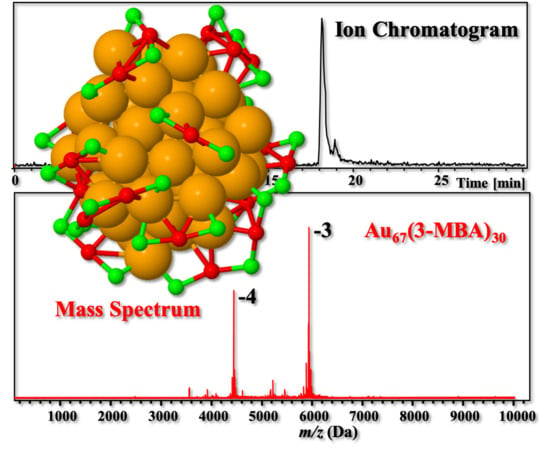
New Evidence of the Bidentate Binding Mode in 3-MBA Protected Gold Clusters: Analysis of Aqueous 13–18 kDa Gold-Thiolate Clusters by HPLC-ESI-MS Reveals Special Compositions Aun(3-MBA)p, (n = 48–67, p = 26–30)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. 3-MBA/Au System Characterization
2.2.1. Coupled Chromatography—ESI-MS
2.2.2. HP-LC–ESI-MS Sample Preparation
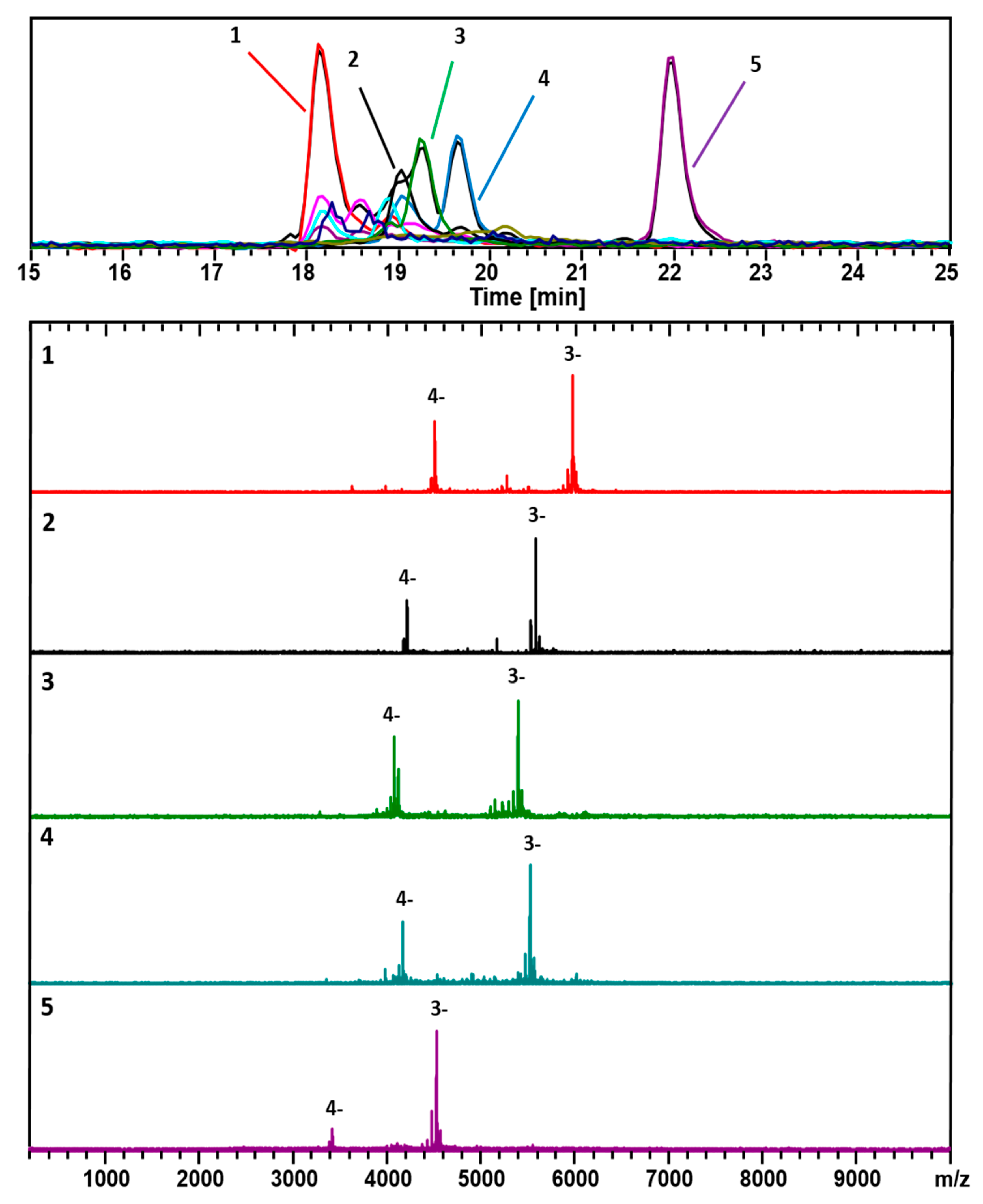
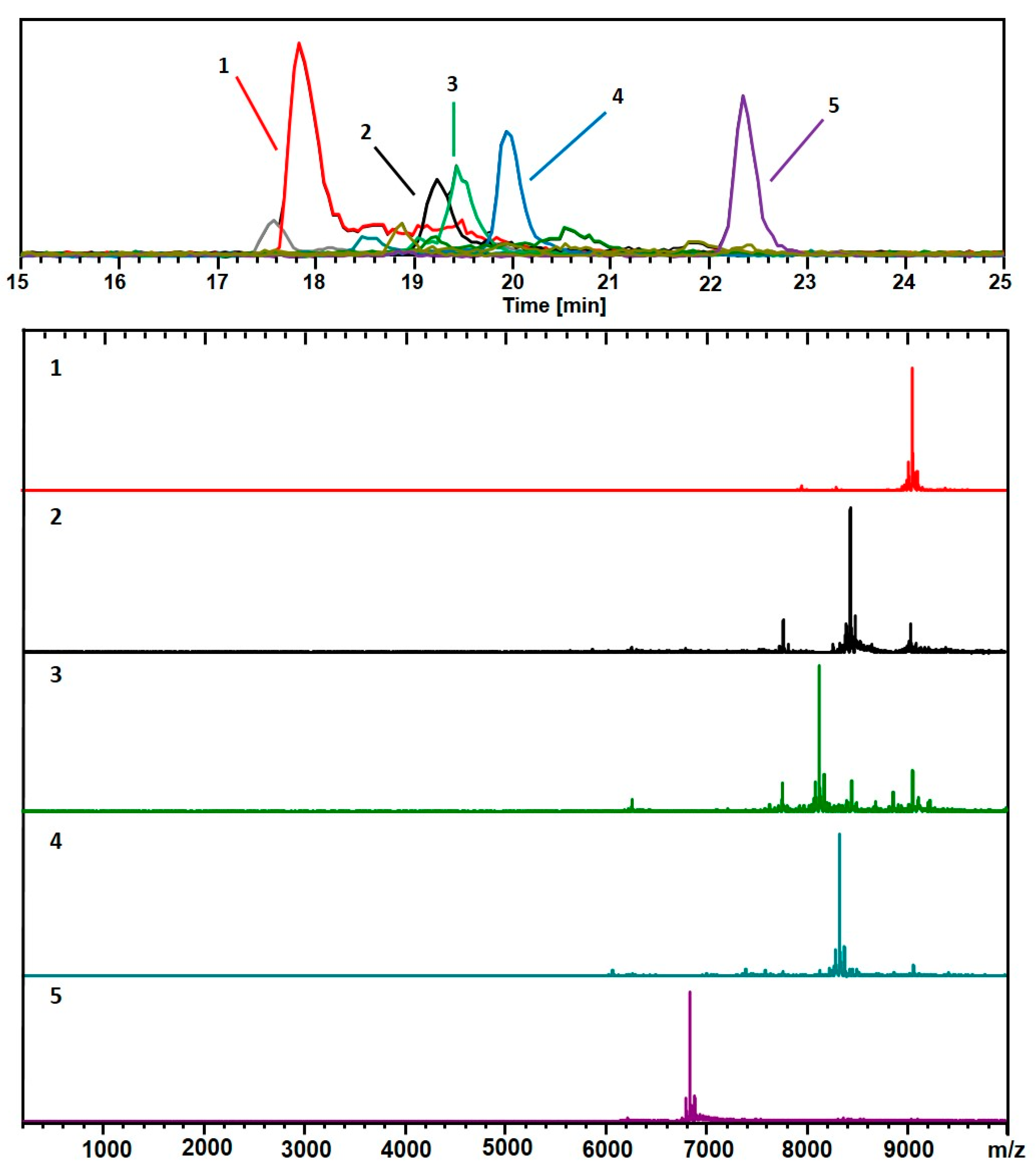
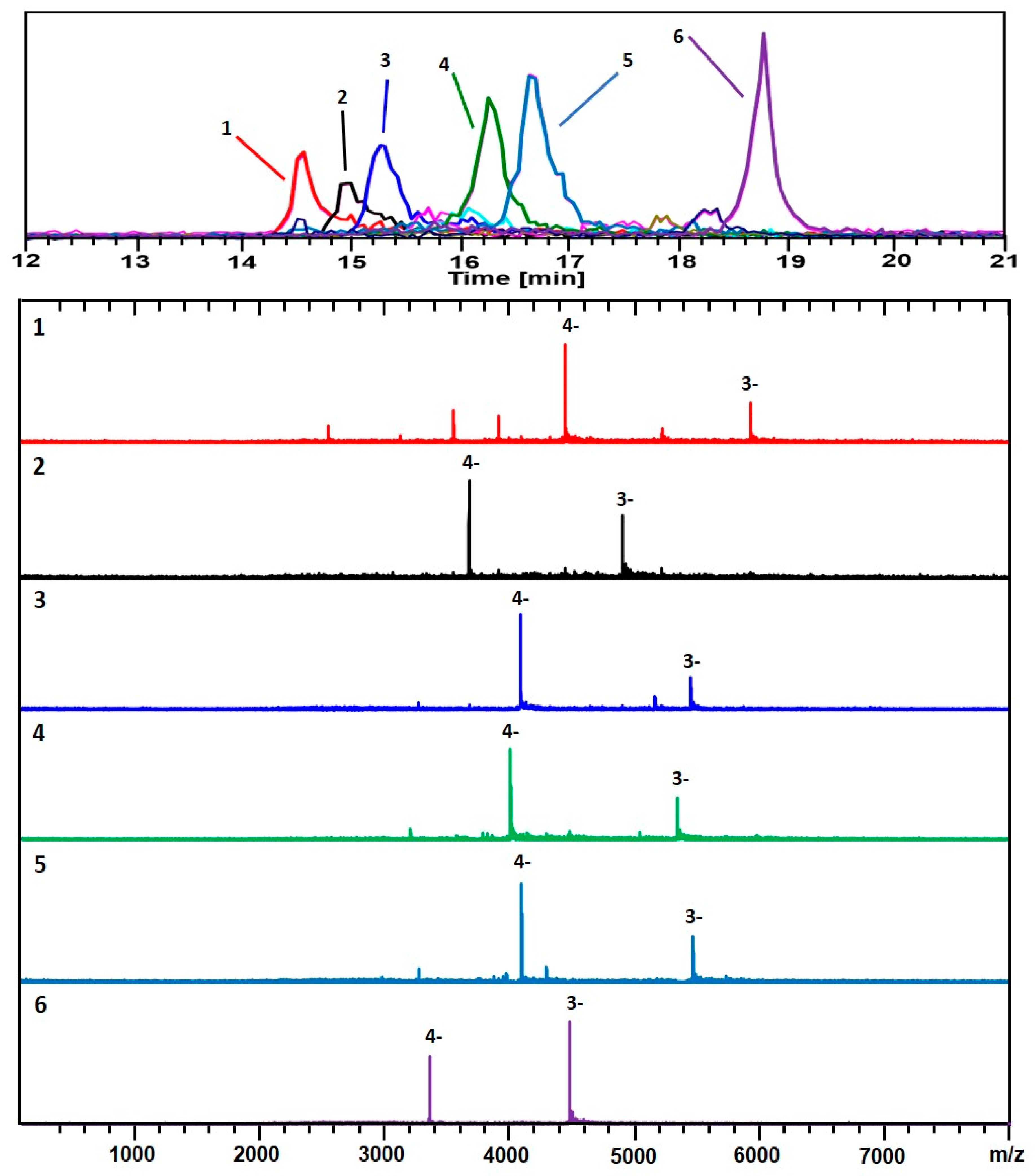
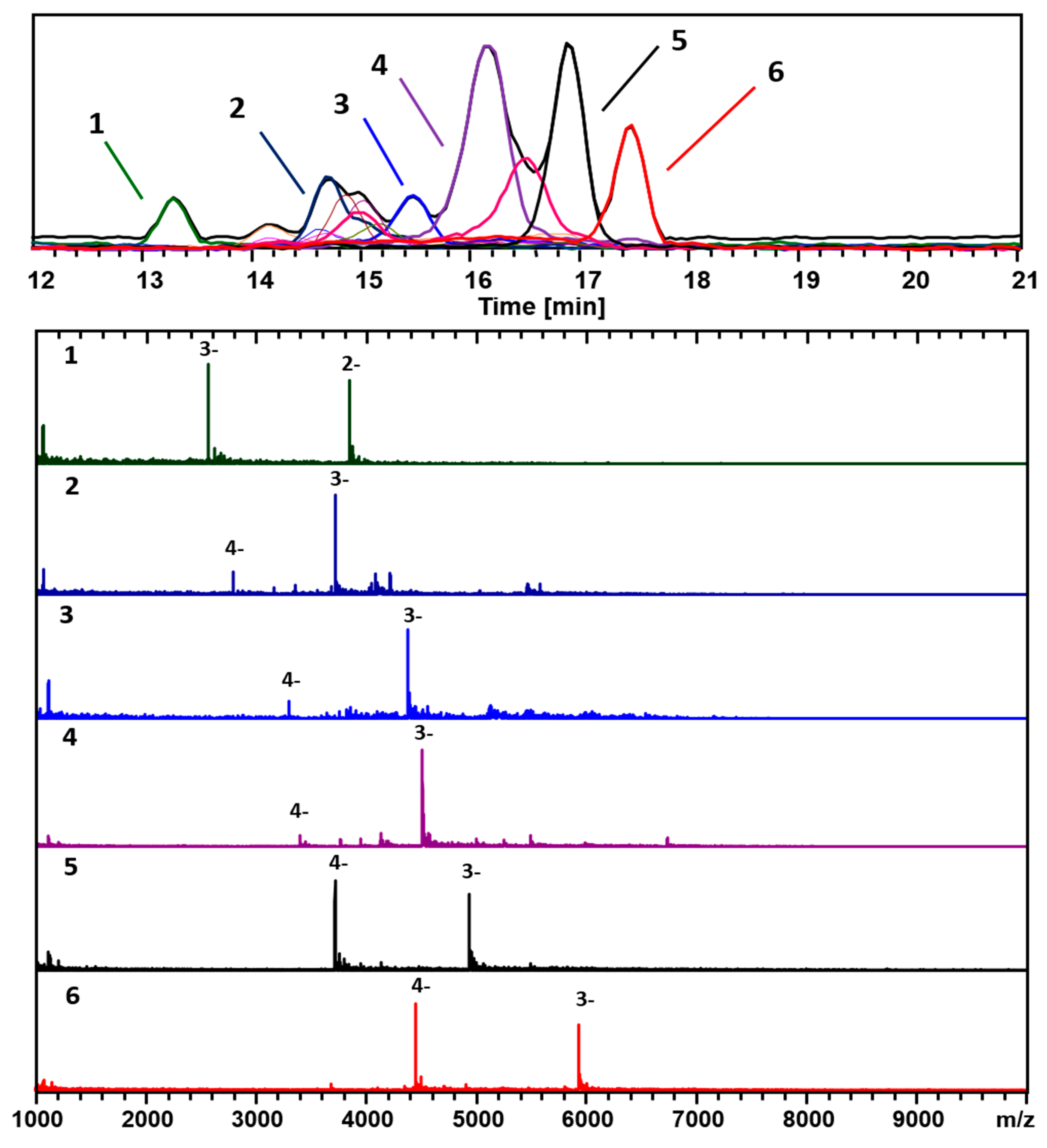
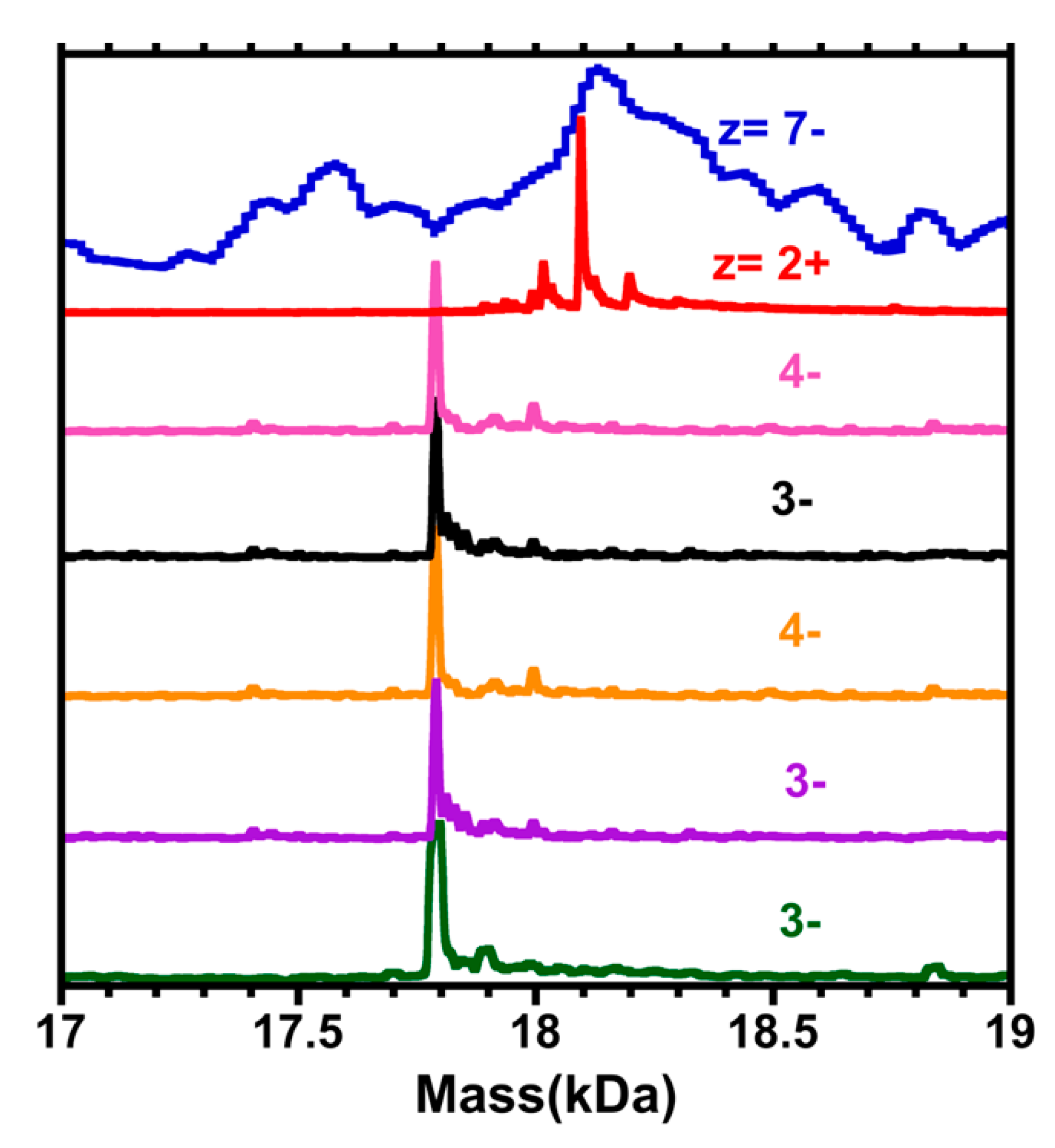
3. Results
4. Discussion
4.1. General Remarks
4.2. Contrasting 3-MBA (or Meta-MBA) and 4-MBA (Aka Para-MBA)
- There has been no total-structure determination of any 3-MBA protected gold clusters.
- There has been no adequately resolved ESI-MS identification of any of these: no composition-determination by any standard analytical method.
- Electron microscopy (or diffraction) provides the gold structure and atom count, in both (2) reported cases. (Ligands/S-atoms are not located by this method). Models are then constructed, which include the ligands, and these are tested (refined) by DFT computations.
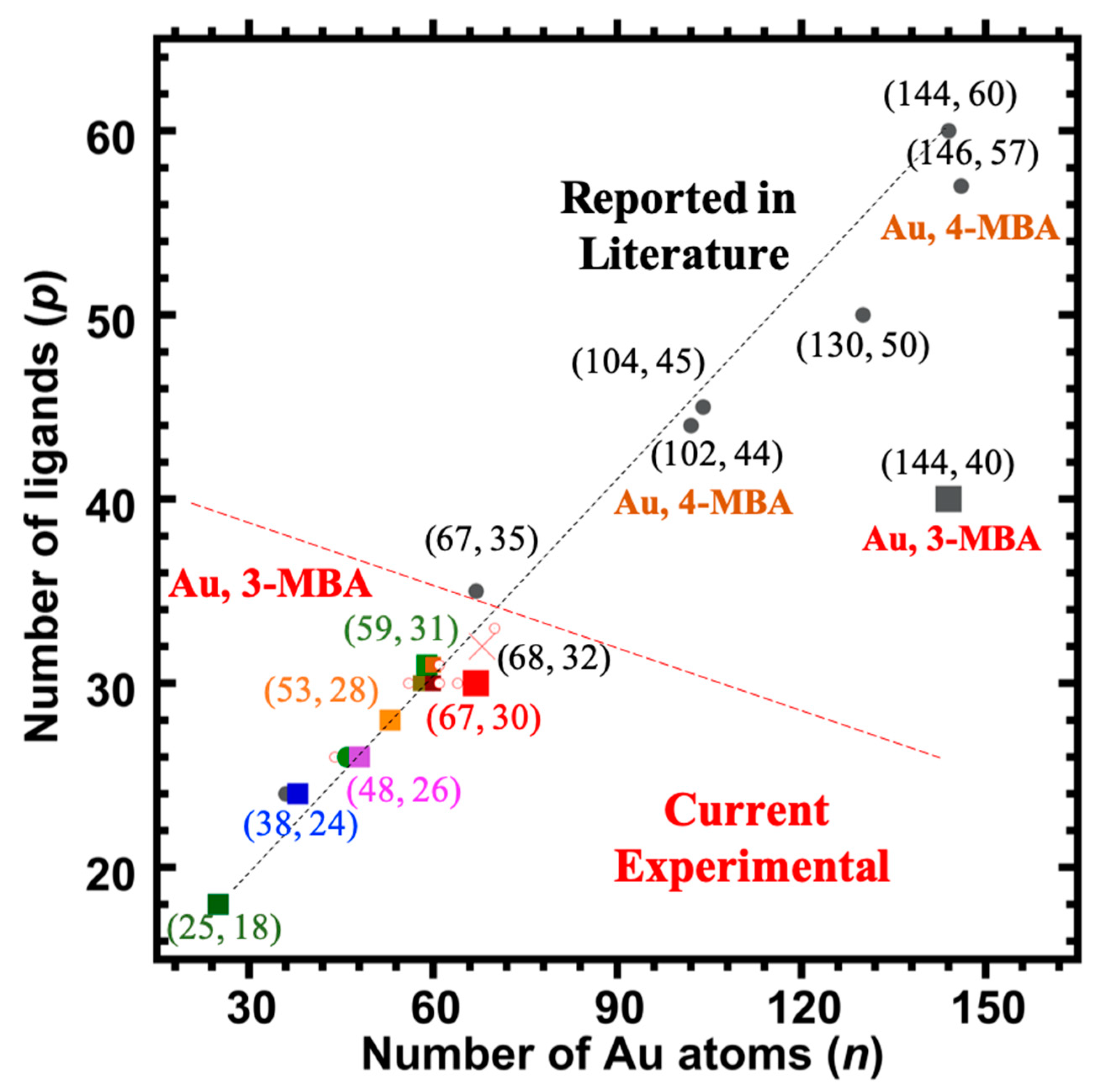
- The compositions arrived at by these procedures, (68, 32) and (144, ~40), are respectively distinctly and strikingly different from those determined previously for aliphatic ligands, i.e., (67, 35) and (144, 60), or from the more directly relevant water-soluble aromatic pMBA ligand (146, 57). [Figure 6 presents these compositions in a graphical format.]
- In the carbonyl (C=O) stretching region, the vibrational FTIR spectra show “distinct peak[s] around 1730 cm−1, observable only in 3-MBA-passivated clusters, and interpreted as the signal of the O=C−OH···Au interaction.” [4].
- Molecular dynamic (MD) simulations were based on structure models for each cluster. “Visual inspection of MD trajectories revealed several weak interactions in the ligand layer and at the ligand−gold interface, such as formation of inter-ligand hydrogen bonds, inter-ligand π stacking (aromatic contacts), π−Au interaction where the aromatic ring lies “flat” on the gold core, and hydrogen bonding-like O=C−OH···Au interaction when the hydroxyl group is rotated toward the gold core.” “We thus assigned the highest frequency observed for both Au144(3-MBA)∼40 and Au68(3-MBA)32 to the O=C−OH···Au interaction visualized … This interaction at the ligand−metal interface has not been reported before for any thiolate protected gold nanocluster.” [4].
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Azubel, M.; Koivisto, J.; Malola, S.; Bushnell, D.; Hura, G.L.; Koh, A.L.; Tsunoyama, H.; Tsukuda, T.; Pettersson, M.; Häkkinen, H.; et al. Electron microscopy of gold nanoparticles at atomic resolution. Science 2014, 345, 909–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azubel, M.; Kornberg, R.D. Synthesis of water-soluble, thiolate-protected gold nanoparticles uniform in size. Nano. Lett. 2016, 16, 3348–3351. [Google Scholar] [CrossRef] [PubMed]
- Azubel, M.; Koh, A.L.; Koyasu, K.; Tsukuda, T.; Kornberg, R.D. Structure determination of a water-soluble 144-gold atom particle at atomic resolution by aberration-corrected electron microscopy. ACS Nano 2017, 11, 11866–11871. [Google Scholar] [CrossRef] [PubMed]
- Tero, T.-R.; Malola, S.; Koncz, B.; Pohjolainen, E.; Lautala, S.; Mustalahti, S.; Permi, P.; Groenhof, G.; Pettersson, M.; Häkkinen, H. Dynamic stabilization of the ligand–metal interface in atomically precise gold nanoclusters Au68 and Au144 protected by meta-mercaptobenzoic acid. ACS Nano 2017, 11, 11872–11879. [Google Scholar] [CrossRef] [PubMed]
- Vergara, S.; Lukes, D.A.; Martynowycz, M.W.; Santiago, U.; Plascencia-Villa, G.; Weiss, S.C.; de la Cruz, M.J.; Black, D.M.; Alvarez, M.M.; López-Lozano, X.; et al. MicroED Structure of Au146(p-MBA)57 at Subatomic Resolution Reveals a Twinned FCC Cluster. J. Phys. Chem. Lett. 2017, 8, 5523–5530. [Google Scholar] [CrossRef] [PubMed]
- Kumara, C.; Hoque, M.M.; Zuo, X.; Cullen, D.A.; Whetten, R.L.; Dass, A. Isolation of a 300-kDa, Au~1400 Gold Compound, the Standard 3.6-nm Capstone to a Series of Plasmonic Nanocrystals Protected by Aliphatic-Like-Thiolates. J. Phys. Chem. Lett. 2018, 9, 6825–6832. [Google Scholar] [CrossRef] [PubMed]
- Aikens, C.M. Electronic and Geometric Structure, Optical Properties, and Excited State Behavior in Atomically Precise Thiolate-Stabilized Noble Metal Nanoclusters. Acc. Chem. Res. 2018, 51, 3065–3073. [Google Scholar] [CrossRef]
- Templeton, A.C.; Wuelfing, W.P.; Murray, R.W. Monolayer-Protected Cluster Molecules. Acc. Chem. Res. 2000, 33, 27–36. [Google Scholar] [CrossRef]
- Hoque, M.M.; Mayer, K.M.; Ponce, A.; Alvarez, M.M.; Whetten, R.L. Toward Smaller Aqueous-Phase Plasmonic Gold Nanoparticles: High-Stability Thiolate-Protected ∼4.5 nm Cores. Langmuir 2019, 35, 10610–10617. [Google Scholar] [CrossRef]
- Huang, Z.; Ishida, Y.; Narita, K.; Yonezawa, T. Kinetics of Cationic-Ligand-Exchange Reactions in Au25 Nanoclusters. J. Phys. Chem. C 2018, 122, 18142–18150. [Google Scholar] [CrossRef]
- Ishida, Y.; Narita, K.; Yonezawa, T.; Whetten, R.L. Fully Cationized Gold Clusters: Synthesis of Au25(SR+)18. J. Phys. Chem. Lett. 2016, 7, 3718–3722. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Suzuki, J.; Akita, I.; Yonezawa, T. Ultrarapid Cationization of Gold Nanoparticles via a Single-Step Ligand Exchange Reaction. Langmuir 2018, 34, 10668–10672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, E.; Powell, R.; Furuya, F.; Hainfeld, J.; Schaaff, T.; Shafigullin, M.; Stephens, P.; Whetten, R. Greengold, a giant cluster compound of unusual electronic structure. Eur. Phys. J. D 1999, 9, 647–651. [Google Scholar] [CrossRef]
- Nieto-Ortega, B.; Bürgi, T. Vibrational Properties of Thiolate-Protected Gold Nanoclusters. Acc. Chem. Res. 2018, 51, 2811–2819. [Google Scholar] [CrossRef] [PubMed]
- Hesari, M.; Ding, Z. A grand avenue to Au nanocluster electrochemiluminescence. Acc. Chem. Res. 2017, 50, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Bauld, R.; Hesari, M.; Workentin, M.S.; Fanchini, G. Tessellated gold nanostructures from Au144(SCH2 CH2Ph)60 molecular precursors and their use in organic solar cell enhancement. Nanoscale 2014, 6, 7570–7575. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.M.; Dass, A.; Mayer, K.M.; Whetten, R.L. Protein-Like Large Gold Clusters Based on the ω-Aminothiolate DMAET: Precision Thermal and Reaction Control Leading to Selective Formation of Cationic Gold Clusters in the Critical Size Range, n = 130–144 Gold Atoms. J. Phys. Chem. C 2019, 123, 14871–14879. [Google Scholar] [CrossRef]
- Hoque, M.M.; Black, D.M.; Mayer, K.M.; Dass, A.; Whetten, R.L. Base Side of Noble Metal Clusters: Efficient Route to Captamino-Gold, Aun(−S(CH2)2N(CH3)2)p, n = 25–144. J. Phys. Chem. Lett. 2019, 10, 3307–3311. [Google Scholar] [CrossRef] [PubMed]
- Whetten, R.L.; Weissker, H.-C.; Pelayo, J.J.; Mullins, S.M.; López-Lozano, X.; Garzón, I.L. Chiral-Icosahedral (I) Symmetry in Ubiquitous Metallic Cluster Compounds (145A,60X): Structure and Bonding Principles. Acc. Chem. Res. 2019, 52, 34–43. [Google Scholar] [CrossRef]
- Sakthivel, N.A.; Dass, A. Aromatic Thiolate-Protected Series of Gold Nanomolecules and a Contrary Structural Trend in Size Evolution. Acc. Chem. Res. 2018, 51, 1774–1783. [Google Scholar] [CrossRef]
- Rambukwella, M.; Sakthivel, N.A.; Delcamp, J.H.; Sementa, L.; Fortunelli, A.; Dass, A. Ligand Structure Determines Nanoparticles’ Atomic Structure, Metal-Ligand Interface and Properties. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Xia, N.; Liao, L.; Zhu, M.; Jin, F.; Jin, R.; Wu, Z. Unraveling the long-pursued Au144 structure by x-ray crystallography. Sci. Adv. 2018, 4, eaat7259. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.M.; Vergara, S.; Das, P.P.; Ugarte, D.; Santiago, U.; Kumara, C.; Whetten, R.L.; Dass, A.; Ponce, A. Structural Analysis of Ligand-Protected Smaller Metallic Nanocrystals by Atomic Pair Distribution Function under Precession Electron Diffraction. J. Phys. Chem. C 2019, 123, 19894–19902. [Google Scholar] [CrossRef]
- Sakthivel, N.A.; Theivendran, S.; Ganeshraj, V.; Oliver, A.G.; Dass, A. Crystal Structure of Faradaurate-279: Au279(SPh-tBu)84 Plasmonic Nanocrystal Molecules. J. Am. Chem. Soc. 2017, 139, 15450–15459. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Pradeep, T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef]
- Black, D.M.; Alvarez, M.M.; Yan, F.; Griffith, W.P.; Plascencia-Villa, G.; Bach, S.B.; Whetten, R.L. Triethylamine Solution for the Intractability of Aqueous Gold–Thiolate Cluster Anions: How Ion Pairing Enhances ESI-MS and HPLC of aq-Aun(pMBA)p. J. Phys. Chem. C 2016, 121, 10851–10857. [Google Scholar] [CrossRef]
- Knoppe, S.; Boudon, J.; Dolamic, I.; Dass, A.; Bürgi, T. Size exclusion chromatography for semipreparative scale separation of Au38(SR)24 and Au40(SR)24 and larger clusters. Anal. Chem. 2011, 83, 5056–5061. [Google Scholar] [CrossRef]
- Gies, A.P.; Hercules, D.M.; Gerdon, A.E.; Cliffel, D.E. Electrospray Mass Spectrometry Study of Tiopronin Monolayer-Protected Gold Nanoclusters. J. Am. Chem. Soc. 2007, 129, 1095–1104. [Google Scholar] [CrossRef]
- Ghosh, A.; Hassinen, J.; Pulkkinen, P.; Tenhu, H.; Ras, R.H.; Pradeep, T. Simple and efficient separation of atomically precise noble metal clusters. Anal. Chem. 2014, 86, 12185–12190. [Google Scholar] [CrossRef]
- Schaaff, T.G.; Knight, G.; Shafigullin, M.N.; Borkman, R.F.; Whetten, R.L. Isolation and selected properties of a 10.4 kDa gold: Glutathione cluster compound. J. Phys. Chem. B 1998, 102, 10643–10646. [Google Scholar] [CrossRef]
- Choi, M.M.; Douglas, A.D.; Murray, R.W. Ion-pair chromatographic separation of water-soluble gold monolayer-protected clusters. Anal. Chem. 2006, 78, 2779–2785. [Google Scholar] [CrossRef] [PubMed]
- Niihori, Y.; Shima, D.; Yoshida, K.; Hamada, K.; Nair, L.V.; Hossain, S.; Kurashige, W.; Negishi, Y. High-performance liquid chromatography mass spectrometry of gold and alloy clusters protected by hydrophilic thiolates. Nanoscale 2018, 10, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Niihori, Y.; Kikuchi, Y.; Shima, D.; Uchida, C.; Sharma, S.; Hossain, S.; Kurashige, W.; Negishi, Y. Separation of Glutathionate-Protected Gold Clusters by Reversed-Phase Ion-Pair High-Performance Liquid Chromatography. Ind. Eng. Chem. Res. 2017, 56, 1029–1035. [Google Scholar] [CrossRef]
- Alvarez, M.M.; Chen, J.; Plascencia-Villa, G.; Black, D.M.; Griffith, W.P.; Garzón, I.L.; José-Yacamán, M.; Demeler, B.; Whetten, R.L. Hidden Components in Aqueous “Gold-144” Fractionated by PAGE: High-Resolution Orbitrap ESI-MS Identifies the Gold-102 and Higher All-Aromatic Au-pMBA Cluster Compounds. J. Phys. Chem. B 2016, 120, 6430–6438. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Robles, G.; Lopez, P.; Bach, S.B.H.; Alvarez, M.; Whetten, R.L. Liquid Chromatography Separation and Mass Spectrometry Detection of Silver-Lipoate Ag29(LA)12 Nanoclusters: Evidence of Isomerism in the Solution Phase. Anal. Chem. 2018, 90, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.; Yao, Q.; Chen, T.; Xie, J. Mechanistic exploration and controlled synthesis of precise thiolate-gold nanoclusters. Coord. Chem. Rev. 2016, 329, 1–15. [Google Scholar] [CrossRef]
- Lopez, P.; Lara, H.H.; Mullins, M.S.; Black, M.D.; Ramsower, H.M.; Alvarez, M.M.; Williams, T.L.; Lopez-Lozano, X.; Weissker, H.C.; García, A.P.; et al. Tetrahedral (T) Closed-Shell Cluster of 29 Silver Atoms & 12 Lipoate Ligands, [Ag29(R-α-LA)12](3−): Antibacterial and Antifungal Activity. ACS Appl. Nano. Mater. 2018, 1, 1595–1602. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Black, D.M.; Hoque, M.M.; Plascencia-Villa, G.; Whetten, R.L. New Evidence of the Bidentate Binding Mode in 3-MBA Protected Gold Clusters: Analysis of Aqueous 13–18 kDa Gold-Thiolate Clusters by HPLC-ESI-MS Reveals Special Compositions Aun(3-MBA)p, (n = 48–67, p = 26–30). Nanomaterials 2019, 9, 1303. https://doi.org/10.3390/nano9091303
Black DM, Hoque MM, Plascencia-Villa G, Whetten RL. New Evidence of the Bidentate Binding Mode in 3-MBA Protected Gold Clusters: Analysis of Aqueous 13–18 kDa Gold-Thiolate Clusters by HPLC-ESI-MS Reveals Special Compositions Aun(3-MBA)p, (n = 48–67, p = 26–30). Nanomaterials. 2019; 9(9):1303. https://doi.org/10.3390/nano9091303
Chicago/Turabian StyleBlack, David M., M. Mozammel Hoque, Germán Plascencia-Villa, and Robert L. Whetten. 2019. "New Evidence of the Bidentate Binding Mode in 3-MBA Protected Gold Clusters: Analysis of Aqueous 13–18 kDa Gold-Thiolate Clusters by HPLC-ESI-MS Reveals Special Compositions Aun(3-MBA)p, (n = 48–67, p = 26–30)" Nanomaterials 9, no. 9: 1303. https://doi.org/10.3390/nano9091303
APA StyleBlack, D. M., Hoque, M. M., Plascencia-Villa, G., & Whetten, R. L. (2019). New Evidence of the Bidentate Binding Mode in 3-MBA Protected Gold Clusters: Analysis of Aqueous 13–18 kDa Gold-Thiolate Clusters by HPLC-ESI-MS Reveals Special Compositions Aun(3-MBA)p, (n = 48–67, p = 26–30). Nanomaterials, 9(9), 1303. https://doi.org/10.3390/nano9091303