Application of CuxO-FeyOz Nanocatalysts in Ethynylation of Formaldehyde
Abstract
1. Introduction
2. Materials and Methods
2.1. Preperation of Catalysts
2.2. Characterization of Catalysts
2.3. Catalyst Test of Catalysts
3. Results
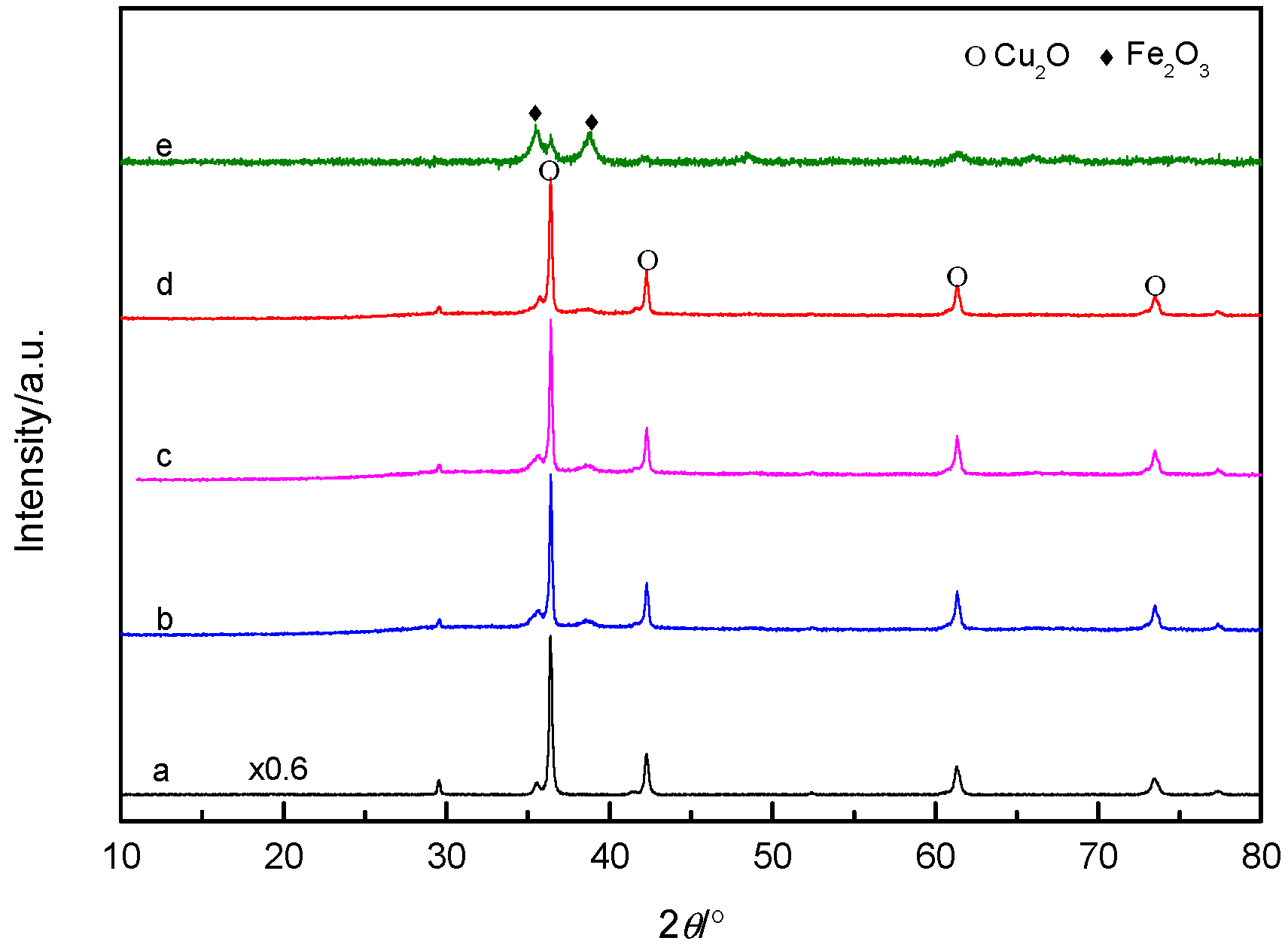
3.1. Structure Analysis of Catalysts
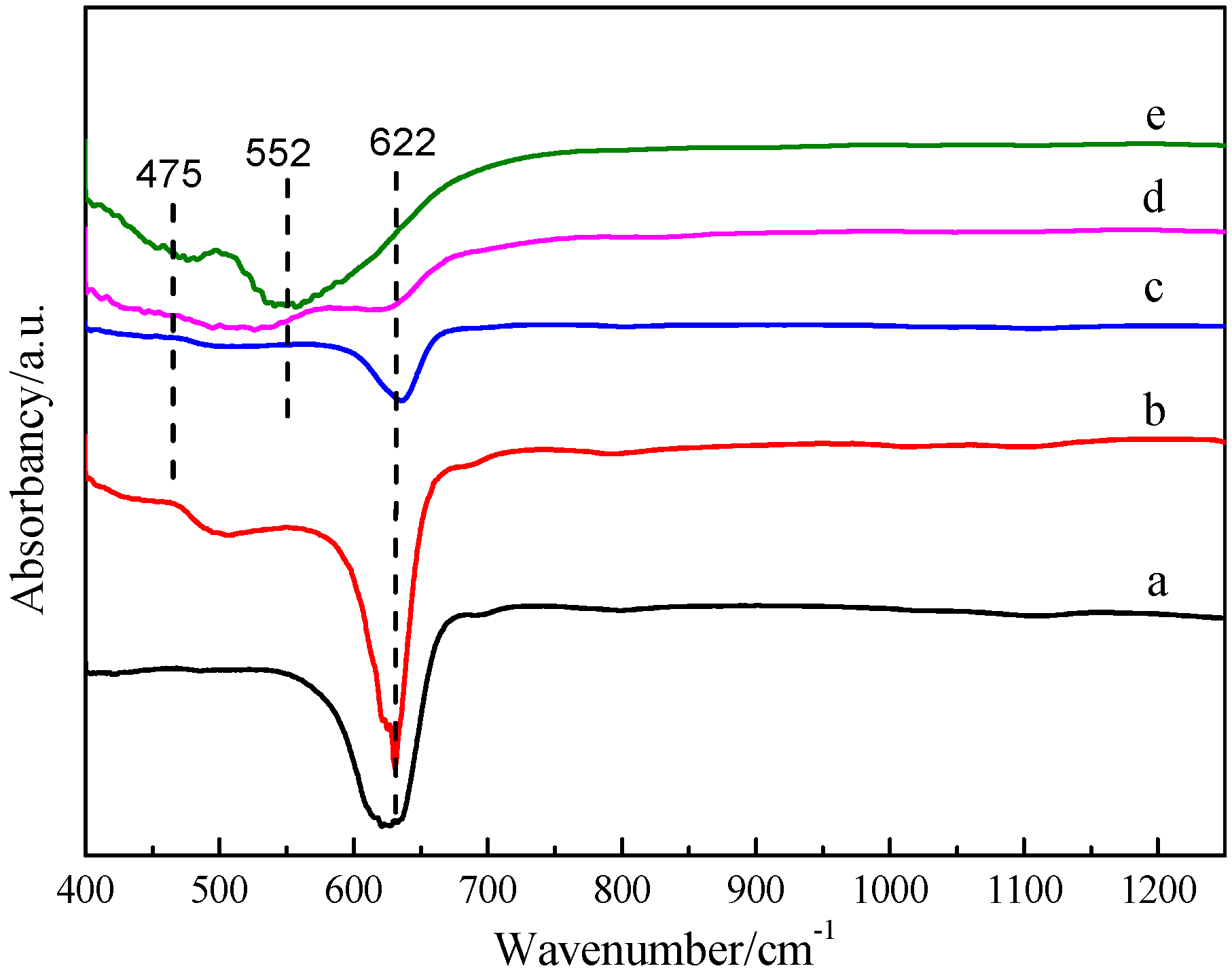
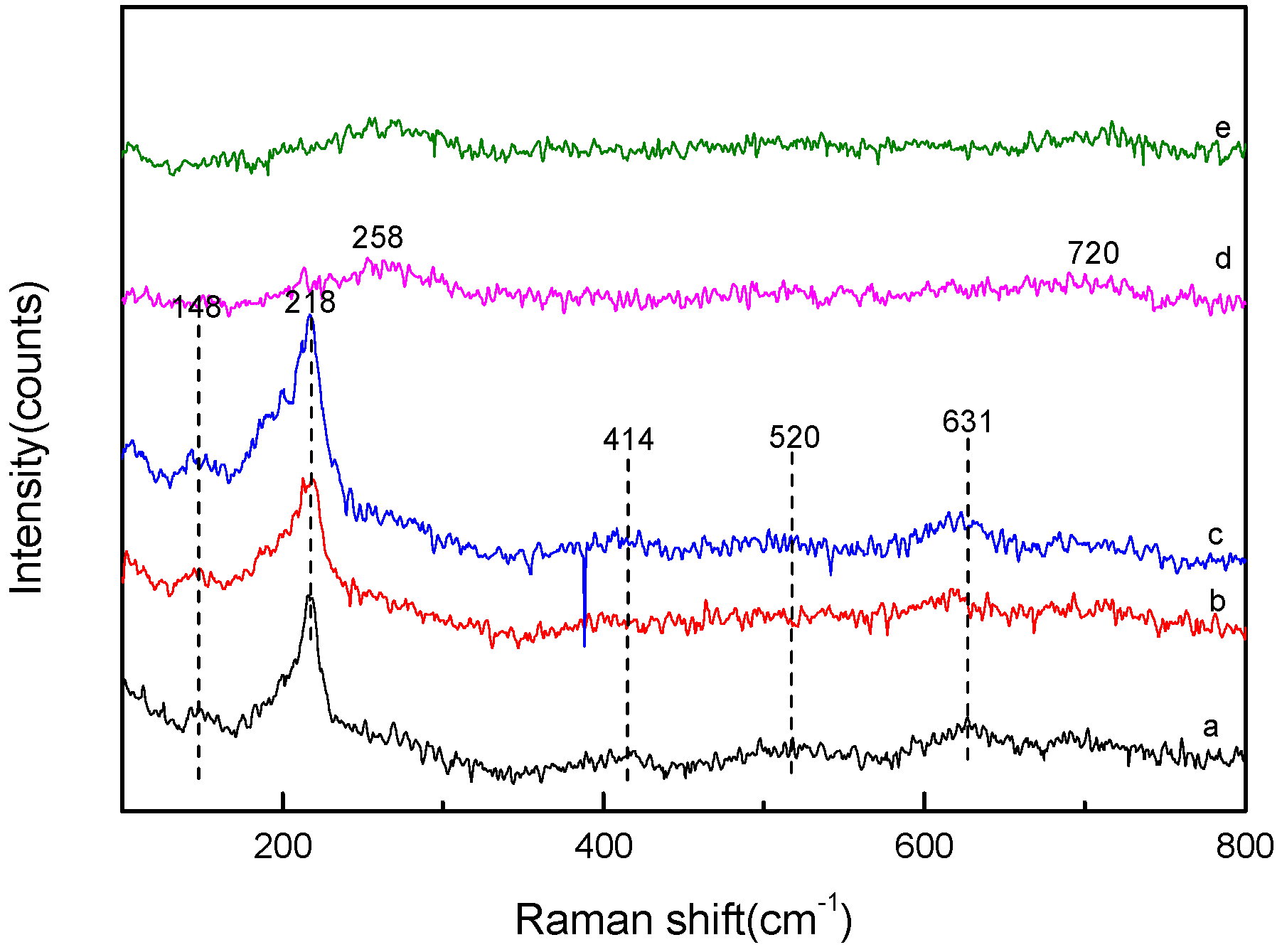
3.2. FT-IR Analysis and Raman Analysis
3.3. Morphological Analysis
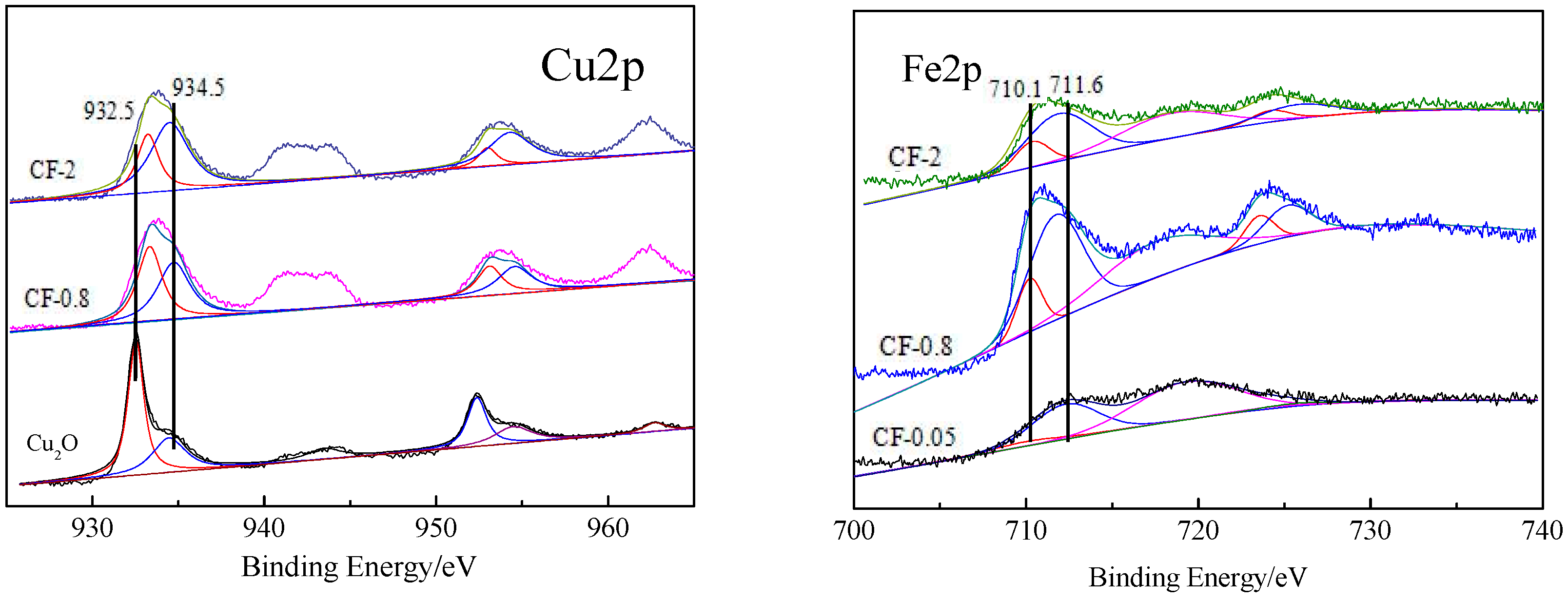
3.4. Surface Composition and Valence Analysis of the Catalyst
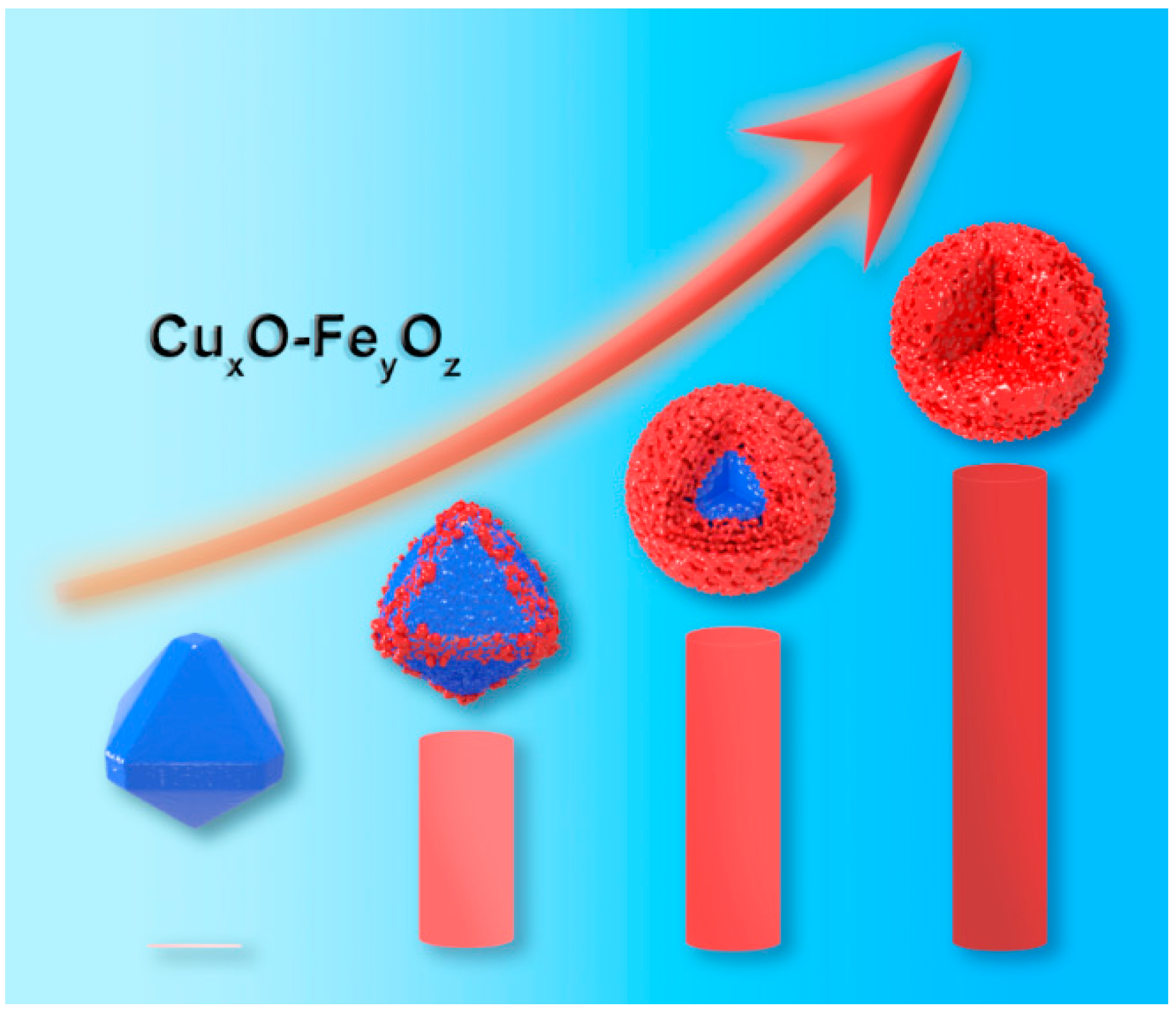
3.5. Discussion on the Formation Mechanism of CuxO-FeyOz Structure
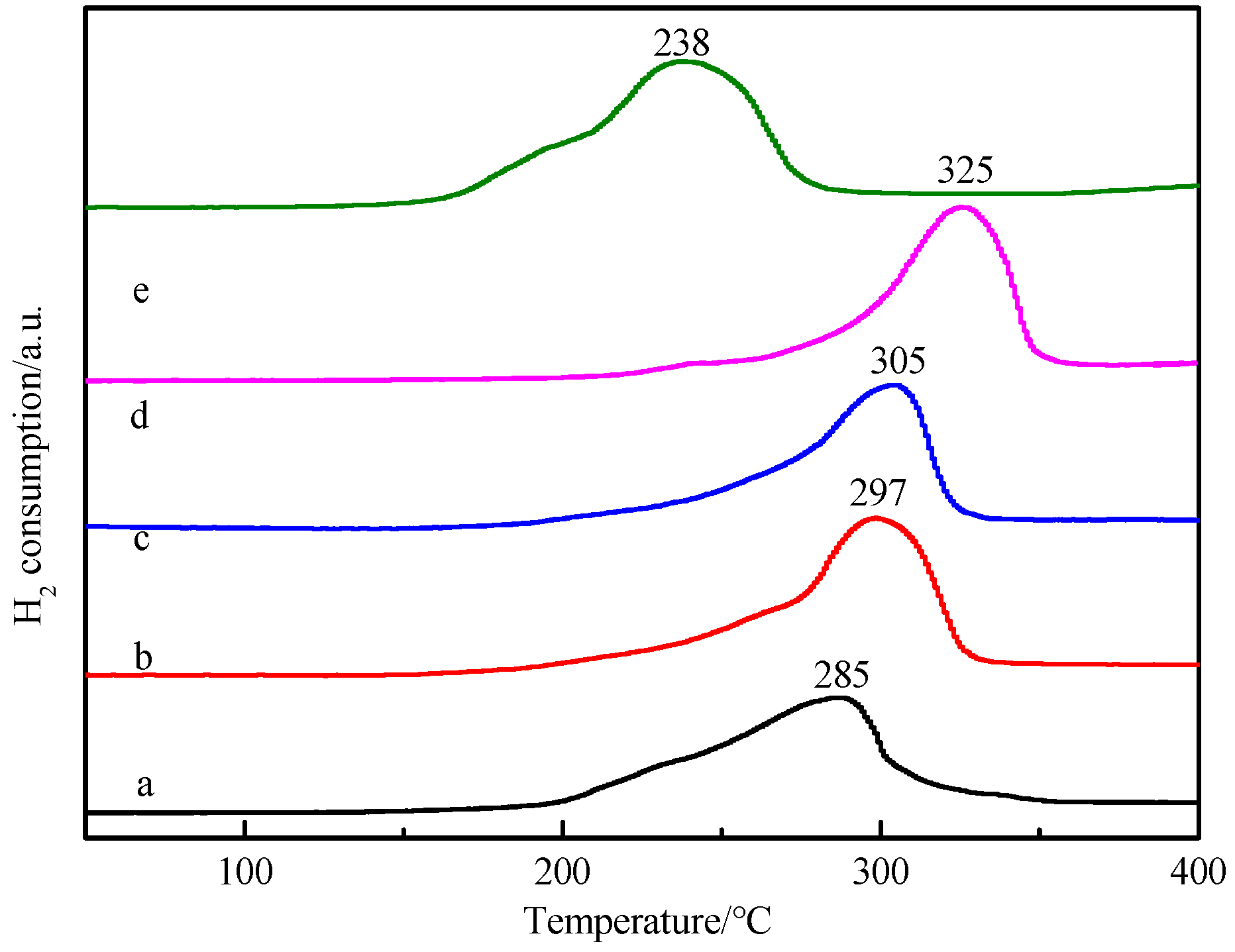
3.6. Analysis of Reduction Behavior of the Catalyst
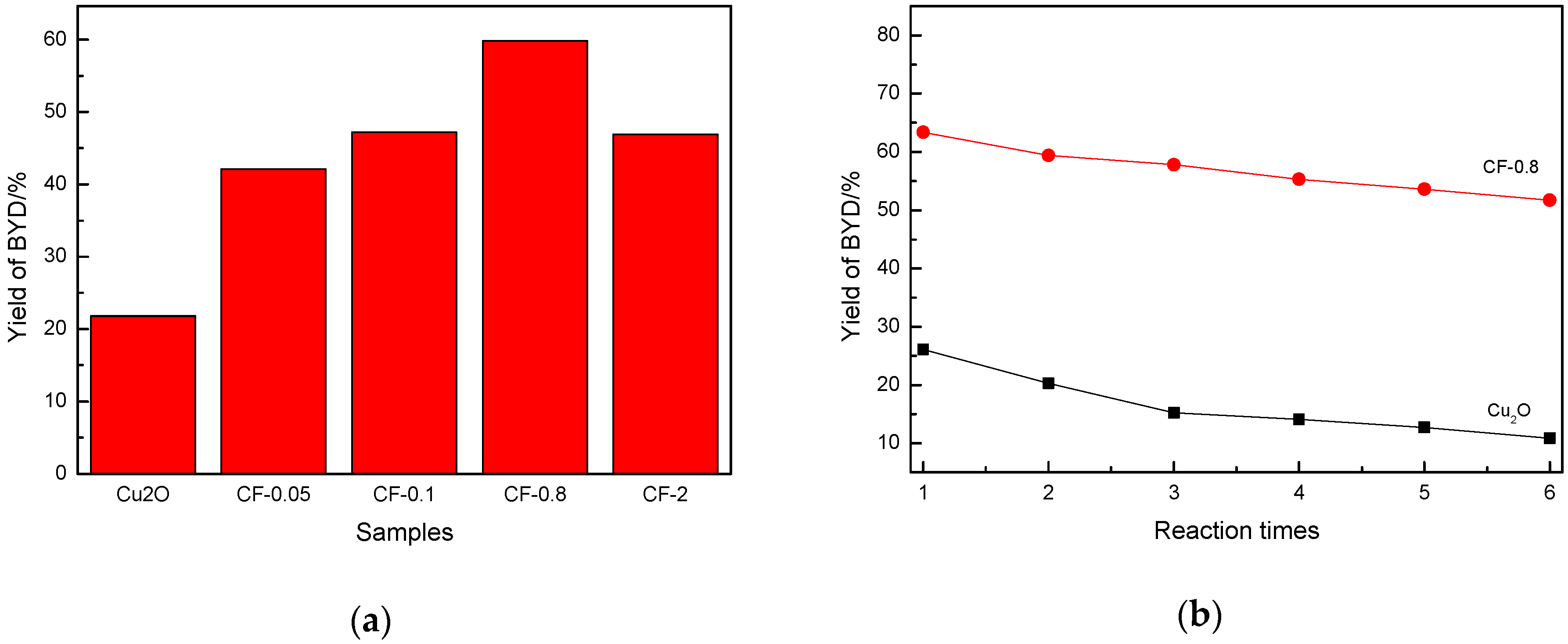
3.7. Evaluation Results of Catalyst Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tanielyan, S.; Schmidt, S.; Marin, N.; Alvez, G.; Augustine, R. Selective hydrogenation of 2-butyne-1,4-diol to 1,4-butanediol over particulate Raney® nickel catalysts. Top. Catal. 2010, 53, 1145–1149. [Google Scholar] [CrossRef]
- Nadgeri, J.M.; Telkar, M.M.; Rode, C.V. Hydrogenation activity and selectivity behavior of supported palladium nanoparticles. Catal. Commun. 2008, 9, 441–446. [Google Scholar] [CrossRef]
- Trotus, I.T.; Zimmermann, T.; Schüth, F. Catalytic reactions of acetylene: A feedstock for the chemical industry revisited. Chem. Rev. 2014, 114, 1761–1782. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, Y.; Gao, C.; Wang, Y.; Sun, Z.; Liang, X. Study on deactivation of Ni/Al2O3 catalyst for liquid phase hydrogenation of crude 1,4-butanediol aqueous solution. Chem. Eng. J. 2012, 181–182, 501–507. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Ikushima, Y.; Arai, M. Hydrogenation of 2-butyne-1,4-diol in supercritical carbon dioxide promoted by stainless steel reactor wall. Catal. Today 2004, 93–95, 439–443. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Li, H.; Gao, C.; Zhao, Y. Heterogeneous CaO-ZrO2 acid-base bifunctional catalysts for vapor-phase selective dehydration of 1,4-butanediol to 3-buten-1-ol. Appl. Catal. A Gen. 2013, 466, 233–239. [Google Scholar] [CrossRef]
- Li, H.T.; Ban, L.J.; Wang, Z.P.; Meng, P.F.; Zhang, Y.; Wu, R.F.; Zhao, Y.X. Regulation of Cu species in CuO/SiO2 and its structural evolution in ethynylation reaction. Nanomaterials 2019, 9, 842. [Google Scholar] [CrossRef]
- Yang, G.F.; Li, H.T.; Zhang, H.X.; Wang, Z.P.; Liu, L.L.; Zhao, Y.X. Effect of NaOH concentration on structure and catalytic performance of Cu2O for formaldehyde ethynylation. J. Mol. Catal. 2016, 30, 540–546. [Google Scholar]
- Eugene, V.H.; Pispacaway, N.J. Ethynylation Catalyst and Process for Production Alkynols by Low Pressure Reactions. US 3920759, 18 November 1975. [Google Scholar]
- Zak, D.J. Butynediol Production. US 4085151, 18 April 1978. [Google Scholar]
- Fremont, J.M. Preparation of Butynediol Using Bismuth Modified Spheroidal Malachite. U.S. Patent 4127,734, 28 November 1978. [Google Scholar]
- Haas, T.; Jaeger, B.; Weber, R.; Mitchell, S.F.; King, C.F. New diol process: 1,3-propanediol and 1,4-butanediol. Appl. Catal. A Gen. 2005, 280, 83–88. [Google Scholar] [CrossRef]
- Ma, Z.Q.; Zhang, H.X.; Li, H.T. Preparation of core-shell CuO-Bi2O3@meso-SiO2 catalyst and its catalytic performance for formaldehyde ethynylation. Ind. Catal. (China) 2015, 23, 344–348. [Google Scholar]
- Luo, M.; Li, H.T.; Ma, Z.Q.; Wang, J.J.; Zhao, Y.X. Researches on activation process of CuO-Bi2O3 /SiO2-MgO catalyst in formaldehyde ethynylation reaction. Ind. Catal. (China) 2014, 22, 363–368. [Google Scholar]
- Zheng, Y.; Sun, Z.J.; Wang, Y.Z.; Li, H.T.; Wang, S.A.; Luo, M.; Zhao, J.L.; Zhao, Y.X. Preparation of CuO-Bi2O3/SiO2-MgO catalyst and its ethynylation performance. J. Mol. Catal. (China) 2012, 26, 233–238. [Google Scholar]
- Wang, J.J.; Li, H.T.; Ma, Z.Q.; Wang, Z.P.; Guo, J.Y.; Zhao, Y.X. Preparation of magnetic CuO-Bi2O3/Fe3O4-SiO2-MgO catalyst and its catalytic performance for formaldehyde ethynylation. J. Chem. Ind. Eng. 2015, 66, 2098–2104. [Google Scholar]
- Wang, Z.P.; Niu, Z.Z.; Hao, Q.A.; Ban, L.J.; Li, H.T.; Zhao, Y.X.; Jiang, Z. Enhancing the ethynylation performance of CuO-Bi2O3 nanocatalysts by tuning Cu-Bi interactions and phase structures. Catalysts 2019, 9, 35. [Google Scholar] [CrossRef]
- Li, H.T.; Niu, Z.Z.; Yang, G.F.; Zhang, H.X.; Wang, Z.P.; Zhao, Y.X. Support effect of Cu2O/TiO2 employed in formaldehyde ethynylation. J. Chem. Ind. Eng. 2018, 69, 2512–2518. [Google Scholar]
- Wang, Z.P.; Ban, L.J.; Meng, P.F.; Li, H.T.; Zhao, Y.X. Ethynylation of Formaldehyde over Binary Cu-Based Catalysts: Study on Synergistic Effect between Cu+ Species and Acid/Base Sites. Nanomaterials 2019, 9, 1038. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Hao, Q.A.; Wang, Z.P.; Ban, L.J.; Meng, P.F. Study on catalytic of CuO-ZnO catalyst prepared by different precipitants. Ind. Catal. (China) 2019, 33, 124–131. [Google Scholar]
- Lakhera, S.K.; Venkataramana, R.; Watts, A.; Anpo, M.; Neppolian, B. Facile synthesis of Fe2O3/Cu2O nanocomposite and its visible light photocatalytic activity for the degradation of cationic dyes. Res. Chem. Intermed. 2017, 43, 5091–5102. [Google Scholar] [CrossRef]
- Jiang, H.; Dai, Y.; Hu, Y.; Chen, W.; Li, C. Nanostructured ternary nanocomposite of rGO/CNTs/MnO2 for high-rate supercapacitors. ACS Sustain. Chem. Eng. 2014, 2, 70–74. [Google Scholar] [CrossRef]
- Pal, J.; Ganguly, M.; Dutta, S.; Mondal, C.; Negishi, Y.; Pal, T. Hierarchical Au-CuO nanocomposite from redox transformation reaction for surface enhanced Raman scattering and clock reaction. CrystEngComm 2014, 16, 883–893. [Google Scholar] [CrossRef]
- Wang, Z.; Luan, D.; Li, C.M.; Zhang, M.; Lei, D.; Yin, X.; Chen, L.; Li, Q.; Wang, Y.; Wang, T. Magnetite/Graphene composites: Microwave irradiation synthesis and enhanced cycling and rate performances for lithium ion batteries. J. Mater. Chem. 2010, 20, 5538–5543. [Google Scholar]
- Zhao, Z.; Liu, J.; Cui, F.; Feng, H.; Zhang, L. One-pot synthesis of tunable Fe3O4–MnO2 core–shell nanoplates and their applications for water purification. J. Mater. Chem. 2012, 22, 9052–9057. [Google Scholar] [CrossRef]
- Choi, Y.; Hong, S.; Liu, L.; Kim, S.K.; Park, S. Galvanically replaced hollow Au–Ag nanospheres: Study of their surface plasmon resonance. Langmuir 2012, 28, 6670–6676. [Google Scholar] [CrossRef] [PubMed]
- Pal, J.; Mondal, C.; Sasmal, A.K.; Ganguly, M.; Negishi, Y.; Pal, T. Account of nitroarene reduction with size- and facet-controlled CuO–MnO2 nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 9173–9184. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, Z.; Yao, W.; Wang, P.; Yu, S.; Wang, X. Decorating of Ag and CuO on Cu nanoparticles for enhanced high catalytic activity to the degradation of organic pollutants. Langmuir 2017, 33, 7606–7614. [Google Scholar] [CrossRef] [PubMed]
- Kaviyarasan, K.; Anandan, S.; Mangalaraja, R.V.; Sivasankar, T.; Ashokkumar, M. Sonochemical synthesis of Cu2O nanocubes for enhanced chemiluminescence applications. Ultrason. Sonochem. 2016, 29, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Lakheraa, S.K.; Watts, A.; Haffeez, H.Y.; Neppolian, B. Interparticle double charge transfer mechanism of heterojunction α-Fe2O3/Cu2O mixed oxide catalysts and its visible light photocatalytic activity. Catal. Today 2018, 300, 58–70. [Google Scholar] [CrossRef]
- Huang, K. The long wave modes of the Cu2O Lattice. Zeitschrift für Physik 1963, 171, 213–225. [Google Scholar] [CrossRef]
- Dawson, P.; Hargreave, M.M.; Wilkinson, G.R. The dielectric and lattice vibrational spectrum of cuprous oxide. J. Phys. Chem. Solids 1973, 34, 2201–2208. [Google Scholar] [CrossRef]
- Singhal, A.; Pai, M.R.; Rao, R.; Pillai, K.T.; Lieberwirth, I.; Tyagi, A.K. Copper(I) oxide nanocrystals-one step synthesis, characterization, formation mechanism and photocatalytic properties. Eur. J. Inorg. Chem. 2013, 14, 2640–2651. [Google Scholar] [CrossRef]
- Rodriguez, R.D.; Sheremet, E.; Deckert-Gaudig, T.; Chaneac, C.; Hietschold, M.; Deckert, V.; Zahn, D.R. Surface- and tip-enhanced Raman spectroscopy reveals spin-waves in iron oxide nanoparticles. Nanoscale 2015, 7, 9545–9551. [Google Scholar] [CrossRef] [PubMed]
- Bersani, D.; Lottici, P.P.; Montenero, A. A micro-Raman study of iron-titanium oxides obtained by sol-gel synthesis. J. Mater. Sci. 2000, 35, 4301–4305. [Google Scholar] [CrossRef]
- Huang, W.C.; Lyu, L.M.; Yang, Y.C.; Huang, M.H. Synthesis of Cu2O nanocrystals from cubic to rhombic dodecahedral structures and their comparative photocatalytic activity. J. Am. Chem. Soc. 2012, 134, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Tian, Y.L.; Zhang, J.D. Construction of p-n heterojunction film of Cu2O/a-Fe2O3 for efficiently photoelectrocatalytic degradation of oxytetracycline. J. Colloid Interface Sci. 2018, 526, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.H.; Zhang, X.M.; Xu, J.; Han, Y.F. Effects of preparation methods on the activity of CuO/CeO2 catalysts for CO oxidation. Front. Chem. Sci. Eng. 2017, 11, 603–612. [Google Scholar] [CrossRef]
- Wang, J.C.; Zhang, L.; Fang, W.X.; Ren, J.; Li, Y.Y.; Yao, H.C.; Wang, J.S.; Li, Z.J. Enhanced photoreduction CO2 activity over direct z-scheme α-Fe2O3/Cu2O heterostructures under visible light lrradiation. ACS Appl. Mater. Interfaces 2015, 7, 8631–8639. [Google Scholar] [CrossRef]
- Wilson, D.; Langell, M.A. XPS analysis of oleylamine/oleic acid capped Fe3O4 nanoparticles as a function of temperature. Appl. Surf. Sci. 2014, 303, 6–13. [Google Scholar] [CrossRef]
- Kuo, C.H.; Huang, M.H. Facile synthesis of Cu2O nanocrystals with systematic shape evolution from cubic to octahedral structures. J. Phys. Chem. C 2008, 112, 18355–18360. [Google Scholar] [CrossRef]
- Ho, J.Y.; Huang, M.H. Synthesis of submicrometer-sized Cu2O crystals with morphological evolution from cubic to hexapod structures and their comparative photocatalytic activity. J. Phys. Chem. C 2009, 113, 14159–14164. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.C.; Kim, A.; Kim, A.Y.; Lee, H.J.; Song, H.; Park, K.H. Cu2O nanocube-catalyzed cross-coupling of aryl halides with phenols via ullmann coupling. Eur. J. Inorg. Chem. 2009, 28, 4219–4223. [Google Scholar] [CrossRef]
- Colacino, E.; Villebrun, L.; Martinez, J.; Lamaty, F. PEG3400-Cu2O-Cs2CO3: An efficient and recyclable microwave-enhanced catalytic system for ligand-free ullmann arylation of indole and benzimidazol. Tetrahedron 2010, 66, 3730–3735. [Google Scholar] [CrossRef]
- Kiss, J.; Óvári, L.; Oszkó, A.; Pótári, G.; Tóth, M.; Baán, K.; Erdóhelyi, A. Structure and reactivity of Au-Rh bimetallic clusters on titanate nanowires, nanotubes and TiO2(110). Catal. Today 2012, 181, 163–170. [Google Scholar] [CrossRef]
- Óvári, L.; Berkó, A.; Balázs, N.; Majzik, Z.; Kiss, J. Formation of Rh-Au Core-Shell Nanoparticles on TiO2(110) Surface Studied by STM and LEIS. Langmuir 2010, 26, 2167–2175. [Google Scholar] [CrossRef]
- Zhu, H.; Du, M.L.; Wang, Y.; Wang, L.N.; Zou, M.L.; Zhang, M.; Fu, Y.Q. A new strategy for the surface-free-energy-distribution induced selective growth and controlled formation of Cu2O-Au hierarchical heterostructures with a series of morphological evolutions. J. Mater. Chem. A 2013, 1, 919–929. [Google Scholar] [CrossRef]
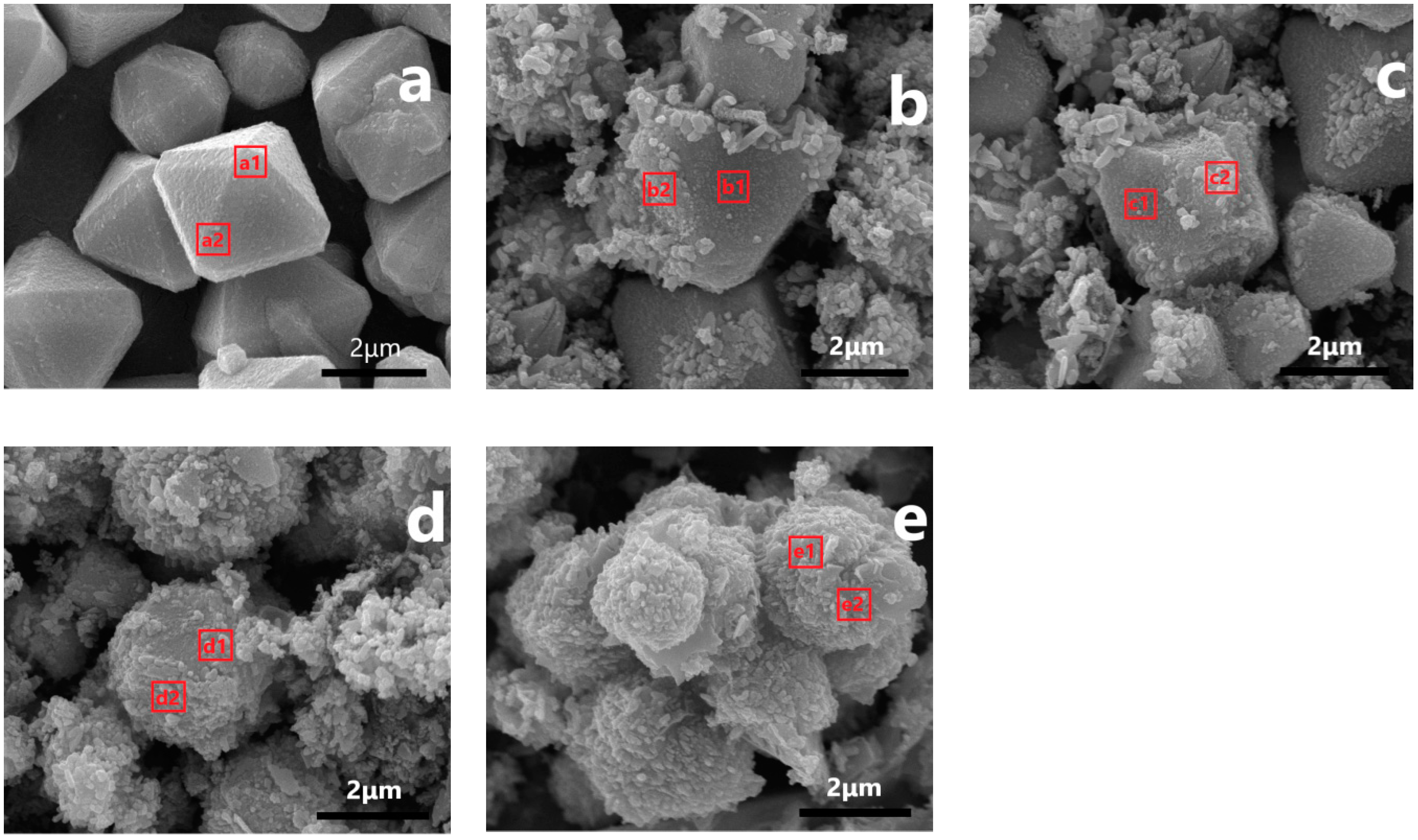
| Catalysts | Region | AT% | ||
|---|---|---|---|---|
| OK | FeK | CuK | ||
| Cu2O | a1 | 7.67 | 0 | 92.33 |
| a2 | 8.97 | 0 | 91.03 | |
| CF-0.05 | b1 | 7.85 | 0 | 92.15 |
| b2 | 13.91 | 10.46 | 75.63 | |
| CF-0.1 | c1 | 8.67 | 0 | 91.33 |
| c2 | 17.36 | 14.84 | 67.80 | |
| CF-0.8 | d1 | 29.33 | 35.74 | 34.92 |
| d2 | 29.12 | 35.97 | 34.91 | |
| CF-2 | e1 | 33.23 | 41.96 | 24.81 |
| e2 | 34.12 | 41.72 | 24.16 | |
| Catalysts | Fe(eV) | Fe2+/Fe3+ (Atomic) | Cu(eV) | Cu2+/Cu+ (Atomic) | Fe/Cu (Atomic) | ||
|---|---|---|---|---|---|---|---|
| Fe2+2p3/2 | Fe3+2p3/2 | Cu+2p3/2 | Cu2+2p3/2 | ||||
| Cu2O | - | - | - | 932.4 | 934.7 | 0.31 | - |
| CF-0.05 | 710.1 | 711.6 | 0.07 | - | - | - | 0.26 |
| CF-0.8 | 710.1 | 711.6 | 0.26 | 932.8 | 934.7 | 0.83 | 0.79 |
| CF-2 | 710.1 | 711.6 | 0.32 | 932.8 | 934.7 | 2.3 | 1.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Ban, L.; Niu, Z.; Huang, X.; Meng, P.; Han, X.; Zhang, Y.; Zhang, H.; Zhao, Y. Application of CuxO-FeyOz Nanocatalysts in Ethynylation of Formaldehyde. Nanomaterials 2019, 9, 1301. https://doi.org/10.3390/nano9091301
Li H, Ban L, Niu Z, Huang X, Meng P, Han X, Zhang Y, Zhang H, Zhao Y. Application of CuxO-FeyOz Nanocatalysts in Ethynylation of Formaldehyde. Nanomaterials. 2019; 9(9):1301. https://doi.org/10.3390/nano9091301
Chicago/Turabian StyleLi, Haitao, Lijun Ban, Zhuzhu Niu, Xin Huang, Pingfan Meng, Xudong Han, Yin Zhang, Hongxi Zhang, and Yongxiang Zhao. 2019. "Application of CuxO-FeyOz Nanocatalysts in Ethynylation of Formaldehyde" Nanomaterials 9, no. 9: 1301. https://doi.org/10.3390/nano9091301
APA StyleLi, H., Ban, L., Niu, Z., Huang, X., Meng, P., Han, X., Zhang, Y., Zhang, H., & Zhao, Y. (2019). Application of CuxO-FeyOz Nanocatalysts in Ethynylation of Formaldehyde. Nanomaterials, 9(9), 1301. https://doi.org/10.3390/nano9091301





