Wu, P.; Chen, W.; Zhang, Y.-F.; Xia, X.-H. Electrochemical DNA Biosensor for the Detection of Short DNA Species of Chronic Myelogenous Leukemia by Using Methylene Blue. Talanta 2007, 72, 468–471. [Google Scholar] [CrossRef]
- Bohunicky, B.; Mousa, S.A. Biosensors: The New Wave in Cancer Diagnosis. Nanotechnol. Sci. Appl. 2010, 4, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laboria, N.; Fragoso, A.; Kemmner, W.; Latta, D.; Nilsson, O.; Botero, M.L.; Drese, K.; O’Sullivan, C.K. Amperometric Immunosensor for Carcinoembryonic Antigen in Colon Cancer Samples Based on Monolayers of Dendritic Bipodal Scaffolds. Anal. Chem. 2010, 82, 1712–1719. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, D.; Weng, L.; Wang, L. Early Lung Cancer Diagnosis by Biosensors. Int. J. Mol. Sci. 2013, 14, 15479–15509. [Google Scholar] [CrossRef]
- Jha, M.; Gupta, R.; Saxena, R. A Review on Noninvasive Biosensors for Early Detection of Lung Cancer. In Proceedings of the 2020 6th International Conference on Signal Processing and Communication (ICSC), Noida, India, 5–7 March 2020; pp. 162–166. [Google Scholar]
- Chaudhuri, A.A.; Chabon, J.J.; Lovejoy, A.F.; Newman, A.; Stehr, H.; Azad, T.; Khodadoust, M.S.; Esfahani, M.S.; Liu, C.L.; Zhou, L.; et al. Early Detection of Molecular Residual Disease in Localized Lung Cancer by Circulating Tumor DNA Profiling. Cancer Discov. 2017, 7, 1394–1403. [Google Scholar] [CrossRef] [Green Version]
- Galvano, A.; Gristina, V.; Insalaco, L.; La Mantia, M.; Perez, A.; Barraco, N.; Castellana, L.; Bono, M.; Cusenza, S.; Castiglia, M.; et al. P07.02 Detection of Molecular Residual Disease (MRD) using ctDNA in NSCLC: A Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2021, 16, S278. [Google Scholar] [CrossRef]
- Mak, R.H.; Hermann, G.; Aerts, H.J.; Baldini, E.H.; Chen, A.B.; Kozono, D.; Rabin, M.S.; Swanson, S.J.; Chen, Y.-H.; Catalano, P.; et al. Outcomes by EGFR, KRAS, and ALK Genotype after Combined Modality Therapy for Locally Advanced Non–Small-Cell Lung Cancer. JCO Precis. Oncol. 2018, 2, 1–18. [Google Scholar] [CrossRef]
- Li, H.-J.; Ke, F.-Y.; Lin, C.-C.; Lu, M.-Y.; Kuo, Y.-H.; Wang, Y.-P.; Liang, K.-H.; Lin, S.-C.; Chang, Y.-H.; Chen, H.-Y.; et al. ENO1 Promotes Lung Cancer Metastasis via HGFR and WNT Signaling–Driven Epithelial-to-Mesenchymal Transition. Cancer Res. 2021, 81, 4094–4109. [Google Scholar] [CrossRef]
- Li, P.; Zhang, B.; Cui, T. Towards Intrinsic Graphene Biosensor: A Label-Free, Suspended Single Crystalline Graphene Sensor for Multiplex Lung Cancer Tumor Markers Detection. Biosens. Bioelectron. 2015, 72, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Ohara, S.; Suda, K.; Sakai, K.; Nishino, M.; Chiba, M.; Shimoji, M.; Takemoto, T.; Fujino, T.; Koga, T.; Hamada, A.; et al. Prognostic Implications of Preoperative Versus Postoperative Circulating Tumor DNA in Surgically Resected Lung Cancer Patients: A Pilot Study. Transl. Lung Cancer Res. 2020, 9, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Killock, D. DYNAMIC Insights into MRD Responses Early after Resection of NSCLC. Nat. Rev. Clin. Oncol. 2019, 16, 661. [Google Scholar] [CrossRef]
- Peters, S.; Spigel, D.; Ahn, M.; Tsuboi, M.; Chaft, J.; Harpole, D.; Goss, G.; Barlesi, F.; Abbosh, C.; Poole, L.; et al. P03.03 MERMAID-1: A Phase III Study of Adjuvant Durvalumab plus Chemotherapy in Resected NSCLC Patients with MRD+ Post-Surgery. J. Thorac. Oncol. 2021, 16, S258–S259. [Google Scholar] [CrossRef]
- Chen, M.; Wu, D.; Tu, S.; Yang, C.; Chen, D.; Xu, Y. A Novel Biosensor for the Ultrasensitive Detection of the Lncrna Biomarker MALAT1 in Non-Small Cell Lung Cancer. Sci. Rep. 2021, 11, 3666. [Google Scholar] [CrossRef]
- Pu, D.; Liang, H.; Wei, F.; Akin, D.; Feng, Z.; Yan, Q.; Li, Y.; Zhen, Y.; Xu, L.; Dong, G.; et al. Evaluation of a Novel Saliva-Based Epidermal Growth Factor Receptor Mutation Detection for Lung Cancer: A Pilot Study. Thorac. Cancer 2016, 7, 428–436. [Google Scholar] [CrossRef]
- Cavallaro, S.; Horak, J.; Hååg, P.; Gupta, D.; Stiller, C.; Sahu, S.S.; Görgens, A.; Gatty, H.K.; Viktorsson, K.; El-Andaloussi, S.; et al. Label-Free Surface Protein Profiling of Extracellular Vesicles by an Electrokinetic Sensor. ACS Sens. 2019, 4, 1399–1408. [Google Scholar] [CrossRef]
- Wei, F.; Yang, J.; Wong, D.T.W. Detection of Exosomal Biomarker by Electric Field-Induced Release and Measurement (EFIRM). Biosens. Bioelectron. 2013, 44, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.-W.; Weng, X.-H.; Wang, C.-L.; Lin, W.-W.; Liu, A.-L.; Chen, W.; Lin, X.-H. Detection EGFR Exon 19 Status of Lung Cancer Patients by DNA Electrochemical Biosensor. Biosens. Bioelectron. 2016, 80, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Bao, J.; Huo, D.; Zeng, Y.; Wang, X.; Samalo, M.; Zhao, J.; Zhang, S.; Shen, C.; Hou, C. Au Doped Poly-Thionine and Poly-M-Cresol Purple: Synthesis and their Application in Simultaneously Electrochemical Detection of Two Lung Cancer Markers CEA and CYFRA21-1. Talanta 2020, 224, 121816. [Google Scholar] [CrossRef]
- Feng, J.; Wu, T.; Cheng, Q.; Ma, H.; Ren, X.; Wang, X.; Lee, J.Y.; Wei, Q.; Ju, H. A Microfluidic Cathodic Photoelectrochemical Biosensor Chip for the Targeted Detection of Cytokeratin 19 Fragments 21-1. Lab Chip 2021, 21, 378–384. [Google Scholar] [CrossRef]
- Jian, L.; Wang, X.; Hao, L.; Liu, Y.; Yang, H.; Zheng, X.; Feng, W. Electrochemiluminescence Immunosensor for Cytokeratin Fragment Antigen 21-1 Detection Using Electrochemically Mediated Atom Transfer Radical Polymerization. Microchim. Acta 2021, 188, 115. [Google Scholar] [CrossRef]
- Sahu, S.S.; Cavallaro, S.; Hååg, P.; Nagy, Á.; Karlström, A.E.; Lewensohn, R.; Viktorsson, K.; Linnros, J.; Dev, A. Exploiting Electrostatic Interaction for Highly Sensitive Detection of Tumor-Derived Extracellular Vesicles by an Electrokinetic Sensor. ACS Appl. Mater. Interfaces 2021, 13, 42513–42521. [Google Scholar] [CrossRef]
- Wu, W.; Yu, X.; Wu, J.; Wu, T.; Fan, Y.; Chen, W.; Zhao, M.; Wu, H.; Li, X.; Ding, S. Surface Plasmon Resonance Imaging-Based Biosensor for Multiplex and Ultrasensitive Detection of NSCLC-Associated Exosomal Mirnas Using DNA Programmed Heterostructure of Au-On-Ag. Biosens. Bioelectron. 2021, 175, 112835. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Duan, X.; Zhao, M.; Wei, X.; Wu, J.; Chen, W.; Liu, P.; Cheng, W.; Cheng, Q.; Ding, S. High-Sensitive and Multiplex Biosensing Assay of NSCLC-Derived Exosomes via Different Recognition Sites Based on Spri Array. Biosens. Bioelectron. 2020, 154, 112066. [Google Scholar] [CrossRef] [PubMed]
- Ladd, J.; Taylor, A.D.; Piliarik, M.; Homola, J.; Jiang, S. Label-Free Detection of Cancer Biomarker Candidates Using Surface Plasmon Resonance Imaging. Anal. Bioanal. Chem. 2009, 393, 1157–1163. [Google Scholar] [CrossRef]
- Liu, C.; Zeng, X.; An, Z.; Yang, Y.; Eisenbaum, M.; Gu, X.; Jornet, J.M.; Dy, G.K.; Reid, M.E.; Gan, Q.; et al. Sensitive Detection of Exosomal Proteins via a Compact Surface Plasmon Resonance Biosensor for Cancer Diagnosis. ACS Sens. 2018, 3, 1471–1479. [Google Scholar] [CrossRef]
- Sun, Y.; Ge, S.; Xue, J.; Zhou, X.; Lu, W.; Li, G.; Cao, X. Highly Sensitive Detection of Cytochrome C in the NSCLC Serum Using a Hydrophobic Paper Based–Gold Nanourchin Substrate. Biomed. Opt. Express 2020, 11, 7062–7078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, R.; Gao, M.; Zhang, X. Novel Nitrocellulose Membrane Substrate for Efficient Analysis of Circulating Tumor Cells Coupled with Surface-Enhanced Raman Scattering Imaging. ACS Appl. Mater. Interfaces 2014, 6, 370–376. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Crow, J.; Roth, M.; Zeng, Y.; Godwin, A.K. Integrated Immunoisolation and Protein Analysis of Circulating Exosomes Using Microfluidic Technology. Lab Chip 2014, 14, 3773–3780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soamalala, J.; Diot, S.; Pellerano, M.; Blanquart, C.; Galibert, M.; Jullian, M.; Puget, K.; Morris, M.C. Fluorescent Peptide Biosensor for Probing CDK6 Kinase Activity in Lung Cancer Cell Extracts. ChemBioChem 2021, 22, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Earhart, C.M.; Hughes, C.E.; Gaster, R.S.; Ooi, C.C.; Wilson, R.J.; Zhou, L.Y.; Humke, E.W.; Xu, L.; Wong, D.J.; Willingham, S.B.; et al. Isolation and Mutational Analysis of Circulating Tumor Cells from Lung Cancer Patients with Magnetic Sifters and Biochips. Lab Chip 2014, 14, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Zhao, Q.; Wang, S.; Zhao, S.; Zhang, S.; Yin, Y.; Dong, Y. Development of a Lateral Flow Aptamer Assay Strip for Facile Identification of Theranostic Exosomes Isolated from Human Lung Carcinoma Cells. Anal. Biochem. 2020, 594, 113591. [Google Scholar] [CrossRef] [PubMed]
- Ghazani, A.A.; Pectasides, M.; Sharma, A.; Castro, C.M.; Mino-Kenudson, M.; Lee, H.; Shepard, J.-A.O.; Weissleder, R. Molecular Characterization of Scant Lung Tumor Cells Using Iron-Oxide Nanoparticles and Micro-Nuclear Magnetic Resonance. Nanomedicine 2013, 10, 661–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wuethrich, A.; Rajkumar, A.R.; Shanmugasundaram, K.B.; Reza, K.K.; Dey, S.; Howard, C.B.; Ibn Sina, A.A.; Trau, M. Single Droplet Detection of Immune Checkpoints on a Multiplexed Electrohydrodynamic Biosensor. Analyst 2019, 144, 6914–6921. [Google Scholar] [CrossRef]
- Cirmena, G.; Garuti, A.; De Mariano, M.; Coco, S.; Ferrando, L.; Isnaldi, E.; Barbero, V.; Fregatti, P.; Del Mastro, L.; Ferrando, F.; et al. Circulating Tumor DNA Using Tagged Targeted Deep Sequencing to Assess Minimal Residual Disease in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. J. Oncol. 2020, 2020, 8132507. [Google Scholar] [CrossRef]
- Dizdar, L.; Fluegen, G.; van Dalum, G.; Honisch, E.; Neves, R.P.; Niederacher, D.; Neubauer, H.; Fehm, T.; Rehders, A.; Krieg, A.; et al. Detection of Circulating Tumor Cells in Colorectal Cancer Patients Using the GILUPI CellCollector: Results from a Prospective, Single-Center Study. Mol. Oncol. 2019, 13, 1548–1558. [Google Scholar] [CrossRef] [Green Version]
- Chen, E.; Cario, C.L.; Leong, L.; Lopez, K.; Márquez, C.P.; Chu, C.; Li, P.S.; Oropeza, E.; Tenggara, I.; Cowan, J.; et al. Cell-free DNA Concentration and Fragment Size as a Biomarker for Prostate Cancer. Sci. Rep. 2021, 11, 5040. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Su, L.; Qian, C. Circulating Tumor DNA: A Promising Biomarker in the Liquid Biopsy of Cancer. Oncotarget 2015, 7, 48832–48841. [Google Scholar] [CrossRef] [Green Version]
- Russano, M.; Napolitano, A.; Ribelli, G.; Iuliani, M.; Simonetti, S.; Citarella, F.; Pantano, F.; Dell’Aquila, E.; Anesi, C.; Silvestris, N.; et al. Liquid Biopsy and Tumor Heterogeneity in Metastatic Solid Tumors: The Potentiality of Blood Samples. J. Exp. Clin. Cancer Res. 2020, 39, 95. [Google Scholar] [CrossRef] [PubMed]
- Cucchiara, F.; Del Re, M.; Valleggi, S.; Romei, C.; Petrini, I.; Lucchesi, M.; Crucitta, S.; Rofi, E.; De Liperi, A.; Chella, A.; et al. Integrating Liquid Biopsy and Radiomics to Monitor Clonal Heterogeneity of EGFR-Positive Non-Small Cell Lung Cancer. Front. Oncol. 2020, 10, 2664. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Huang, Q.; Yin, W.; Tan, S.; Chen, C.; Liu, W.; Tang, J.; Wang, X.; Zhang, B.; Zou, M.; et al. Circulating Tumor DNA as a Prognostic Biomarker in Localized Non-small Cell Lung Cancer. Front. Oncol. 2020, 10, 561598. [Google Scholar] [CrossRef]
- Abbosh, C.; Birkbak, N.J.; Wilson, G.A.; Jamal-Hanjani, M.; Constantin, T.; Salari, R.; Le Quesne, J.; Moore, D.A.; Veeriah, S.; Rosenthal, R.; et al. Phylogenetic Ctdna Analysis Depicts Early-Stage Lung Cancer Evolution. Nature 2017, 545, 446–451. [Google Scholar] [CrossRef]
- Chen, L.; Bode, A.M.; Dong, Z. Circulating Tumor Cells: Moving Biological Insights into Detection. Theranostics 2017, 7, 2606–2619. [Google Scholar] [CrossRef]
- Tamminga, M.; de Wit, S.; Hiltermann, T.J.N.; Timens, W.; Schuuring, E.; Terstappen, L.; Groen, H.J. Circulating Tumor Cells in Advanced Non-Small Cell Lung Cancer Patients are Associated with Worse Tumor Response to Checkpoint Inhibitors. J. Immunother. 2019, 7, 173. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, T.; Lv, X.; Li, R.; Yuan, L.; Shen, J.; Li, Y.; Yan, T.; Liu, B.; Wang, L. Detection of PD-L1 Expression and Its Clinical Significance in Circulating Tumor Cells from Patients with Non-Small-Cell Lung Cancer. Cancer Manag. Res. 2020, 12, 2069–2078. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.; Zhang, W.; Yang, Z.; Li, G.; Cheng, S.; Zhang, K.; Feng, L. Correlation between PD-L1 Expression ON CTCs and Prognosis of Patients with Cancer: A Systematic Review and Meta-Analysis. OncoImmunology 2021, 10, 1938476. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Lee, C.-L.; Fu, J.-Y.; Yang, C.-T.; Wen, C.-T.; Liu, Y.-H.; Liu, H.-P.; Hsieh, J.C.-H.; Wu, C.-F. Circulating Tumor Cells as a Tool of Minimal Residual Disease Can Predict Lung Cancer Recurrence: A Longitudinal, Prospective Trial. Diagnostics 2020, 10, 144. [Google Scholar] [CrossRef] [Green Version]
- Li, M.-Y.; Liu, L.-Z.; Dong, M. Progress on Pivotal Role and Application of Exosome in Lung Cancer Carcinogenesis, Diagnosis, Therapy and Prognosis. Mol. Cancer 2021, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Li, S.; Jin, Y.; Xian, Z.; Yang, D.; Zhou, W.; Mangaran, F.; Leung, F.; Sithamparanathan, G.; Kerman, K. Nanomaterial-Based Biosensors for Biological Detections. Adv. Heal. Care Technol. 2017, 3, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Aykaç, A.; Gergeroglu, H.; Beşli, B.; Akkaş, E.; Yavaş, A.; Güler, S.; Güneş, F.; Erol, M. An Overview on Recent Progress of Metal Oxide/Graphene/CNTs-Based Nanobiosensors. Nanoscale Res. Lett. 2021, 16, 65. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, Properties, and Applications in Bionanotechnology. Nanoscale 2011, 4, 1871–1880. [Google Scholar] [CrossRef]
- Tîlmaciu, C.-M.; Morris, M.C. Carbon Nanotube Biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muley, T.; He, Y.; Rolny, V.; Wehnl, B.; Escherich, A.; Warth, A.; Stolp, C.; Schneider, M.A.; Meister, M.; Herth, F.J.; et al. Potential for the Blood-Based Biomarkers Cytokeratin 19 Fragment (CYFRA 21-1) and Human Epididymal Protein 4 (HE4) to Detect Recurrence during Monitoring after Surgical Resection of Adenocarcinoma of the Lung. Lung Cancer 2019, 130, 194–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yola, M.L.; Atar, N.; Özcan, N. A Novel Electrochemical Lung Cancer Biomarker Cytokeratin 19 Fragment Antigen 21-1 Immunosensor Based on Si3N4/Mos2 Incorporated Mwcnts and Core–Shell Type Magnetic Nanoparticles. Nanoscale 2021, 13, 4660–4669. [Google Scholar] [CrossRef] [PubMed]
- Dall’Olio, F.G.; Abbati, F.; Facchinetti, F.; Massucci, M.; Melotti, B.; Squadrilli, A.; Buti, S.; Formica, F.; Tiseo, M.; Ardizzoni, A. CEA and CYFRA 21-1 as Prognostic Biomarker and as a Tool for Treatment Monitoring in Advanced NSCLC Treated with Immune Checkpoint Inhibitors. Adv. Med. Oncol. 2020, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Kim, T.H.; Zhang, Z.; Azizi, E.; Pham, T.M.; Paoletti, C.; Lin, J.; Ramnath, N.; Wicha, M.S.; Hayes, D.F.; et al. Sensitive Capture of Circulating Tumour Cells by Functionalized Graphene Oxide Nanosheets. Nat. Nanotechnol. 2013, 8, 735–741. [Google Scholar] [CrossRef]
- Bolat, G.; Vural, O.A.; Yaman, Y.T.; Abaci, S. Polydopamine Nanoparticles-Assisted Impedimetric Sensor Towards Label-Free Lung Cancer Cell Detection. Mater. Sci. Eng. C 2021, 119, 111549. [Google Scholar] [CrossRef]
- Nguyen, N.-V.; Jen, C.-P. Selective Detection of Human Lung Adenocarcinoma Cells Based on the Aptamer-Conjugated Self-Assembled Monolayer of Gold Nanoparticles. Micromachines 2019, 10, 195. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.; Lin, C.-C.; Joon, A.; Feng, Z.; Troche, G.; Lira, M.E.; Chia, D.; Mao, M.; Ho, C.L.; Su, W.C.; et al. Noninvasive Saliva-Based EGFR Gene Mutation Detection in Patients with Lung Cancer. Am. J. Respir. Crit. Care Med. 2014, 15, 1117–1126. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Wei, F.; Huang, W.-L.; Lin, C.-C.; Li, L.; Shen, M.M.; Yan, Q.; Liao, W.; Chia, W.; Tu, M.; et al. Ultra-Short Circulating Tumor DNA (usctDNA) in Plasma and Saliva of Non-Small Cell Lung Cancer (NSCLC) Patients. Cancers 2020, 12, 2041. [Google Scholar] [CrossRef]
- Li, N.; Guha, U.; Kim, C.; Ye, L.; Cheng, J.; Li, F.; Chia, D.; Wei, F.; Wong, D.T.W. Longitudinal Monitoring of EGFR and PIK3CA Mutations by Saliva-Based EFIRM in Advanced NSCLC Patients with Local Ablative Therapy and Osimertinib Treatment: Two Case Reports. Front. Oncol. 2020, 10, 1240. [Google Scholar] [CrossRef]
- Lindeman, L.R.; Jones, K.M.; High, R.A.; Howison, C.M.; Shubitz, L.F.; Pagel, M.D. Differentiating Lung Cancer and Infection Based on Measurements of Extracellular pH with acidoCEST MRI. Sci. Rep. 2019, 9, 13002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mani, G.K.; Morohoshi, M.; Yasoda, Y.; Yokoyama, S.; Kimura, H.; Tsuchiya, K. ZnO-Based Microfluidic pH Sensor: A Versatile Approach for Quick Recognition of Circulating Tumor Cells in Blood. ACS Appl. Mater. 2017, 9, 5193–5203. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical Biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, S.; Hååg, P.; Sahu, S.S.; Berisha, L.; Kaminskyy, V.O.; Ekman, S.; Lewensohn, R.; Linnros, J.; Viktorsson, K.; Dev, A. Multiplexed Electrokinetic Sensor for Detection and Therapy Monitoring of Extracellular Vesicles from Liquid Biopsies of Non-Small-Cell Lung Cancer Patients. Biosens. Bioelectron. 2021, 193, 113568. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.S.; Stiller, C.; Cavallaro, S.; Karlström, A.E.; Linnros, J.; Dev, A. Influence of Molecular Size and Zeta Potential in Electrokinetic Biosensing. Biosens. Bioelectron. 2020, 152, 11200. [Google Scholar] [CrossRef]
- Nabaei, V.; Chandrawati, R.; Heidari, H. Magnetic Biosensors: Modelling and Simulation. Biosens. Bioelectron. 2018, 103, 69–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, V.S.; Beggs, M.; Yu, H.; Carbonell, L.; Wang, S.X.; Vachani, A. Validation of Plasma TIMP-1 to Identify Lung Cancer in Smokers. In D99 Clinically Informative Biomarkers in Lung Cancer: A Needle in a Haystack; American Thoracic Society: New York, NY, USA, 2018; p. A7415. [Google Scholar] [CrossRef]
- Pesta, M.; Kulda, V.; Kucera, R.; Pesek, M.; Vrzalova, J.; Liska, V.; Pecen, L.; Treska, V.; Safranek, J.; Prazakova, M.; et al. Prognostic Significance of TIMP-1 in Non-Small Cell Lung Cancer. Anticancer. Res. 2011, 31, 4031–4038. [Google Scholar]
- Selvaraj, G.; Kaliamurthi, S.; Lin, S.; Gu, K.; Wei, D. Prognostic Impact of Tissue Inhibitor of Metalloproteinase-1 in Non- Small Cell Lung Cancer: Systematic Review and Meta-Analysis. Curr. Med. Chem. 2019, 26, 7694–7713. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Chiu, Y.-J.; Cheng, H.-C.; Liu, F.-J.; Lai, W.-W.; Chang, H.-J.; Liao, P.-C. Down-Regulation of TIMP-1 Inhibits Cell Migration, Invasion, and Metastatic Colonization in Lung Adenocarcinoma. Tumor Biol. 2015, 36, 3957–3967. [Google Scholar] [CrossRef]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical Biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Long, F.; Chen, W.; Chen, J.; Chu, P.K.; Wang, H. Fundamentals and Applications of Surface-Enhanced Raman Spectroscopy–Based Biosensors. Curr. Opin. Biomed. Eng. 2019, 13, 51–59. [Google Scholar] [CrossRef]
- Pang, Y.; Shi, J.; Yang, X.; Wang, C.; Sun, Z.; Xiao, R. Personalized detection of circling exosomal PD-L1 based on Fe3O4@TiO2 isolation and SERS immunoassay. Biosens. Bioelectron. 2020, 148, 111800. [Google Scholar] [CrossRef]
- Lin, J.; Zheng, J.; Wu, A. An Efficient Strategy for Circulating Tumor Cell Detection: Surface-Enhanced Raman Spectroscopy. J. Mater. Chem. B 2020, 8, 3316–3326. [Google Scholar] [CrossRef]
- Song, C.Y.; Yang, Y.J.; Yang, B.Y.; Sun, Y.Z.; Zhao, Y.P.; Wang, L.H. An Ultrasensitive SERS Sensor for Simultaneous Detection of Multiple Cancer-Related miRNAs. Nanoscale 2016, 8, 17365–17373. [Google Scholar] [CrossRef]
- Shin, S.; Park, Y.H.; Jung, S.-H.; Jang, S.-H.; Kim, M.Y.; Lee, J.Y.; Chung, Y. Urinary Exosome Microrna Signatures as a Noninvasive Prognostic Biomarker for Prostate Cancer. npj Genom. Med. 2021, 6, 45. [Google Scholar] [CrossRef]
- Hornick, N.I.; Huan, J.; Doron, B.; Goloviznina, N.; Lapidus, J.; Chang, B.; Kurre, P. Serum Exosome MicroRNA as a Minimally-Invasive Early Biomarker of AML. Sci. Rep. 2015, 5, 11295. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.C.; Fedoriw, Y.; Pulley, W.; Zeidner, J.; Coombs, C.C.; Mirkin, E.; Zomorrodi, M.; Toughiri, R.; Bartakova, A.; Carson, C.; et al. Detection of Measurable Residual Disease (MRD) in Peripheral Blood: First Report of a Novel Microfluidic Platform in Patients with Acute Myeloid Leukemia (AML). Blood 2019, 134, 1417. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Gupta, R.C. Exosomal miRNAs as Biomarkers of Recurrent Lung Cancer. Tumor Biol. 2016, 37, 10703–10714. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shen, Z. Exosomal miRNAs as Biomarkers for Diagnostic and Prognostic in Lung Cancer. Cancer Med. 2020, 9, 6909–6922. [Google Scholar] [CrossRef] [PubMed]
- Strianese, M.; Staiano, M.; Ruggiero, G.; Labella, T.; Pellecchia, C.; D’Auria, S. Fluorescence-Based Biosensors. Methods Mol. Biol. 2012, 875, 193–216. [Google Scholar] [PubMed]
- Bai, Y.; Lu, Y.; Wang, K.; Cheng, Z.; Qu, Y.; Qiu, S.; Zhou, L.; Wu, Z.; Liu, H.; Zhao, J.; et al. Rapid Isolation and Multiplexed Detection of Exosome Tumor Markers via Queued Beads Combined with Quantum Dots in a Microarray. Nano-Micro Lett. 2019, 11, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.D.; Shandilya, R.; Bhargava, A.; Kumar, R.; Tiwari, R.; Chaudhury, K.; Srivastava, R.K.; Goryacheva, I.; Mishra, P.K. Quantum Dot Based Nano-Biosensors for Detection of Circulating Cell Free miRNAs in Lung Carcinogenesis: From Biology to Clinical Translation. Front. Genet. 2018, 9, 616. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Han, D.; Wang, W.; Zhang, Y.; Li, D.; Dai, C.; Qian, L.; Lin, W. Decline in Serum Progastrin-Releasing Peptide Predicts the Response of Patients with Small Cell Lung Cancer to Chemotherapy. Oncol. Lett. 2020, 20, 301. [Google Scholar] [CrossRef]
- Barchiesi, V.; Simeon, V.; Sandomenico, C.; Cantile, M.; Cerasuolo, D.; Chiodini, P.; Morabito, A.; Cavalcanti, E. Circulating Progastrin-Releasing Peptide in the Diagnosis of Small Cell Lung Cancer (SCLC) and in Therapeutic Monitoring. J. Circ. Biomark. 2021, 10, 9–13. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, G.; Yang, E.; Zhang, M.; Luo, D.; Liu, J.; Zhao, C.; Chen, Q.; Ran, F. Utilizing DNase I and Graphene Oxide Modified Magnetic Nanoparticles for Sensing PD-L1 in Human Plasma. Sens. Rev. 2021, 41, 229–234. [Google Scholar] [CrossRef]
- Yang, M.; Shen, H.; Qiu, C.; Ni, Y.; Wang, L.; Dong, W.; Liao, Y.; Du, J. High Expression of Mir-21 and Mir-155 Predicts Recurrence and Unfavourable Survival in Non-Small Cell Lung Cancer. Eur. J. Cancer 2013, 49, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Hanafi, A.R.; Jayusman, A.M.; Alfasunu, S.; Sadewa, A.H.; Pramono, D.; Heriyanto, D.S.; Haryana, S.M. Serum MiRNA as Predictive and Prognosis Biomarker in Advanced Stage Non-small Cell Lung Cancer in Indonesia. Zhongguo Fei Ai Za Zhi 2020, 23, 321–332. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, J.; Li, Y.; Xu, Y.; Chen, L.; Chen, W.; Liu, A.; Lin, X.; Weng, S. Dual-Probe Fluorescent Biosensor Based on T7 Exonuclease-Assisted Target Recycling Amplification for Simultaneous Sensitive Detection of Microrna-21 and Microrna-155. Anal. Bioanal. Chem. 2021, 413, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, L.; Weisser, J.; Kohl, M.; Deigner, H.-P. Small Molecule Detection with Aptamer Based Lateral Flow Assays: Applying Aptamer-C-Reactive Protein Cross-Recognition for Ampicillin Detection. Sci. Rep. 2018, 8, 5628. [Google Scholar] [CrossRef]
- Koh, H.; An, H.; Jung, J.; Song, D. The Prognostic Significance of CD63 Expression in Patients with Non-Small Cell Lung Cancer. Pol. J. Pathol. 2019, 70, 183–188. [Google Scholar] [CrossRef]
- Moyano, A.; Serrano-Pertierra, E.; Duque, J.; Ramos, V.; Teruel-Barandiarán, E.; Fernández-Sánchez, M.; Salvador, M.; Martínez-García, J.; Sánchez, L.; García-Flórez, L.; et al. Magnetic Lateral Flow Immunoassay for Small Extracellular Vesicles Quantification: Application to Colorectal Cancer Biomarker Detection. Sensors 2021, 21, 3756. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhuang, X.; Tian, C.; Fu, X.; Luan, F. Ru(bpy)32+ Encapsulated Cyclodextrin Based Metal Organic Framework with Improved Biocompatibility for Sensitive Electrochemiluminescence Detection of CYFRA21-1 In Cell. Biosens. Bioelectron. 2021, 190, 113371. [Google Scholar] [CrossRef]
- Ramanathan, S.; Gopinath, S.C.B.; Arshad, M.K.M.; Poopalan, P.; Anbu, P.; Lakshmipriya, T.; Kasim, F.H. Aluminosilicate Nanocomposite on Genosensor: A Prospective Voltammetry Platform for Epidermal Growth Factor Receptor Mutant Analysis in Non-small Cell Lung Cancer. Sci. Rep. 2019, 9, 17013. [Google Scholar] [CrossRef]
- Radovich, M.; Jiang, G.; Hancock, B.A.; Chitambar, C.; Nanda, R.; Falkson, C.; Lynce, F.C.; Gallagher, C.; Isaacs, I.; Blaya, M.; et al. Association of Circulating Tumor DNA and Circulating Tumor Cells after Neoadjuvant Chemotherapy with Disease Recurrence in Patients with Triple-Negative Breast Cancer: Preplanned Secondary Analysis of the BRE12-158 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Haglund, T.A.; Rogers, A.J.; Ghanim, A.F.; Sethu, P. Review: Microfluidics Technologies for Blood-Based Cancer Liquid Biopsies. Anal. Chim. Acta 2018, 1012, 10–29. [Google Scholar] [CrossRef]
- Kabza, A.M.; Sczepanski, J.T. l-DNA-Based Catalytic Hairpin Assembly Circuit. Molecules 2020, 25, 947. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Y.; Zhang, T.; Zhang, S.; Johnston, M.; Zheng, X.; Shan, Y.; Liu, T.; Huang, Z.; Qian, F.; Xie, Z.; et al. A CRISPR/Cas13a-Powered Catalytic Electrochemical Biosensor for Successive and Highly Sensitive RNA Diagnostics. Biosens. Bioelectron. 2021, 178, 113027. [Google Scholar] [CrossRef]
- Winer-Jones, J.P.; Vahidi, B.; Arquilevich, N.; Fang, C.; Ferguson, S.; Harkins, D.; Hill, C.; Klem, E.; Pagano, P.C.; Peasley, C.; et al. Circulating Tumor Cells: Clinically Relevant Molecular Access Based on a Novel CTC Flow Cell. PLoS ONE 2014, 9, e86717. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhao, H.; Shi, Y.; Yang, F.; Wang, L.T.; Kang, G.; Nie, Y.; Wang, J. Perioperative Dynamic Changes in Circulating Tumor DNA in Patients with Lung Cancer (DYNAMIC). Clin. Cancer Res. 2019, 25, 7058–7067. [Google Scholar] [CrossRef] [Green Version]
- Severson, E.A.; Riedlinger, G.M.; Connelly, C.F.; Vergilio, J.-A.; Goldfinger, M.; Ramkissoon, S.; Frampton, G.M.; Ross, J.S.; Fratella-Calabrese, A.; Gay, L.; et al. Detection of Clonal Hematopoiesis of Indeterminate Potential in Clinical Sequencing of Solid Tumor Specimens. Blood 2018, 131, 2501–2505. [Google Scholar] [CrossRef] [Green Version]
- Yaung, S.J.; Fuhlbrück, F.; Peterson, M.; Zou, W.; Palma, J.F.; Patil, N.S.; Jiang, Y. Clonal Hematopoiesis in Late-Stage Non–Small-Cell Lung Cancer and Its Impact on Targeted Panel Next-Generation Sequencing. JCO Precis. Oncol. 2020, 1271–1279. [Google Scholar] [CrossRef]
- Si, H.; Du, D.; Li, W.; Li, Q.; Li, J.; Zhao, D.; Li, L.; Tang, B. Sputum-Based Tumor Fluid Biopsy: Isolation and High-Throughput Single-Cell Analysis of Exfoliated Tumor Cells for Lung Cancer Diagnosis. Anal. Chem. 2021, 93, 10477–10486. [Google Scholar] [CrossRef] [PubMed]
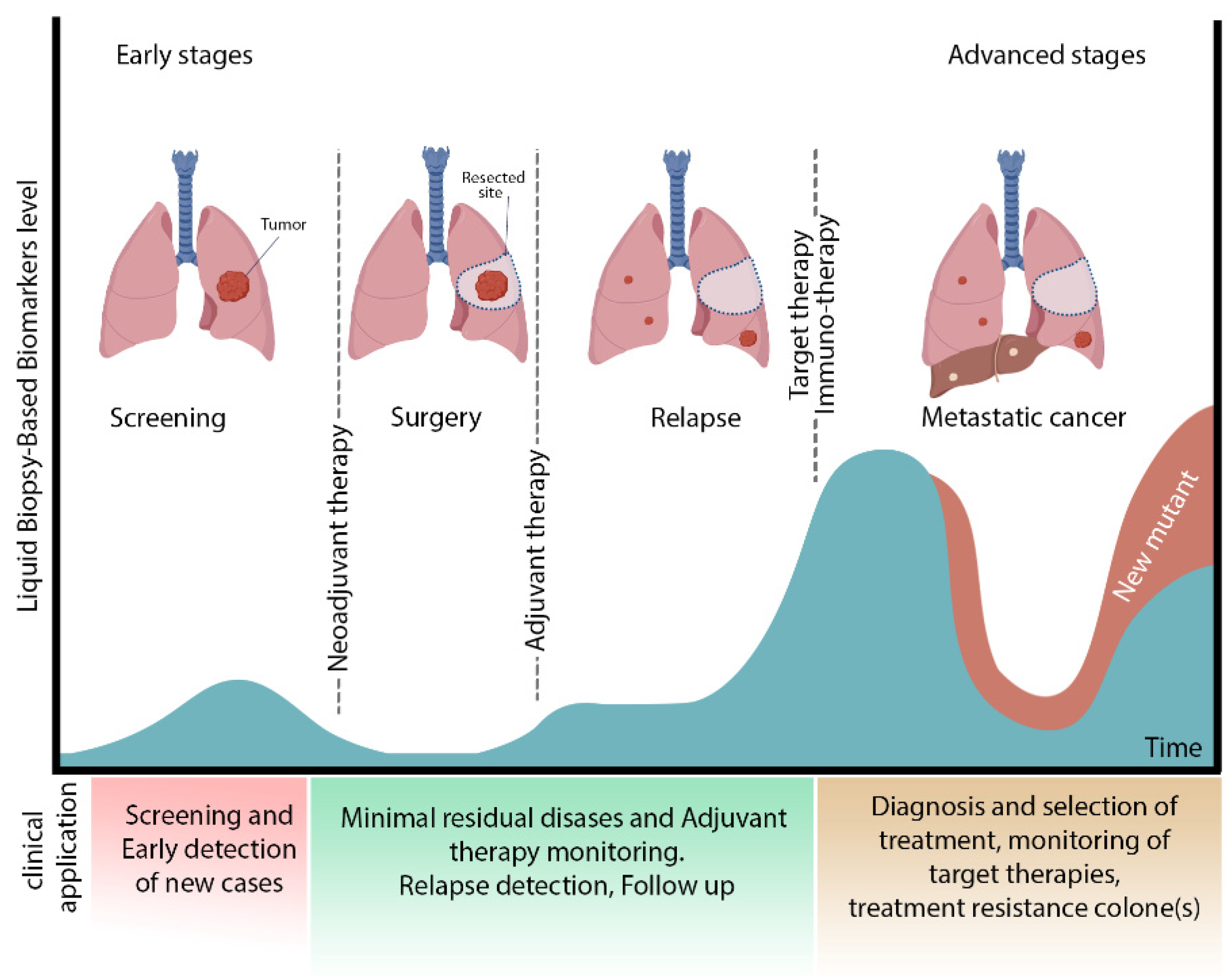

| Principle of Method | Advantages | Disadvantages | Application in MRD of Lung Cancer | |
|---|---|---|---|---|
| Traditional Biopsy and morphological-based test | A piece of lung tissue is taken from the body for histopathology evaluation. | Clinically validated | Invasive and risky. Unpleasant for the patient. Inter and intra laboratory error during reporting of the result. Failure to detect metastasis in other tissues. Serial sampling is challenging. Low sensitivity. | Traditional methods for MRD detection. Immunohistochemistry staining to identify and monitor biomarkers |
| ddPCR | Digital PCR (dPCR) divides a bulk PCR reaction into millions of nanolitre-scale microreactions, each containing zero, one, or only a few DNA molecules. Absolute quantification of the material by dPCR is accomplished by counting positive nano responses and using Poisson statistics. | Costly; ultra-sensitive; no need for a calibrator for quantification. Absolut quantification; fast. | False-positive and negative results. Requires a skilled technician. Not routinely available in many clinical laboratories. Sophisticated instruments are required | It is an ultrasensitive method for detecting pre-defined mutations. MRD quantification and treatment monitoring are possible without the need for a calibrator curve. |
| NGS | Next-generation sequencing (NGS) is a massively parallel sequencing technology to large-scale DNA sequencing. | Applicable to all known and new mutations. | Well-trained technicians are needed. Analysing the results is time-consuming. Costly, requires high DNA input, low sensitivity, rapid turnaround times. | Unlike other methods, it is possible to analyse at the genome-wide level. |
| Biosensor | Simple; ultra-sensitivity; disposable test. Applicable as a POCT test. | Clinically not well validated. False result | As a new and low-cost method, it can perform tests at consecutive times. Different bioreceptors can detect MRD on liquid biopsy. As a POCT test, it is possible to perform the test at the bedside. |
| Detection Method | Sample | Biomarkers | Biosensor | Limit of Defection (LoD) | References |
|---|---|---|---|---|---|
| Electrochemical | Synthetic lncRNAs | MALAT1 | SPCE | 42.8 fM | [40] |
| Saliva and plasma | EGFR L858R and exon 19 del mutations | EFIRM | NA | [41] | |
| Small extracellular vesicles (sEVs) | EGFR | Electrokinetic Sensor | 2.8 × 108 particles/mL | [42] | |
| Serum and saliva | - | EFIRM | NA | [43] | |
| Synthetic DNA | EGFR | sandwich-assays | NA | [44] | |
| Serum | CYFRA21-1 and CEA | 3D graphene (3D-G), poly-thionine (pThi) and poly-m-Cresol purple (pMCP) | 0.18 ng/mL (CYFRA21-1) 0.31 ng/mL (CEA) | [45] | |
| Serum | CYFRA21-1 | Microfluidic | 0.026 pg mL−1 | [46] | |
| Serum | CYFRA 21-1 | ECL and eATRP signal amplification | 0.8 fg mL−1 | [47] | |
| Exosomes | EGFR and PD-L1 | Electro-kinetic | 4.9 × 106 particles/mL | [48] | |
| SPR | Exosomes miRNAs | miRNA-21, 378, 200, 139 | SPR | 1.68 fM | [49] |
| Exosomes | Anti-EGFR and anti-EpCAM | SPRi | 2.37 × 104 particles/μL | [50] | |
| Serum (Protein) | ALCAM, TAGLN2 | SPR imaging sensor with polarisation contrast | 6 ng/mL (ALCAM) 3 ng/mL (TAGLN2) | [51] | |
| Exosomes | EGFR, PD-L1 | Nanoplasmonic exosome (nPLEX) assay | 9.258 × 103%/RIU | [52] | |
| SERS | Serum | Cytochrome c (Cyt c) | Aptasensor | 1.79 pg/mL (Serum) 1.148 pg/mL (PBS) | [53] |
| CTCs | EpCAM | antibody-adsorbed nitrocellulose membrane | NA | [54] | |
| Fluorescence | Tumour-derived exosomes (Plasma) | IGF-1R | Microfluidic device | 0.28–0.38 pg/mL | [55] |
| cell extracts | CDK6 Kinase | fluorescent peptide biosensor | NA | [56] | |
| Magnetic | CTCs | EGFR | Immunomagnetic and Magnetic Sifter | NA | [57] |
| Aptamer | Exosomes | Identification of A549 exosomes | lateral flow aptamer assay | 6.4 × 109 particles/mL | [58] |
| Micronuclear magnetic resonance | CTCs | EGFR, EpCAM, HER-2, MUC-1 | μNMR | NA | [59] |
| colorimetric | Serum | Monitoring soluble immune checkpoints (PD-L1, PD-1), (LAG-3) | microfluidic sandwich immunoassay (multiplexed immune checkpoint biosensor (MICB) | 5 pg mL−1 (PD-1 and PD-L1) 50 pg mL−1 (LAG-3) | [60] |
| Transduce | Principle | Advantages | Disadvantages | Application in Lung Cancer MRD |
|---|---|---|---|---|
| Electrochemical sensor | Convert the biochemical interaction to electrical signals |
|
|
|
| Magnetic biosensor | Applying paramagnetic particles to detect biological interactions by monitoring magnetic property changes |
|
|
|
| Surface-enhanced Raman spectroscopy (SERS) | Using molecules adsorbing on rough metal surfaces to generate Raman scattering |
|
|
|
| Surface plasmon resonance biosensors (SPR) | Alteration of SPR angle due to increasing of refractive index during binding of biomolecules on the sensor surface |
|
|
|
| Fluorescence-based biosensing | An analytical signal of a photoluminescence emission mechanism |
|
|
|
| Lateral flow immunoassay (LFIA) | Optical properties |
|
|
|
| Chemiluminescence | Electrochemistry and visual luminescence measurements |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardarabadi, P.; Kojabad, A.A.; Jafari, D.; Liu, C.-H. Liquid Biopsy-Based Biosensors for MRD Detection and Treatment Monitoring in Non-Small Cell Lung Cancer (NSCLC). Biosensors 2021, 11, 394. https://doi.org/10.3390/bios11100394
Sardarabadi P, Kojabad AA, Jafari D, Liu C-H. Liquid Biopsy-Based Biosensors for MRD Detection and Treatment Monitoring in Non-Small Cell Lung Cancer (NSCLC). Biosensors. 2021; 11(10):394. https://doi.org/10.3390/bios11100394
Chicago/Turabian StyleSardarabadi, Parvaneh, Amir Asri Kojabad, Davod Jafari, and Cheng-Hsien Liu. 2021. "Liquid Biopsy-Based Biosensors for MRD Detection and Treatment Monitoring in Non-Small Cell Lung Cancer (NSCLC)" Biosensors 11, no. 10: 394. https://doi.org/10.3390/bios11100394
APA StyleSardarabadi, P., Kojabad, A. A., Jafari, D., & Liu, C.-H. (2021). Liquid Biopsy-Based Biosensors for MRD Detection and Treatment Monitoring in Non-Small Cell Lung Cancer (NSCLC). Biosensors, 11(10), 394. https://doi.org/10.3390/bios11100394






